Abstract
We have followed sex and second chromosome disjunction, and the effects of these chromosomes on sperm function, in four genotypes: wild-type males, males deficient for the Y-linked crystal locus, males with an X chromosome heterochromatic deficiency that deletes all X–Y pairing sites, and males with both deficiencies. Both mutant situations provoke chromosome misbehavior, but the disjunctional defects are quite different. Deficiency of the X heterochromatin, consonant with the lack of pairing sites, mostly disrupts X–Y disjunction with a decidedly second-level effect on major autosome behavior. Deleting crystal, consonant with the cytological picture of postpairing chromatin-condensation problems, disrupts sex and autosome disjunction equally. Even when the mutant-induced nondisjunction has very different mechanics, however, and even more importantly, even in the wild type, there is strong, and similar, meiotic drive. The presence of meiotic drive when disjunction is disrupted by distinctly different mechanisms supports the notion that drive is a normal cellular response to meiotic problems rather than a direct effect of particular mutants. Most surprisingly, in both wild-type and crystal-deficient males the Y chromosome moves to the opposite pole from a pair of nondisjoined second chromosomes nearly 100% of the time. This nonhomologous interaction is, however, absent when the X heterochromatin is deleted. The nonhomologous disjunction of the sex and second chromosomes may be the genetic consequence of the chromosomal compartmentalization seen by deconvolution microscopy, and the absence of Y–2 disjunction when the X heterochromatin is deleted suggests that XY pairing itself, or a previously unrecognized heterochromatic function, is prerequisite to this macrostructural organization of the chromosomes.
AFTER a century of using Drosophila melanogaster as a model organism for eukaryotic transmission genetics we do not expect major surprises in our understanding of basic meiotic processes: homologs disjoin, nonhomologs move independently, and different gamete genotypes are functionally equal. Normal meiosis and gametogenesis thus yield balanced segregation and independent assortment. Exceptions are of course known. In Drosophila females, nonexchange chromosome have a propensity to nondisjoin, and nonexchange, nonhomologs may disjoin from each other. In Drosophila males, various examples of meiotic drive, the unequal recovery of reciprocal gamete classes, are well known. Nevertheless, these are exceptional situations, and strange disjunctions and distorted ratios of reciprocal classes are usually interpreted as the effects of mutant expression. Recently, however, there has been the delightfully surprising observation from deconvolution microscopy of separation of the X chromosome and major autosomes into distinct compartments during first prophase of male meiosis to which we now add some equally surprising genetic inferences. In wild-type as well as some mutant males, the sex chromosomes and major autosomes regularly end up at opposite poles if the autosomes happen to nondisjoin, and, among sperm arising from those cells, again even without the presence of any mutant to provoke it, the recovery of reciprocal classes is highly distorted.
High frequencies of nondisjunction and strong meiotic drive are found in a variety of mutant situations in D. melanogaster males (Lyttle 1993). This experiment started out as an attempt to clarify the relationship between two of these mutants by examining their separate and combined effects on behavior of the large second chromosome. The two mutant situations are deletion of the X chromosome ribosomal DNA (rDNA) and surrounding heterochromatin (Mckee and Lindsley 1987) and deletion of the Y-linked cry locus (Hardy et al. 1984) that interacts with the X-linked Ste clusters (Livak 1984; Shevelyov 1992; Palumbo et al. 1994).
Distinct heterochromatic X–Y pairing sites (collochores) were first described in fixed material (Cooper 1964) and deconvolution microscopy (Vazquez et al. 2002) is revealing the temporal dynamics of the pairing. From the genetic standpoint, it has long been known that X-heterochromatic deletions, particularly In(1)sc4Lsc8R that removes all of the rDNA, provoke elevated nondisjunction of the X and Y (Gershenson 1933) and distorted recovery of reciprocal sperm genotypes (Sandler and Braver 1954). Sperm function depends on chromosome content, with relative recoveries being 0 > X > Y ≫ XY (Kastenbaum 1958). A few copies of a short rDNA intergenic spacer sequence are sufficient to yield normal disjunction and balanced segregation (Ren et al. 1997), but other, weaker, collochores are also present distal to the rDNA (Cooper 1964; Mckee and Lindsley 1987). Moreover, the rDNA is implicated in the meiotic drive produced by some translocations (Mckee 1987) and in chromosome-based male sterility (Mckee et al. 1998). There do not seem to be any published studies of autosomal nondisjunction in rDNA or X-heterochromatin deficiencies, but Mckee (1984), by using an autosomal translocation, demonstrated that autosomal content also affects relative sperm recovery, i.e., drive, in rDNA− males.
The cry-Ste interaction is quite different. Spermatocytes of males deficient for the Y-linked cry locus contain crystalline accretions of an analog of the β-subunit of casein kinase II encoded by the multicopy X-linked Ste loci (Bozzetti et al. 1995). With high Ste copy number, cry− males are sterile, while with low Ste copy number, cry− males are fertile but sex chromosome nondisjunction is elevated and there is strong meiotic drive (again, following the rule that sperm recoveries are 0 > X > Y ≫ XY). Sex chromosome nondisjunction is tightly correlated with Ste copy number (Palumbo et al. 1994), while meiotic drive responds only slightly to Ste copy number (Robbins et al. 1996) and remains strong even in the absence of all X-linked copies (Belloni et al. 2002). Second chromosome nondisjunction also occurs, in Ste copy-number-correlated fashion, in cry− males (Palumbo et al. 1994). Cytologically, pairing appears to be normal in these males, but chromosomes become decondensed and fail to disjoin (Palumbo et al. 1994), and the same cytological picture is seen in males homozygous for homeless (Stapleton et al. 2001), one of several autosomal recessive mutants that closely mimic the Ste hyperexpression, nondisjunction, and meiotic drive produced by the cry deficiency (Tritto et al. 2003).
The results reported here clearly discriminate between the disjunctional defects of the X heterochromatin and cry deficiencies, confirming cytological indications that, while the X heterochromatin is responsible for X–Y pairing, the cry-Ste interaction causes nondisjunction by interfering with postsynaptic processes. Deficiency of the X heterochromatin mostly disrupts X–Y disjunction and hardly affects behavior of the major autosomes, while deleting crystal disrupts sex and autosome disjunction equally. Even when the mutant-induced nondisjunction has very different mechanics, however, and more importantly, even in the wild type, there is strong, and similar, meiotic drive. Most surprisingly, in the pairing-intact situations, i.e., in wild type and in the crystal deficiency, the Y chromosome preferentially moves to the opposite pole from a pair of nondisjoined second chromosomes, but deletion of the X heterochromatin results in equivalent disjunctional behavior of the two sex chromosomes with respect to the nondisjoining seconds and may actually suppress all nonhomologous disjunction.
MATERIALS AND METHODS
Mutant characteristics and experimental design:
Except as described here, details of markers and chromosomes may be found in Lindsley and Zimm (1992) or at the FlyBase web site. The four males tested were: wild type, SteW12/BSY·y+; cry−, SteW12/BScry1Y·y+; collochore−, Df(1)X1, SteW12 Bx/BSY·y+; and the double mutant cry− collochore−, Df(1)X1, SteW12 Bx/BScry1Y·y+. For convenience, Df(1)X1 is referred to as Xh− in the remainder of the text. It should be kept in mind that Df(1)X1 lacks not only the rDNA but all of the heterochromatin distal to the rDNA and much of the rDNA-proximal block as well. For comparability of the crosses, the X chromosomes all carried the same Ste allele (W12) and the test males were generated with some effort to keep a consistent genetic background; the Xh deficiency was introduced into the wild-type chromosome by a single crossover and the autosomal background was homogenized by several generations of backcrosses, and the cry+ and cry− test males were half-sibs bearing either BScry+Yy+ or BScry1Yy+ chromosomes from intercrossed attached-X stocks. Ste copy number does differ between the Xh+ and Xh− chromosomes because the latter lacks the heterochromatic Ste cluster, but we have already demonstrated that this does not change the level of cry−-induced meiotic drive (Belloni et al. 2002). To completely sample sperm, we used an excess of attached-2 [C(2)EN, bw sp] females, and because fecundity of these crosses is quite low, groups of five males and 15 females were placed in each vial.
Measures of disjunction and meiotic drive in attached-2 crosses:
Because the recovery of each progeny class depends on both disjunction and drive, there are few comparisons of observed numbers that lend themselves to simple interpretations. Just as a simple 9:3:3:1 dihybrid F2 ratio makes sense only if understood in terms of the underlying properties of gene transmission (1:1 segregation and 1:1 parental:recombinant) and the underlying properties of gene expression (viability, dominance, and absence of gene interaction), we can best understand the gamete frequencies produced by these experiments if we disentangle the disjunctional events and the effects on sperm function that underlie them.
Each cross produces eight progeny classes, seven of them independent, and the algebraic description of disjunctional events and chromosome-specific sperm recoveries has six parameters. Hence, unlike crosses in which only the sex chromosomes are followed (Mckee 1984), there is no set of unique solutions for the parameters in terms of the observed numbers. Maximum-likelihood estimates of the parameters were therefore found using the MLIKELY.PAS program (Robbins 2000). Although the excess of independent observations over parameters makes likelihood analysis necessary, it also means that there is 1 d.f. available (for each cross) for asking whether the model adequately describes the biology. Goodness-of-fit was tested by χ2 and hypotheses were compared using the G-statistic. A detailed explanation of the likelihood analysis may be found in supplemental materials at http://www.genetics.org/supplemental/.
RESULTS
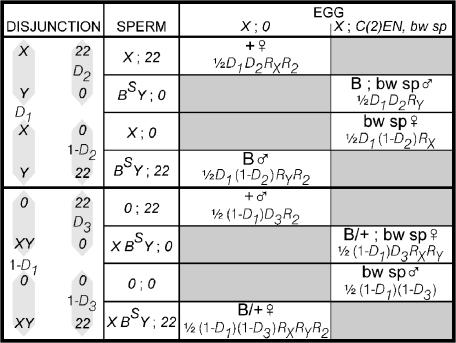
As illustrated in Figure 1, the eight progeny types produced in crosses to attached-2 females come only from spermatocytes in which the second chromosomes nondisjoin. Proceeding from left to right in Figure 1, we can describe their origins as follows. The X and Y chromosomes disjoin (Figure 1, top half, probability D1) or nondisjoin (Figure 1, bottom half, probability 1 − D1). Disjunction of the two second chromosomes yields only aneuploid, lethal zygotes, but a nondisjunctional pair of second chromosomes may disjoin from the Y (with probability D2 if the X and Y have disjoined or probability D3 if the X and Y have nondisjoined) or go to the same pole as the Y (with probabilities 1 − D2 and 1 − D3). If behavior of the sex chromosomes and autosomes is independent, both D2 and D3 will be 0.5.
Figure 1.
Origins and phenotypes of progeny produced in crosses to attached-2 females.
The proportions of zygote types then depend on the functionality of the various sperm. As in Mckee's (1984) formulation for sex chromosome disjunction and drive, we assume that the effects of the various chromosomes on sperm function are independent. Mckee has experimentally shown this to be true for the X and Y chromosomes in rDNA− males, but the consequences of relaxing this assumption for our crosses are considered below. Hence, assigning RX to the recovery of sperm that bear an X chromosome, RY to the recovery of sperm that contain a Y chromosome, and R2 to the recovery of sperm with two second chromosomes, we multiply the probabilities of the disjunctional events by the reduced recovery caused by the presence of each chromosome to arrive at the expected frequencies of the functional gametes. Note that these are frequencies among all gametes, including those that we never recover because of drive, and that they do not sum to one.
This descriptive model is simplified; it assumes Mendelian ratios among the eggs produced by attached-2 females, but a few percent X chromosome nondisjunction and a tendency for nondisjoined X chromosomes and attached autosomes to go to opposite poles has been reported for both C(3L);C(3R) (Harger and Holm 1980) and C(2)EN (Falk 1983) females. The products of maternal nondisjunction are not distinguishable with the markers used, but the effect of confounding maternal and paternal events is conservative; that is, it biases the parameter estimates toward Mendelian behavior in the males. Moreover, maximum-likelihood analysis using the excess degrees of freedom to deduce the frequencies of maternal events shows that their effects are, in any case, insignificant for this data set. The following refers to the simplified model, but details of the complete descriptive model and its analysis may be found in supplemental materials at http://www.genetics.org/supplemental/.
Frequencies of autosomal and sex chromosome nondisjunction:
The data are shown in Table 1. For comparison of autosomal and sex chromosome behavior, Table 2 shows data for crosses of the same genotype males to free-2 females.
TABLE 1.
Sperm genotypes and descriptive parameters for progeny of crosses to attached-2 females
| Malea | Nb | X22 | X0 | Y22 | Y0 | XY22 | XY0 | 022 | 00 | NDc | D1d | D2d | D3d | RXd | RYd | R2d |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| +/cry+Y | 3245 | 29 | 40 | 0 | 42 | 0 | 10 | 0 | 1 | 0.08 | 0.88 | 0.99 | 1.00 | 1.00 | 0.01 | 0.01 |
| (0.80–0.94) | (0.81–1.00) | (0.90–1.00) | (0.79–1.00) | (0.00–0.30) | (0.00–0.17) | |||||||||||
| Xh−/cry+Y | 1230 | 5 | 51 | 1 | 40 | 1 | 19 | 2 | 15 | 0.22 | 0.71 | 0.61 | 0.71 | 1.00 | 0.51 | 0.06 |
| (0.62–0.79) | (0.42–0.81) | (0.42–0.89) | (0.57–1.00) | (0.19–1.00) | (0.02–0.12) | |||||||||||
| +/cry−Y | 835 | 110 | 554 | 4 | 446 | 1 | 97 | 104 | 36 | 3.24 | 0.73 | 0.81 | 0.98 | 0.49 | 0.09 | 0.05 |
| (0.69–0.76) | (0.74–0.88) | (0.97–0.99) | (0.41–0.58) | (0.05–0.14) | (0.03–0.07) | |||||||||||
| Xh−/cry−Y | 125 | 1 | 96 | 1 | 43 | 1 | 21 | 2 | 79 | 3.90 | 0.61 | 0.40 | 0.46 | 0.68 | 0.46 | 0.02 |
| (0.54–0.69) | (0.18–0.70) | (0.17–0.85) | (0.25–1.00) | (0.16–1.00) | (0.01–0.05) |
+, SteW12; Xh−, Df(1)X1, SteW12 Bx; cry+Y, BSY y+; cry−Y, BScry1Y y+.
Number of males crossed. Matings were peformed in vials with five males and 15 females.
2 × progeny/male.
Maximum-likelihood estimates of disjunctional frequencies and chromosome-specific sperm recoveries (see text). Ninety-five percent support intervals are in parentheses.
TABLE 2.
Sperm genotypes and descriptive parameters for progeny of crosses to normal females
| Malea | Nb | X2 | Y2 | XY2 | 02 | NDc | Dd | RXd | RYd |
|---|---|---|---|---|---|---|---|---|---|
| +/cry+Y | 160 | 13326 | 11471 | 1 | 3 | 0.025 | 1.00 | 0.62 | 0.54 |
| (1.00–1.00) | (0.14–1.0) | (0.12–0.88) | |||||||
| Xh−/cry+Y | 165 | 1318 | 436 | 76 | 3115 | 19.3 | 0.61 | 0.27 | 0.09 |
| (0.58–0.64) | (0.24–0.31) | (0.08–0.10) | |||||||
| +/cry−Ye | 121 | 9843 | 6600 | 40 | 254 | 2.4 | 0.99 | 0.49 | 0.33 |
| (0.99–0.99) | (0.41–0.57) | (0.27–0.38) | |||||||
| Xh−/cry−Y | 164 | 1022 | 221 | 29 | 2845 | 17.5 | 0.62 | 0.22 | 0.05 |
| (0.58–0.67) | (0.18–0.26) | (0.04–0.06) |
+, SteW12; Xh−, Df(1)X1, SteW12 Bx;cry+Y, BSY y+; cry−Y, BScry1Y y+.
Number of males crossed. Matings were performed with one male and three females.
(Exceptional progeny)/male.
X–Y disjunction and chromosome-specific sperm recoveries (see text): 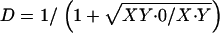 ,
, 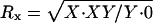 , and
, and 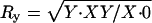 . Support intervals found using the method of maximum likelihood are shown in parentheses.
. Support intervals found using the method of maximum likelihood are shown in parentheses.
Results from Belloni et al. (2000).
Because only second chromosome nondisjunctional zygotes survive in attached-2 crosses, we can neither directly measure the frequency of second chromosome exceptions nor estimate a meiotic rate of second chromosome nondisjunction. The fecundity of the crosses, however, provides a relative measure of second chromosome nondisjunction. [We did not use the alternative measure of progeny per total eggs laid for several reasons: (1) The low frequency of nondisjunction in the control would require counting an enormous number of eggs; (2) meiotic drive implies the presence of nonfunctional sperm, and an egg count, without an accurate estimate of the fraction of unfertilized eggs, would have little meaning; and (3) we deliberately used a threefold excess of females in the crosses to exhaustively sample the sperm, further exacerbating the problem of unfertilized eggs. In other words, the simpler measure of progeny per parental male, while crude, has fewer uncertainties than an estimate based on egg counts.] We can compare autosomal with sex chromosome nondisjunction in Tables 1 and 2 by comparing twice the progeny per male for the attached-2 crosses with exceptional offspring per male for the free-2 crosses. The factor of two is needed because half of the fertilizations by diplo-2 and nullo-2 sperm yield aneuploid, hence lethal, zygotes, but all XY and nullo-X nullo-Y sperm in the crosses to free-2 females yield viable XXY females and X0 males.
We can also compare sex chromosome nondisjunction among autosomally nondisjunctional (Table 1) and autosomally disjunctional cells (Table 2), but for this we use the meiotic rates of nondisjunction (1 − D1 and 1 − D, respectively) as this does not confound disjunction with meiotic drive. The second- and sex-chromosome nondisjunction frequencies and sex chromosome meiotic nondisjunction rates are included in Tables 1 and 2 and compared graphically in Figures 2 and 3.
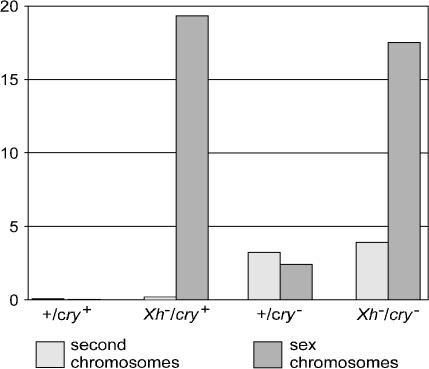
Figure 2.
Frequencies per parental male of second chromosome and sex chromosome nondisjunction. Second chromosome nondisjunction per male is calculated as (2 × progeny)/male for the crosses to C(2)EN females, and sex chromosome nondisjunction per male is calculated as (exceptional progeny)/male for the free-2 crosses (see text).
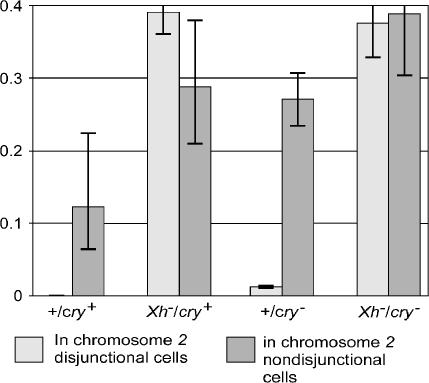
Figure 3.
Meiotic rate of sex chromosome nondisjunction in cells in which the second chromosomes have disjoined normally and in cells in which the second chromosomes have nondisjoined. Maximum-likelihood estimates were obtained numerically using the MLIKELY.PAS program. The 95% support intervals shown are the nondisjunction rates at which likelihood-ratio comparisons with the best estimates yield G > 3.842.
The effects of the mutants on overall sex chromosome and autosome nondisjunction frequency are seen in Figure 2. Both XY and autosomal nondisjunction are very infrequent in wild type. Deleting the X heterochromatin causes only a slight (<3-fold) increase in second-chromosome nondisjunction, although XY nondisjunction increases >700-fold. In contrast, deleting cry yields substantially elevated levels of both second-chromosome and XY nondisjunction. As suggested by the cytology, nondisjunction is initiated by different mechanisms in the two cases, one chromosome specific and the other apparently cell-wide. Moreover, the two pathways leading to nondisjunction appear to be independent: the double deficiency produces the simple sum of the two effects, XY nondisjunction is equal to that produced by deleting the X heterochromatin and autosomal nondisjunction is at the level produced by deleting cry.
Sex chromosome behavior in the subpopulations of second chromosome normal and second chromosome nondisjunctional cells is compared in Figure 3. In wild-type males, although sex chromosome and autosomal nondisjunction are individually rare, in those cells in which the second chromosomes nondisjoin the sex chromosomes frequently fail to disjoin as well (1 − D is near 0, but 1 − D1 = 0.12). The same strong interaction of the sex and second chromosomes is found in cry− males (1 − D = 0.01 vs. 1 − D1 = 0.29). In contrast, XY nondisjunction is quite frequent in both Xh− genotypes, but is independent of the disjunctional state of the second chromosomes. In those males, the frequency of sex chromosome nondisjunction is no higher among autosomal-exceptional cells than among autosomal-normal cells.
Nature of the autosome–sex chromosome interaction:
The high frequency of XY nondisjunction in second chromosome nondisjunctional cells in wild-type and cry− males could simply signal the existence of a subpopulation of particularly sick cells in which both the sex chromosomes and autosomes misbehave or could result from truly nonindependent behavior of nonhomologs. In the first case, the absence of this increase in Xh− males would merely indicate a greater uniformity in the germ-line population; sex chromosome behavior is already so abnormal in the Xh− genotypes that a proportionately much smaller increase in XY nondisjunction among second chromosome nondisjunctional cells might simply go undetected. In the second case, the absence of an increased frequency of sex chromosome nondisjunction in Xh− 2-exceptional cells would imply a role for the X heterochromatin in the nonhomologous interaction.
A hint that the difference between 2-regular and 2-exceptional cells is not merely a consequence of division of the meiotic population into relatively healthy and relatively sick cells, but is produced by disjunction of nonhomologs to opposite poles, has already been noted in Palumbo et al. (1994), and the +/cry− data in Table 1 show the same peculiarity as did the smaller crosses of cry− males carrying five different Ste alleles reported there. Despite strong meiotic drive that yields a nearly 5:1 overall ratio between recovered nullo-2 and diplo-2 sperm, the ratio of 0;0 to 0;22 sperm is inverted to ∼1:3. Palumbo et al. (1994) also noted that the same discrepancy was present in the translocation-generated cry-deficiency crosses of Hardy et al. (1984). Regular separation of the two pairs of nondisjunctional chromosomes, XY to one pole and 22 to the other, could yield an excess of 0;22 sperm despite the presence of meiotic drive because there would be few 0;0 nuclei to begin with. We do not discount the notion that there are two populations of cells, some sick and some more-or-less healthy. Cells in which the second chromosomes nondisjoin are, by definition, sick meiocytes. What this disparity shows, however, is that the elevated rate of XY nondisjunction among 2-exceptional cells is not just an artifact of population structure.
Note that most simple ratios among progeny classes confound both disjunctional pattern and drive effects. For example, if we knew that there were no meiotic drive, an excess of (0;22 + XY;0) compared to (XY;22 + 0;0) would suggest nonhomologous disjunction, but given the possibility of drive, and its roughly inverse relationship between chromatin content and sperm recovery, a distortion of this ratio could result from nonrandom disjunction, meiotic drive, or both. An excess of 0;22 vs. 0;0 is actually the only situation for which an “eyeball” inspection yields an obvious inference—independent disjunction in the absence of drive would have given equality, and independent disjunction plus drive would have given an excess of 0;0. Hence, the observed inequality directly implies that there is a disjunctional interaction between the sex and second chromosomes. In the other genotype for which nondisjunction frequencies suggest a sex chromosome-second chromosome interaction, the wild-type males, there were so few offspring in these two classes that we cannot infer anything from their ratio. For the two Xh− crosses in contrast, we observe an excess of 0;0 vs. 0;22, but that disparity says only that drive is strong; it does not rule out the occurrence of nonhomologous disjunction as well. There are two ways in which we can examine this further. We can evaluate separately the effects of disjunction and drive in the attached-2 crosses, and we can compare ratios of pairs of classes between the attached-2 and free-2 crosses.
Disjunction in the attached-2 crosses:
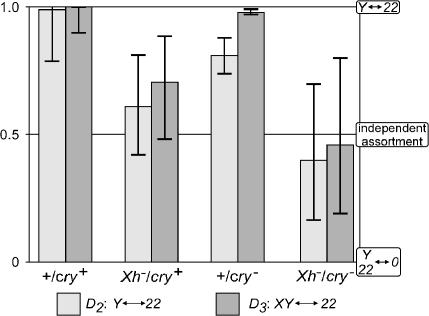
Disjunctional and drive effects were disentangled for the attached-2 crosses using maximum-likelihood estimation of the descriptive parameters illustrated in Figure 1. The results for disjunction are shown in Figure 4, where the maximum-likelihood estimates of D2 and D3 are indicated along with 95% support intervals to indicate the precision of these estimates. Among cells in which the X and Y chromosomes disjoin, the Y chromosome and second chromosomes preferentially go to opposite poles (i.e., D2 > 0.5) in both wild-type and cry− males, the two genotypes in which the frequency of sex chromosome nondisjunction is higher in 2-nondisjunctional cells. The support intervals for D2 for cry− and wild-type males largely overlap, and the small difference is not significantly significant (G = 2.687, 1 d.f., P = 0.101).
Figure 4.
Disjunctional interactions between the sex and second chromosomes. Maximum-likelihood estimates and support intervals for the rates of separation of the Y and second chromosomes to opposite poles (D2) or of nondisjunctional sex chromosomes and the second chromosomes to opposite poles (D3) were found as described in Figure 3.
In Xh− males, however, the frequency of XY nondisjunction is the same in 2-regular and 2-nondisjunctional cells (Figure 3), and here we see that there is also no indication of Y–2 disjunction when the X heterochromatin is deleted. The best estimates of D2 for the Xh−/cry+ and Xh−/cry− crosses are not significantly different from each other (G = 1.392, 1 d.f., P = 0.238) and they are not significantly different from D2 = 0.5 (G = 1.747, 2 d.f., P = 0.417). The difference between the Xh+ and Xh− crosses is, however, highly significant (G = 10.5, 1 d.f., P = 0.001). The Y and second chromosomes go to opposite poles in Xh+ males, but a nondisjunctional pair of second chromosomes is equally likely to be at the pole opposite the X chromosome or that opposite the Y chromosome in Xh− males.
The disjunctional behavior of the sex chromosomes and autosomes in XY-nondisjunctional cells gives a similar result. D3 is near 1 in both wild-type and Xh+/cry− males; the XY and 22 pairs almost always go to opposite poles. These nonhomologs behave independently, however, if the X heterochromatin is deleted. D3 for Xh−/cry+ and Xh−/cry− males are not significantly different from each other (G = 1.303, 1 d.f., P = 0.254), nor do they differ from D3 = 0.5 (G = 3.443, 2 d.f., P = 0.179). Once again, the difference between the Xh+ and Xh− genotypes is highly significant (G = 35.1, 1 d.f., P = 3.2 × 10−9).
Note that the two nonhomologous-disjunction parameters have somewhat different meanings. D2 measures the tendency of the Y chromosome and nondisjoined 22 pair to go to opposite poles; it therefore measures imbalance in the interactions of the two sex chromosomes with the second. D3, in contrast, measures the frequency with which a nondisjoined XY pair ends up at the opposite pole from the 22 pair and says nothing about differential behavior of the X and Y. That both D2 and D3 significantly exceed 0.5 in the two Xh+ crosses indicates that there is nonhomologous disjunction of the sex and second chromosomes and that the Y has a substantially stronger interaction with the second than does the X. That D2 is indistinguishable from 0.5 in the Xh− crosses implies that the X and Y chromosomes behave similarly—either they do not interact with the second at all or they interact equally. That D3 is not significantly different from 0.5 in the Xh− crosses implies that deleting the X heterochromatin suppresses nonhomologous interaction rather than promoting equally likely disjunction of both the X and Y chromosomes from the autosomes.
Inferences about disjunction from comparison of attached-2 and free-2 crosses:
Although simple progeny ratios confound drive and disjunctional parameters, comparing certain ratios in the two series of crosses can isolate some of them if the other parameters cancel in taking the ratio of ratios. In particular, if we assume that chromosome-specific effects on drive are the same in both sets of crosses (but see below), and we compare free-2 and attached-2 crosses in which the frequency of sex chromosome nondisjunction is the same, which is approximately the case for the Xh−/cry+ and Xh−/cry− crosses, but decidedly not the case for the wild-type and Xh+/cry− crosses, we should be able to detect X–22 disjunction, Y–22 disjunction, and XY–22 disjunction. For example, if there is no X–22 nonhomologous disjunction, the ratio (X;2)/(0;2) in a free-2 cross should be the same as the ratio (X;0)/(0;0) in an attached-2 cross; all other parameters cancel out if X chromosome drive and XY nondisjunction are the same in both crosses. In formal terms, we can see this relationship, and estimate the degree of X–2 disjunction, in the following way. The ratio (X;2)/(0;2) for a free-2 cross is RXD/(1 − D), where RX measures the effect of the X chromosome on sperm function and D is the frequency of disjunction of the X and Y chromosomes. The ratio (X;0)/(0;0) for an attached-2 cross is RXDDX/(1 − D)(1 − DX) in which DX is the frequency of X–22 disjunction. Dividing these two, we get (1 – DX)/DX, which is 1 if DX = 0.5, and from which we can find DX if this ratio is not 1. For Xh−/cry+ males (X;2)/(0;2) = 1318/3115 = 0.42, and (X;0)/(0;0) = 51/15 = 3.4; and these are clearly not equal (contingency χ2 = 69, 1 d.f., P < 10−10). Calculating DX, we get DX = 0.89, a substantial tendency for the X and nondisjunctional second chromosomes to separate from each other. The ratios of diplo-2 classes could also be used in these calculations, but very few diplo-2 sperm were recovered in these two crosses and their use would not materially change these estimates. In the same manner, we can estimate DY, the frequency of Y–22 disjunction, = 0.95, and DXY, the frequency of XY–22 disjunction, = 0.98, for Xh−/cry+ males. Both the X chromosome and the Y chromosome seem to disjoin equally from the second chromosome, instead of there being an absence of nonhomologous disjunction. The maximum-likelihood estimates of chromosome-specific sperm recoveries are not the same in the two sets of crosses, however, and if we recalculate the values of DX, DY, and DXY taking these differences into account, we get DX = 0.68, DY = 0.77, and DXY = 0.71, a good bit closer to the suppression of nonhomologous disjunction indicated by the analysis of the attached-2 crosses alone.
Comparing the attached-2 and free-2 crosses for Xh−/cry− males, we get DX = 0.77, DY = 0.98, and DXY = 0.96 if we do not take account of the observed differences in drive levels between the attached-2 and free-2 crosses, but DX = 0.52, DY = 0.43, and DXY = 0.48 if we do take account of these differences. In other words, when differences in drive levels are accounted for, the within-attached-2-cross analysis and the comparison of attached-2 and free-2 crosses give equivalent results.
To summarize, sex chromosome nondisjunction is substantially elevated among 2-nondisjunctional meioses in wild-type and in Xh+/cry− males, and the excess of 0;22 (compared with 0;0) sperm in Xh+/cry− males suggested that simultaneous nondisjunction of the sex and second chromosomes is accompanied by separation of the two pairs of errant chromosomes to opposite poles. Extraction of the disjunctional parameters from the attached-2 data showed that this is also true for the Y and second chromosomes in cells in which the sex chromosomes have disjoined normally, that it occurs in wild-type as well as in cry− males, and that the bias toward a Y–2 rather than an X–2 interaction disappears if the X heterochromatin is deleted. The doubly nondisjunctional classes suggest a suppression of all nonhomologous interactions, rather than equality of X–2 and Y–2 interactions, in males lacking the X heterochromatin. Comparison of the attached-2 and free-2 data, if done taking account of differences in drive level, also indicates that there is little (in Xh−/cry+ males) or no (in Xh−/cry− males) nonhomologous disjunction of either the X or the Y chromosomes when the X heterochromatin is absent.
Meiotic drive:
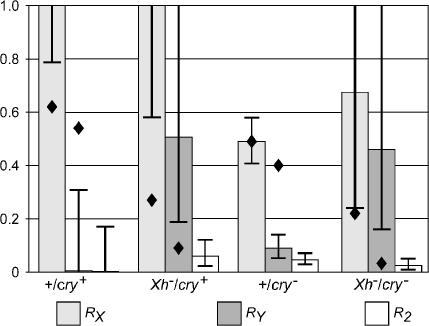
The same analysis that extracts disjunction from the confounded effects of disjunction and drive also separates the effects of each chromosome on sperm recovery. These results are illustrated in Figure 5. In all of the genotypes, RX > RY, and R2 is not far from zero. An excess of haplo-2 sperm could be explained by loss of the 2 from what should have been haplo-2 nuclei, but this would not explain sex chromosome drive. The behavior of these normal, but nondisjoined, second chromosomes is quite similar to the nearly complete sperm lethality of some attached-2 chromosomes (Dernburg et al. 1996a). The values of RX and RY estimated for the free-2 crosses (Figure 5, diamonds) also follow the RX > RY ≫ RXRY rule, but the levels of drive are quantitatively rather different for some of the genotypes.
Figure 5.
Chromosome-specific effects on sperm function. Maximum-likelihood estimates and support intervals for RX, RY, and R2 were found as indicated in Figure 3. Values of RX and RY for the crosses to free-2 females are indicated by diamonds; the values for the wild-type × free-2 cross are particularly unreliable because that cross produced only four nondisjunctional offspring.
Within the attached-2 data, there are also some statistically significant quantitative differences. For the X chromosome, there is a significant difference (G = 18.0, 3 d.f., P = 0.00045) ascribable to a difference between the pair of cry+ and the pair of cry− genotypes. There is also a significant difference for the Y chromosome (G = 15.1, 3 d.f., P = 0.0017), but in this case it is the pairs of Xh+ and Xh− genotypes that differ. For the second chromosome, there is no significant difference at all (G = 3.2, 3 d.f., P = 0.36). Whether these small and contradictory differences actually indicate slight differences in how drive is produced or merely the presence of extraneous factors such as marker effects on viability, we do not know. It is nevertheless clear that there is meiotic drive in all four genotypes and that the overall properties of the drive are similar in all of them and qualitatively similar to the drive seen in free-2 crosses.
One might wonder how these males could be at all fertile given the large fraction of nonfunctional sperm among these gametes or whether we are grossly underestimating the frequency of autosomal nondisjunction when we count offspring per father because only a fraction of 2-nondisjunctional events yield functional sperm. Estimates of the numbers of sperm affected, however, show that loss of fertility is not massive and that the rate of autosomal nondisjunction has not been grossly underestimated (a step-by-step example of the calculation is given in supplemental materials at http://www.genetics.org/supplemental/).
The healthiest of these genotypes is the Xh+/cry+ wild type. For these, ∼57% of the 2-exceptional sperm are nonfunctional, and we estimate the total frequency of chromosome 2 nondisjunction as 0.17 sperm per male. Presumably, chromosome 3 nondisjunction would yield a similarly modest amount of sperm mortality. In any case, few sperm are nonfunctional and there is no contradiction between this analysis and the fertility of wild-type males.
All of the other male genotypes are sick and not fully fertile, and neither the sperm wastage nor the frequency of autosomal nondisjunction is out of reason. For Xh−/cry+ males, nonfunctional exceptional-2 sperm are ∼1 per male. For Xh+/cry− males, total 2-nondisjunctional sperm are also roughly five times the number of recovered 2-nondisjunctional zygotes, and second chromosome nondisjunction would reduce fecundity in crosses to free-2 females by ∼15 sperm per male. Finally, in Xh−/cry− males total 2-nondisjunctional sperm are roughly four times the number of 2-nondisjunctional zygotes for a total of 16 dead sperm per male. In all of these genotypes, autosomal nondisjunction and drive account for little of the observed loss of fertility.
Adequacy of the descriptive model for attached-2 crosses, sources of uncertainties, and consideration of some alternative hypotheses:
The most striking result of the foregoing analysis is the apparent nonhomologous disjunction of the Y and second chromosomes, but that result depends on the reasonableness of the model used to describe disjunction and sperm survival and on the absence of reasonable alternatives.
Is this a reasonable description? In this model, no constraints were placed on disjunctional events, but it was assumed that the effects of each chromosome on sperm function are independent and do not change in different disjunctional subclasses. After estimating the six parameters, we have 1 d.f. left over for each cross, 4 d.f. for the entire set, and we can compare expected numbers derived from the maximum-likelihood parameter estimates with the observed numbers by χ2. There appears to be a significant difference between observed and expected numbers (χ2 = 19.1, 4 d.f., P = 0.0007), but nearly all of the difference is in two classes for which the expected numbers are so small that χ2 is unreliable (E = 3.58, O = 0 for 0;22 in the +/cry+Y cross, and E = 0.08, O = 1 for XY;22 in the +/cry−Y cross). Summing the observed and expected numbers for those classes with the numbers for the reciprocal genotypes yields a nonsignificant difference (χ2 = 2.59, 2 d.f., P = 0.27), as does simply excluding those two classes (χ2 = 4.61, 2 d.f., P = 0.1).
Although differences involving small expected numbers are intrinsically unreliable, we can use the remaining degree of freedom to relax, one at a time, each of the model's constraints. Allowing different effects of the Y on sperm survival in Y-only sperm and in XY sperm, we get χ2 = 12.3. Allowing different effects of the X on sperm survival in X-only and XY sperm, we get χ2 = 19.1. Finally, allowing different effects of the 22 pair on sperm survival in XY-disjunctional and XY-nondisjunctional cells, we get χ2 = 0.7. Only the last of these suggests a possible source of the lack of fit, but what is the magnitude of the deviation from independence? For +/cry+ males, R2 assuming independence is 0.004, while the separate values for XY-disjunctional and XY-nondisjunctional cells are 0.005 and 0.000. For Xh−/cry+ males, the estimates are 0.06 (assuming independence) and 0.057 and 0.068 (without independence). For the other two genotypes fit was near perfect to begin with, so the maximum-likelihood estimates of R2 are the same whether or not independence is assumed. These differences in survival of 22 sperm coming from XY-normal and XY-nondisjunctional cells, even if one were tempted to give any weight to “significant differences” that involve small expected numbers, are hardly of any biological importance; “statistically significant” is clearly not the same thing as “strong” or “important.” Hence, we can conclude that the model, if not perfect, is at least reasonable.
The disjunctional parameters estimated using this model point toward a difference in the sex chromosome/autosome interaction in Xh+ and Xh− males. D2, the frequency with which a disjunctional Y chromosome and nondisjunctional 22-pair go to opposite poles, is close to 1 in the Xh+ crosses and not significantly different from 0.5 in the Xh− crosses; the two sex chromosomes behave differently in the former, but equivalently in the latter. D3, the frequency with which a nondisjunctional XY-pair and nondisjunctional 22-pair disjoin, is also close to 1 in the Xh+ crosses and not significantly different from 0.5 in the Xh− crosses; it appears that the sex chromosome/autosome interaction disappears in Xh− males. What we do not know is how sensitive the estimates of one parameter are to the uncertainties in the estimates of the others. We have found support intervals, in many cases rather broad, for each parameter, but we do not know what range of values would be plausible for D3 with all possible combinations of acceptable values for the other parameters. It is possible that a difference between the true population value and the best estimate of one or more of the other parameters biased D3 toward 0.5. We can be reasonably confident that the sex chromosomes interact differently with nondisjunctional second chromosomes in Xh+ and Xh− males, but we have less confidence in the inference that deleting Xh eliminates nonhomologous disjunction.
Is there an alternative to nonhomologous disjunction? In other words, is it possible that the sex chromosomes and autosomes move independently, but that the effects of genotype on sperm function do not follow the usual rules? We have explored this possibility by evaluating three alternative hypotheses that each remove most of the constraints on drive, but assume independent disjunction of nonhomologs. For the first alternative, six parameters were also used to describe each cross: D, the frequency of XY nondisjunction; RX, recovery of sperm that bear an X (but not a Y) chromosome; RY, the recovery of sperm that bear a Y (but not an X) chromosome; RXY, the recovery of sperm that bear both sex chromosomes; R2a, the recovery of sperm with two second chromosomes coming from cells in which the sex chromosomes have disjoined; and R2b, the recovery of sperm with two second chromosomes coming from cells in which the sex chromosomes have nondisjoined. The only constraint on drive remaining in this hypothesis is a partial requirement for independence of effects of the second and sex chromosomes on sperm function, partial because the recovery of 2-bearing sperm was allowed to differ between XY-regular and XY-nondisjunctional classes. This hypothesis quite clearly does not fit the data: χ2 = 231.7, 4 d.f., P < 1 × 10−10 with the deviations between observed and expected being unacceptably large for all of the classes in the Xh+ crosses (χ2 = 228.5, 2 d.f., P <1 × 10−10) and quite small for the Xh− crosses (χ2 = 3.12, 2 d.f., P = 0.21) in which there is no indication of nonhomologous disjunction in any case.
For the second alternative with no nonhomologous disjunction, the effect of a pair of second chromosomes on sperm function was allowed to differ for each sex chromosome genotype. In this way, we require independence only for the effects of the X and the Y chromosomes in nullo-2 sperm. The parameters were: D, the frequency of XY nondisjunction; RX, the effect of an X chromosome on nullo-2 sperm recovery; RY, the effect of a Y chromosome on nullo-2 sperm recovery; RX2, recovery of X;22 sperm; RY2, recovery of Y;22 sperm; RXY2, recovery of XY;22 sperm; and R02, recovery of 0;22 sperm. These seven parameters, each of which is a probability and hence between 0 and 1, use up all of the degrees of freedom and should give a perfect, or nearly perfect, fit to the data unless the model is completely absurd. Indeed, this hypothesis is absurd; with the parameters constrained to the 0–1 interval, we get χ2 = 50.9 rather than the perfect fit of χ2 = 0. As for the preceding alternative without nonhomologous disjunction, this hypothesis adequately describes the situation in Xh− males, but is patently incompatible with the wild-type and Xh+/cry− results.
For the third attempt at a hypothesis without nonhomologous disjunction, we assumed independent effects of the X and Y chromosomes on sperm function, but allowed both to differ depending on whether they were accompanied by two second chromosomes or none, and allowed different effects of the second in XY-disjunctional and XY-nondisjunctional products. This hypothesis, again with seven parameters, should also give a perfect fit but does not (χ2 = 134.8), and once again it is the wild-type and Xh+/cry− crosses that are incompatible with independent movement of the sex and second chromosomes. In summary, we have not found any way to explain the wild-type and cry− results except by invoking preferential Y–2 nonhomologous disjunction, a bias that disappears if the X heterochromatin is deleted.
Uncertainties intrinsic to the comparison of attached-2 and free-2 crosses:
If we could assume that nonhomologous disjunction is the only parameter that differs between the attached-2 and free-2 crosses, comparisons between them would suggest that deleting Xh balances nonhomologous disjunction rather than eliminating it. There are two senses in which this assumption is unreasonable. First, there are experimental differences. The two sets of crosses were not contemporaneous, the males did not necessarily share common genetic backgrounds, markers were necessarily different, and there is a gross difference in the basic health of attached-2 crosses in which most zygotes are aneuploid lethal and free-2 crosses in which almost none of the zygotes are aneuploid lethal. Second, there are observed differences. Although, for these two male genotypes, the frequencies of XY nondisjunction were similar in both the attached-2 and free-2 crosses, they were not identical. More importantly, although chromosome-specific sperm recoveries follow the usual order of RX > RY ≫ RXY in both data sets, the quantitative differences are more than minimal. Once one takes account of the differences in RX and RY between the two series, the appearance of equal X-22 and Y-22 nonhomologous disjunction mostly disappears for one Xh− genotype and completely disappears for the other.
DISCUSSION
Two caveats should be noted before proceeding to discussion of these results. First, the data presented are formal genetic data, unsupported by any physical or molecular observations. With these alone, we can suggest only a formal mechanism for the observed ratios, and the underlying cellular events remain, of course, unknown. Moreover, we have observed only a small minority of meiotic cells, those in which the second chromosomes have nondisjoined, and the inferences may apply only to that subpopulation of cells.
Second, although we have attempted to honestly consider alternative hypotheses and sources of error in the analyses, it is doubtful that we have thought of all possible alternatives. In addition to the possibilities of nonindependence of chromosome effects on sperm function and alternatives to nonhomologous disjunction described above, we have also tested and discarded some wildly ad hoc hypotheses, hypotheses in which one or more gamete classes in one or another cross follow rules completely different from the others. We offer what we think is a reasonable explanation for the genetic observations, but the validity of that explanation must await experimental tests, whether physical or formal, of predictions based on that hypothesis.
The cry− and Xh− meiotic defects:
Both the timing and mechanisms of nondisjunction are different in these two aberrant situations. Deleting the X heterochromatin leads to near-random movement of the sex chromosomes, causes only a slight increase in second chromosome nondisjunction, and either sex and autosome behavior are independent or, less likely, the X and Y chromosomes interact equally with the second chromosome; all results consonant with defective X–Y pairing. Deleting cry, in contrast, increases both sex chromosome and second chromosome nondisjunction, and there is a strong Y chromosome–autosome interaction. That interaction, however, is not specifically induced by deleting cry, it is also present in wild type (see below). These genetic observations accord well with cytology: the cry− defect is postpairing and seemingly cellwide. The only caveat to this is the observation (Hardy et al. 1984) that fourth chromosome nondisjunction is not elevated in cry− males, an observation that we have confirmed for cry− males carrying diverse Ste copy-number X chromosomes (data not shown). Why the tiny fourth chromosome escapes we do not know.
Nonhomologous disjunction:
The unscrambling of disjunctional and sperm recovery parameters done here can be likened to the separation of out-of-phase photons in deconvolution microscopy and yields an interesting genetic parallel to the compartmentalization of nonhomologs discovered with that methodology. Sequestering sex chromosomes and autosomes in separate compartments requires some means of distinguishing homolog from nonhomolog. Y–2 nonhomologous disjunction might be compartmentalization's basis, or its consequence, or compartmentalization and nonhomologous disjunction might be two effects of a common cause.
This is not the first report of an interaction between the second and sex chromosomes in D. melanogaster males. Falk (1983) had noted an excess of 0;attached-2 sperm among progeny of C(2)EN males, and Dernburg et al. (1996a), although they did not use marked sex chromosomes in their experiments, found sex-ratio distortions consistent with this. Dernburg et al. ascribed that interaction to the presence of sex chromosome-derived segments in C(2)EN that, they presumed, promoted sex chromosome-C(2)EN pairing. The second chromosomes followed in our experiments, however, were normal chromosomes that do not contain any pieces of either the X or the Y. Nevertheless, in wild-type and Xh+/cry− males second chromosome nondisjunction is accompanied by elevated sex chromosome nondisjunction and the second chromosomes regularly disjoin from the Y chromosome (when the X and Y go to opposite poles) or from the pair of sex chromosomes (if the X and Y chromosomes nondisjoin). Thus, neither a mutant genotype nor transposition of homologous segments is prerequisite to nonhomologous disjunction.
Does autosomal nondisjunction trigger the nonhomologous interaction? Purely genetic experiments cannot reveal a Y–2 interaction without second chromosome nondisjunction to mark the two poles. We cannot, however, exclude Y–autosome separation as a normal part of Drosophila male meiosis and, applying Okham's razor, we suggest that it is more likely a normal process than a process evolved to deal only with decidedly infrequent autosomal nondisjunction.
Obviously, since the prophase compartments and nonhomologous disjunction are newly discovered phenomena, or two aspects of the same unexpected phenomenon, it is time to pose some questions that would not have arisen in the past.
Two of these questions are essentially cytological. The first is whether Y–2 disjunction is pairing based, as is homologous disjunction, but a cytological marker for the Y chromosome that can be observed dynamically in living material is probably needed before this can be answered. Do note that up to here, we have referred only to “nonhomologous disjunction” and have carefully avoided “nonhomologous pairing.” What we have detected is disjunction, and the underlying mechanism might, but need not, involve physical contact of nonhomologs. Nonhomologous univalents disjoin regularly in Drosophila females, yet no physical contact occurs (Dernburg et al. 1996b), and a simple mechanical model based on the peculiarities of the spindle in Drosophila females has been proposed (Hawley and Theurkauf 1993). An example of a nonpairing mechanism in which nonhomologous disjunction is a consequence of domain formation rather than the reverse can be found in supplemental materials (http://www.genetics.org/supplemental/), but it is only a heuristic example and not something to be taken seriously until we know whether pairing is involved or not.
The second cytological issue is whether domain formation proceeds normally in Xh− males. Deleting the X heterochromatin largely eliminates nonhomologous disjunction. Does it also randomize spatial chromatin distribution or inhibit sequestering of sex chromosomes and autosomes in the proper zones?
Other questions are amenable to purely genetic tests: What parts of the Y and second chromosomes are responsible for the nonhomologous interaction? One should at least be able to find out if YL or YS is particularly important.
In Xh+ males, the Y chromosome preferentially disjoins from the second chromosome, but deleting the X heterochromatin obliterates the Y–2 bias and probably eliminates all sex chromosome/autosome nonhomologous disjunction. Our intuitive expectation would have been the reverse, that lack of X–Y pairing when Xh is deleted might lead to a secondary interaction between the Y and (nondisjoining) second chromosomes. Deleting the X heterochromatin changes how the sex chromosomes and autosomes interact, and understanding exactly what it does and how it does it demands new experiments rather than greater analytic artistry. What part of the X heterochromatin is necessary for promoting Y–2 disjunction: the major pairing site in the rDNA, the other collochores distal to the rDNA, the heterochromatic Ste copies that are also deleted in Df(1)X1, or something as yet unrecognized? Would, for example, introducing an rDNA array into a Df(1)X1 chromosome so that the chromosome contains rDNA but lacks most of the remainder of the heterochromatin restore Y–2 disjunction?
How does the X heterochromatin promote Y–2 disjunction? Does Xh “attract” the second away from the Y, change the conformation of the Y chromosome by pairing with it, pull it away from an autosomal compartment by pairing with it, or is some product of Xh required in trans for the interaction between the Y and the second? We cannot offer an answer, but, in addition to the possibility that future cytological observations will resolve this, we can suggest an experiment. If attached-2 crosses were done with a free Xh duplication added to both the Xh− and Xh+ genotypes, one should be able to, at least partially, distinguish between these possibilities.
Although we think that this is the first clear case of nonhomologous disjunction of normal chromosomes in D. melanogaster males and suspect that it is not restricted to the abnormal meioses in which a major autosome nondisjoins, nonhomologous disjunction has been long known in females (for reviews, see Hawley et al. 1993 and Mckim et al. 2002). In females, heterochromatic pairing serves as an imperfect backup mechanism for disjunction of nonexchange chromosomes; and nonexchange, nonhomologs can disjoin from each other. Given that meiosis is achiasmate in D. melanogaster males, Y–2 disjunction in males is not a mistake made by a backup system as it is in females. What function nuclear compartmentalization or nonhomologous disjunction serves must be, for now, a matter of speculation, but Hawley (2002) has suggested an interesting and plausible possibility: that keeping nonhomologs physically well separated helps keep weakly aligned homologs from falling apart or from getting entangled with the other chromosomes before anaphase. In other words, it may substitute for the chiasmata that keep bivalents distinct in females.
Meiotic drive:
The mechanics of nondisjunction are clearly different in cry− and Xh− males, but strong meiotic drive occurs, and has similar properties, in both of these abnormal situations. In these crosses to attached-autosome females, as well in crosses to free-autosome females, sperm recovery follows basically a simple rule: the less chromatin, the better the recovery. Meiotic drive is also detected, and has similar properties, in wild-type males, at least in those rare cells in which the second chromosomes nondisjoin. Drive intensities were different in attached-2 and free-2 crosses, but we are hard pressed to say whether these quantitative differences are meaningful given the enormous differences, both genetic and in the ecology of the cultures tubes, between the two series.
Does meiotic drive also occur when the autosomes disjoin normally? Crosses that expose autosomal nondisjunction usually seem unsatisfying because only nondisjunctional products survive. This was a major advantage for us, however, in that, although we had to cross >3000 males with 9000 females to get a barely adequate wild-type sample, an entirely reasonable amount of work was needed to score the progeny. Obtaining a similar wild-type sample in the absence of autosomal nondisjunction will mean scoring about half a million flies, most of which are uninformative, an exceedingly boring experiment that should nevertheless be done.
With a large enough sample, we have been able to detect meiotic drive in wild type as well as in mutants. Hence, whatever machinery produces drive, it is not a machine created ad hoc by the mutants, but is exposed by their high frequency of meiotic misbehavior. Mckee et al. (1998) have already suggested that the meiotic drive observed in aberrant situations is a symptom of an underlying, normal process that serves to avoid production of aneuploid gametes. In analogy to cell-cycle control, they also suggested that failure of homologous pairing triggers that system. Tomkiel (2000), having found a mutant that produces abundant autosomal nondisjunction but does not evince any drive, suggested that it is sex chromosome pairing failure in particular and not pairing failure in general that triggers drive and that sex chromosome pairing failure causes autosomal drive because unpaired sex chromosomes interact with the autosomes. Our experiments indicate that drive occurs in wild type (among chromosome 2 nondisjunctional products), that one sex chromosome–second chromosome interaction, Y–2 disjunction, also occurs in wild-type males, and that drive occurs both when that interaction occurs and when it is suppressed by deleting Xh. Hence, they do not identify a “trigger,” but raise doubts about both of the proposed triggers.
How can we identify the components of the normal meiotic machinery that produce meiotic drive when chromosomes misbehave? Mutants that yield nondisjunction and drive will not suffice because they expose rather than cause drive. Mutants that yield nondisjunction but do not provoke drive may include mutants actually defective in the drive machinery, but might merely disrupt processes extraneous to drive, and there is no obvious way to distinguish between these two interpretations. We suggest that a search for mutants that suppress drive in a genotype in which it is normally quite evident, such as in Xh−/cry− males, is in order.
A technical note:
Although surely of interest only to die-hard chromosome mechanics, we note that the difficult task of substituting chromosomes in an attached-autosome stock can now be greatly simplified. All that is needed is to have the cry1 Y in the father, and, as long as the X chromosome is not one of the relatively rare ones that carries a high-copy-number Ste allele, nullo-2 (or nullo-3) sperm will be so frequent that a few vials will suffice to transfer new set of X, third (or second), and fourth chromosomes into an attached-autosome female.
Acknowledgments
Research was supported by the Piano di Ateneo per la Ricerca 2001 and 2003 and by a Programmi di Ricerca Scientifica di Rilevante Interesse Nazionale (PRIN) 2004 grant from the Ministero dell'Istruzione dell'Università e della Ricerca.
References
- Belloni, M., P. Tritto, M. P. Bozzetti, G. Palumbo and L. G. Robbins, 2002. Does Stellate cause meiotic drive in Drosophila melanogaster? Genetics 161: 1551–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozzetti, M. P., S. Massari, P. Finelli, F. Meggio, L. A. Pinna et al., 1995. The Ste locus, a component of the parasitic cry-Ste system of Drosophila melanogaster, encodes a protein that forms crystals in primary spermatocytes and mimics properties of the beta subunit of casein kinase 2. Proc. Natl. Acad. Sci. USA 92: 6067–6071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper, K. W., 1964. Meiotic conjunctive elements not involving chiasma. Proc. Natl. Acad. Sci. USA 52: 1248–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dernburg, A. F., D. R. Daily, J. J. Yook, J. A. Crobin, J. W. Sedat et al., 1996. a Selective loss of sperm bearing a compound chromosome in the Drosophila female. Genetics 143: 1629–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dernburg, A. F., J. W. Sedat and R. S. Hawley, 1996. b Direct evidence of a role for heterochromatin in meiotic chromosome segregation. Cell 86: 135–146. [DOI] [PubMed] [Google Scholar]
- Falk, R., 1983. The effect of an unusual chromosome architecture on disjunction and non-disjunction in Drosophila. Genet. Res. 41: 17–28. [Google Scholar]
- Gershenson, S., 1933. Studies on the genetically inert region of the X chromosome of Drosophila. I. Behavior of an X chromosome deficient for a part of the inert region. J. Genet. 28: 297–312. [Google Scholar]
- Hardy, R. W., D. L. Lindsley, K. J. Livak, B. Lewis, L. Siversten et al., 1984. Cytogenetic analysis of a segment of the Y chromosome of Drosophila melanogaster. Genetics 107: 591–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harger, H., and D. G. Holm, 1980. Meiotic behavior of compound autosomes in females of Drosophila melanogaster: interchromosomal effects and the source of spontaneous nonsegregation. Genetics 96: 455–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley, R. S., 2002. Meiosis: how male flies do meiosis. Curr. Biol. 12: 660–662. [DOI] [PubMed] [Google Scholar]
- Hawley, R. S., and W. E. Theurkauf, 1993. Requiem for distributive segregation: achiasmate segregation in Drosophila females. Trends Genet. 9: 310–317. [DOI] [PubMed] [Google Scholar]
- Hawley, R. S., K. S. Mckim and T. Arbel, 1993. Meiotic segregation in Drosophila melanogaster females: molecules, mechanisms and myths. Annu. Rev. Genet. 27: 281–317. [DOI] [PubMed] [Google Scholar]
- Kastenbaum, M. A., 1958. Estimation of relative frequencies of four sperm types in Drosophila melanogaster. Biometrics 14: 223–228. [Google Scholar]
- Lindsley, D. L., and G. G. Zimm, 1992. The Genome of Drosophila melanogaster. Academic Press, San Diego.
- Livak, K. J., 1984. Organization and mapping of a sequence on the Drosophila melanogaster X and Y chromosomes that is transcribed during spermatogenesis. Genetics 107: 611–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyttle, T. W., 1993. Cheaters sometimes prosper: distortion of Mendelian segregation by meiotic drive. Trends Genet. 9: 205–210. [DOI] [PubMed] [Google Scholar]
- Mckee, B. D., 1984. Sex chromosome meiotic drive in Drosophila melanogaster males. Genetics 106: 403–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckee, B., 1987. X–4 translocations and meiotic drive in Drosophila melanogaster males: role of sex chromosome pairing. Genetics 116: 409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckee, B., and D. Lindsley, 1987. Inseparability of X-heterochromatic functions responsible for X:Y pairing, meiotic drive and male fertility in Drosophila melanogaster. Genetics 116: 399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckee, B. D., K. Wilhelm, C. Merrill and X. Ren, 1998. Male sterility and meiotic drive associated with sex chromosome rearrangements in Drosophila: role of X–Y pairing. Genetics 149: 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckim, J. S., J. K. Jang and E. A. Manheim, 2002. Meiotic recombination and chromosome segregation in Drosophila females. Annu. Rev. Genet. 36: 205–232. [DOI] [PubMed] [Google Scholar]
- Palumbo, G., S. Bonaccorsi, L. G. Robbins and S. Pimpinelli, 1994. Genetic analysis of Stellate elements of Drosophila melanogaster. Genetics 138: 1181–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, X., L. Eisenhour, C. Hong, Y. Lee and B. D. Mckee, 1997. Roles of rDNA spacer and transcription unit-sequences in X-Y meiotic chromosome pairing in Drosophila melanogaster males. Chromosoma 106: 29–36. [DOI] [PubMed] [Google Scholar]
- Robbins, L. G., G. Palumbo, S. Bonaccorsi and S. Pimpinelli, 1996. Measuring meiotic drive. Genetics 142: 645–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins, L. G., 2000. Do-it-yourself statistics: a computer-assisted likelihood approach to analysis of data from genetic crosses. Genetics 154: 13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler, L., and G. Braver, 1954. The meiotic loss of unpaired chromosomes in Drosophila melanogaster. Genetics 39: 365–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevelyov, Y. Y., 1992. Copies of a Stellate gene variant are located in the X heterochromatin of Drosophila melanogaster and are probably expressed. Genetics 132: 1033–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton, W., S. Das and B. D. Mckee, 2001. A role of the Drosophila homeless gene in repression of Stellate in male meiosis. Chromosoma 110: 228–240. [DOI] [PubMed] [Google Scholar]
- Tomkiel, J. E., 2000. Chromosomally-induced meiotic drive in Drosophila males: Checkpoint or fallout? Genetica 109: 95–103. [DOI] [PubMed] [Google Scholar]
- Tritto, P., V. Specchia, L. Fanti, M. Berloco, R. D'Alessandro et al., 2003. Structure, regulation and evolution of the crystal-Stellate system. Genetica 117: 301–311. [DOI] [PubMed] [Google Scholar]
- Vazquez, J., A. S. Belmont and J. W. Sedat, 2002. The dynamics of homologous chromosome pairing during male Drosophila meiosis. Curr. Biol. 12: 1473–1483. [DOI] [PubMed] [Google Scholar]