Abstract
Some populations of maize's closest relatives, the annual teosintes of Mexico, are unreceptive to maize pollen. When present in the pistil (silk and ovary) a number of maize genes discriminate against or exclude pollen not carrying the same allele. An analogous gene Tcb1-s was found in some teosinte populations but not in sympatric or parapatric maize. It was polymorphic among populations of teosinte growing wild, but regularly present in populations growing in intimate association with maize as a weed. Introduction of Tcb1-s into maize substantially to fully restored compatibility with Tcb1-s carrying teosintes. Although Tcb1-s pollen can fertilize tcb1 tcb1 maize, it is at a competitive disadvantage relative to tcb1 pollen. Hence, the influence of Tcb1-s on crossability is bidirectional. In the absence of maize, Tcb1-s can increase in teosinte populations without improving their fitness. In the presence of maize, Tcb1-s appears to have been co-opted to provide reproductive isolation for adaptation to a cultivated habitat.
WIND-POLLINATED plants rely on physiological interaction between pollen and pistil to regulate hybridization. At one end of the spectrum of crossability, incompatibility prevents hybridization that would be so disparate as to be dysgenic. At the other end of the spectrum, many outcrossing plants possess a genetic mechanism to prevent self-fertilization and crossing among close relatives, minimizing inbreeding. Between these extremes of cross- and self-incompatibility (CI and SI) lies a window of congruity where structures and products of the pistil and pollen complement one another to achieve fertilization. A growing list of genes involved in this congruous interaction is being enumerated, especially in the self-fertilizing species Arabidopsis (reviewed in Swanson et al. 2004). For several decades, SI has been the object of intensive genetic analysis (summarized in Denettancourt 2001). More recently, it has been characterized in cellular and molecular detail (reviewed in Nasralla 2002; Kao and Tsukamoto 2004). In contrast, understanding of CI remains primitive.
Investigation of CI has relied heavily on a link with SI. Pollen of SI species often can fertilize self-compatible congeners whereas the reciprocal cross fails (Lewis and Crowe 1958). Failure typically is attributable to the dominant alleles of a few genes, of which the SI locus is one (e.g., Bernacchi and Tanksley 1997). Murfett et al. (1996) characterized unilateral compatibility in Nicotina utilizing transformation of the S locus and antisense suppression of its RNase product. For some species, transfer of S RNase was sufficient to confer incompatibility. Others required the presence of additional factors, and for some it was not essential. Multiple rejection pathways were proposed. Studies with other species indicate the timing and nature of the rejection response differs in self- and interspecific incompatibilities (Asher and Peloquin 1968; Liedl et al. 1996).
Of course not all outcrossing species having unilateral crossing behavior are self-incompatible. Cultivated Zea mays is an example. Pollen-pistil incompatibility distorts transmission of alleles at six or more loci (Nelson 1993). If a particular allele of any one of the six is present in the pistil, pollen not having that allele is disadvantaged or excluded. These cryptic factors were discovered by their effect on linked genes: alleles coupled in cis to the pollen-pistil compatibility allele were recovered preferentially. Because behavior of a pollen grain reflects its own genotype, rather than the parent plant's, these loci were designated gametophyte factors (ga). To the extent tested, the genes involved distort transmission independently of one another. Among the six, a strong allele of ga1 has a more overt effect. On Ga1-s Ga1-s pistils, ga1 pollen fails to effect fertilization even in the absence of competing Ga1-s pollen, resulting in barrenness. The same is true for the Tcb1-s allele of the Teosinte crossing barrier-1 locus discovered in a population of annual teosinte (Kermicle and Allen 1990).
The coupling of female preference and pollen competence to the same chromosome region, possibly as pleiotropic effects of the same gene, makes these systems particularly efficient prezygotic barriers to crossing. In a population polymorphic for Ga1-s or Tcb1-s, the advantage of such an allele (generically P for pollen-pistil recognition) is illustrated qualitatively by considering the genotypic classes of seed borne by PP, Pp, and pp plants. Homozygous PP individuals bear only PP offspring since their pistils are unreceptive to p pollen. Pp heterozygotes receive P pollen in preference to p, thereby producing equal numbers of PP and Pp offspring. Only pp plants produce pp offspring. But fertilization by P pollen, which begets Pp progeny, reduces the proportion of pp each generation. Accordingly, p-containing genotypes decrease rapidly over generations, with the P allele driven toward fixation. Like meiotic drive, these systems of transmission ratio distortion affect haploid products. They differ from meiotic drive in that distortion is based on pollen (gametophyte) function rather than on a modified meiosis.
Such P genes could foster reproductive isolation. Some populations of teosinte in Central Mexico grow wild. Others have adapted to cultivation, thriving only as weeds or as ruderals in habitats disturbed by human activity (Sanchez-G. and Ruiz-C. 1997). Early studies showed that although the weedy populations resembled the local maize in plant habit, coloration, and maturity, hybridization was less frequent than in a semiwild population growing outside, but in close proximity to, maize fields (Wilkes 1977). In conjunction with his pioneering observations concerning the natural history of teosinte and maize in Mexico and Central America, Wilkes (1967) suggested that the low frequency of hybrids occurring in teosinte-infested maize fields might be due to pollen-pistil incompatibility of the sort associated with the Ga1-s gene. Because this gene confers a strong to exclusive advantage to pollen to function on pistils also carrying it, if it were present only in teosinte or only in maize, fertilization by the other would be prevented. Expressed in other terms, such systems in plants, like in free-spawning animals, involve sexual selection based on physiologic rather than visual recognition (Skogsmyr and Lankinen 2002).
There has been little concerted effort to determine the P-allelic composition in paired teosinte and sympatric or parapatric maize populations. Recently, Ga1-s was reported in 6 of 14 annual teosinte populations (Kermicle et al. 2005). However, all of the associated landrace maize populations carried the cross-neutral allele Ga1-m, which fertilizes Ga1-s/−, but accepts ga1 pollen. Thus it is not obvious how Ga1-s could serve as a primary barrier to crossing in this circumstance. Thirteen of the 14 teosinte populations were collected in Mexico. The distribution of tcb1 alleles among these 13 populations and their associated maize landraces, relating the different distributions to the wild vs. weedy habit of teosinte, is reported here. The findings implicate Tcb1-s as providing reproductive isolation while promoting its own propagation.
MATERIALS AND METHODS
Sympatric teosinte and maize populations:
Paired collections of teosinte and counterpart maize were assembled from two sources. Eight pairs, numbered as the 100 series in Table 1, were collected by the author under guidance of Suketoshi Taba, director of maize germplasm at the International Maize and Wheat Improvement Center, El Batan, Mexico. For these, seed of the open-pollinated maize landrace was taken from plants growing in the same field for the weedy teosinte populations, and from nearby fields for ruderal and wild teosinte populations. Five teosinte populations (200 series) were obtained from other workers. Maize counterparts for these were furnished by S. Taba as representative of the landraces traditionally grown in these regions. Seed collected by the author was used directly to initiate the experiments reported whereas all but collection 202 of those obtained from others were first propagated one or more generations.
TABLE 1.
Teosinte collections
| Location | Code no. | Z. mays subspecies | Sympatric/parapatric maize population |
|---|---|---|---|
| Cocotitlan, Edo. Mexico | 101 | mexicana | Chalqueño |
| Santiago Zula, Edo. Mexico | 102 | mexicana | Conico |
| Teloloapan, Guerrero | 104 | parviglumis | Pepitilla |
| Erendira, Michoacan | 105 | parviglumis | Conego |
| Hidalgo, Michoacan | 106 | mexicana | Conico |
| Copándaro, Michoacan | 107 | mexicana | Conico Norteno |
| Uriangato, Guanajuato | 109 | mexicana | Conico Norteno |
| Mexicaltzingo, Edo. Mexico | 110 | mexicana | Conico |
| Nobogame, Chihuahua | 201a | mexicana | Chih.-243 |
| Mazatlán, Guerrero | 202b | parviglumis | Guerrero-32 |
| deCuautitlan, Jalisco | 203c | parviglumis | Jalisco-437 |
| Durango, Durango | 205d | mexicana | Durango-201 |
| Degollado, Jalisco | 207e | mexicana | Jalisco-437 |
CIMMYT, W85-2.
G. Beadle, “El Salado.”
R. Guzman, unnumbered.
CIMMYT, W92-1.
L. M. dePuga, no. 11066.
A winter nursery located near Homestead, Florida, served to bridge the difference between the long-day-adapted incompatibility tester stocks and the short-day-adapted Mexican Zea materials. The latter were crossed and then backcrossed once to inbred line W22, which is free of known cross-incompatibility factors, to obtain stocks that could be propagated in summer nurseries at Madison, Wisconsin.
Genotyping cross-incompatibility:
Genotyping teosinte plants according to tcb1, Tcb1-s, and Tcb1-m (an allele having only pollen function, analogous to Ga1-m) proceeded in two stages: first, distinguishing tcb1 tcb1 plants from those carrying Tcb1-m or Tcb1-s, then distinguishing Tcb1-m from Tcb1-s. Three plants representing each teosinte population were hybridized with W22 ga1 tcb1 females, and from 6 to 17 plants in each hybrid family were then crossed onto a Ga1-m Tcb1-s tester. Each testcross samples a gamete derived from the teosinte parent. Progenies in which all or nearly all plants crossed successfully indicated the parental teosinte plant contained Tcb1-m or Tcb1-s, but not tcb1. Heterozygous tcb1 parental plants produced progenies in which one-half of the offspring crossed successfully, compared with none from tcb1 tcb1 teosinte parents. Other plants in the three F1 hybrid progenies were crossed to W22 tcb1 tcb1 to continue the introgression of the Tcb1-m and Tcb1-s alleles into W22. Additional backcrosses produced lineages suitable for distinguishing Tcb1-s from Tcb1-m and for removing Ga1-m or Ga1-s if present.
To distinguish between -m and -s alleles of Tcb1, plants of W22-introgressed lines testing positive for pollen competence were evaluated for the silk barrier by pollinating with a mixture of approximately equal amounts of pollen from two pure-breeding strains (Table 2). One, Ga1-m Tcb1-s, possesses pollen competence for both Ga1-s and Tcb1-s pistil barriers. Pollinations by this stock on the lines derived from teosinte or Mexican maize produce noncolored kernels. Pollen from the second source ga1 tcb1 carried all the dominant factors necessary for pigmenting the outer layer of the kernel's endosperm with anthocyanin. Crosses using the pollen mix onto a colorless, compatible ga1 tcb1 strain estimated the proportion of viable pollen derived from the two sources. Control crosses using the mix on two tester stocks, Ga1-s tcb1 and Ga1-m Tcb1-s, produced few if any colored kernels. Test plants containing the -s allele of either locus likewise discriminate against pollen in the mix that produces colored kernels, whereas plants containing -m alleles do not. To determine whether discrimination against ga1 tcb1 pollen was due to Ga1-s, to Tcb1-s or to both, pollen of the test plants was evaluated for compatibility on Ga1-s tcb1 and Ga1-m Tcb1-s tester females.
TABLE 2.
Compatibility test stocks
| Compatibility phenotype
|
||
|---|---|---|
| Stock | As female | As male |
| ga1 tcb1 | Receptive to all Zea pollen | Unable to fertilize Ga1-s Ga1-s or Tcb1-s/− |
| Ga1-s tcb1 | Homozygotes unreceptive and heterozygotes variably receptive to ga1 pollen | Fertilizes Ga1-s Ga1-s but not Tcb1-s/− |
| Ga1-m tcb1 | Receptive to all Zea pollen | Fertilizes Ga1-s Ga1-s but not Tcb1-s/− |
| Gal-m Tcb1-s | Unreceptive to tcb1 pollen | Fertilizes Ga1-s Ga1-s and Tcb1-s/− |
| ga1 Tcb1-s | Unreceptive to tcb1 pollen | Fertilizes Tcb1-s/− but not Ga1-s Ga1-s |
| ga1 Tcb1-m | Receptive to all Zea pollen | Fertilizes Tcb1-s/− but not Ga1-s Ga1-s |
Parallel procedures were followed in genotyping the Mexican landrace maize populations starting in this case with the first generation backcross lines.
Wind-pollinated tests of compatibility:
To determine compatibility of the teosinte populations with different maize pollinators, plants from each teosinte population were induced to flower under short days, basal shoots (tillers) removed, and the plants divided into two lots. One group was allowed to wind pollinate in a greenhouse section along with plants of other teosinte collections belonging to the same subspecies, that is, Z. mays mexicana or parviglumis. This group provides a baseline of potential seed production. The second lot was reared in another greenhouse, emasculated at least every other day and wind pollinated by interspersed maize plants. Repeated emasculation was necessary because the lateral inflorescences of plants in many teosinte populations have staminate tips partly enclosed in husk leaves, making complete emasculation difficult. Although the two groups representing an individual population were tested in parallel, not all of the populations were tested concurrently.
In one series of experiments the maize pollinator was Ga1-m tcb1, the most common genotype among the present collection of counterpart maize populations. The amount of seed produced relative to that from teosinte intercrosses measured incompatibility due to all sources other than Ga1-s. In a second series, Ga1-m Tcb1-s maize was pollinator. Improvement in set relative to the first series determined the rectification of compatibility due to Tcb1. Sample size ranged from 4 to 14 teosinte plants per collection for each treatment, averaging 7.5.
Large-scale testcrossing of Tcb1-s/tcb1 heterozygotes:
Crossovers in the immediate Tcb1 region of chromosome arm 4S were chosen among the progeny of gl7 tcb1 bm3 su1-B × gl7 tcb1 bm3 su1-B/Gl7 Tcb1-s Bm3 Su1 testcrosses. The gl7 marker resides 2 cM distal to tcb1, whereas bm3 and su1 reside 1 and 6 cM proximal. Initially, sugary kernels (su1-B su1-B) were separated from starchy kernels (Su1 su1-B). Then, recombinant, glossy seedlings grown from starchy kernels, and nonglossy seedlings grown from the sugary class, were selected in greenhouse plantings and transplanted to field plots. (The glossy-7 mutation causes a reduction in cuticular wax in first to fourth seedling leaves.) At flowering, all brown-midrib plants (bm3 bm3) within the nonglossy seedling class and all nonbrown-midrib plants from the glossy class, i.e., recombinants for the markers flanking tcb1, were evaluated for pollen and pistil Tcb1 actions through reciprocal crosses with Tcb1-s Su/tcb1 su1-B testers. Only plants containing Tcb1-s, or possible recombinant Tcb1-m pollen, successfully fertilize the tester female. In the reciprocal cross, Tcb1-s pistil action in any plant in the recombinant population selects for Tcb1-s pollen thereby restricting transmission of su1-B linked to tcb1 in the tester male. Such crosses produce ∼3% sugary kernels, reflecting 6% recombination between Tcb1 and su1 loci (Evans and Kermicle, 2001), rather than the Mendelian F2 ratio of 3 starchy to 1 sugary.
RESULTS
Hybridization of teosinte by maize pollen:
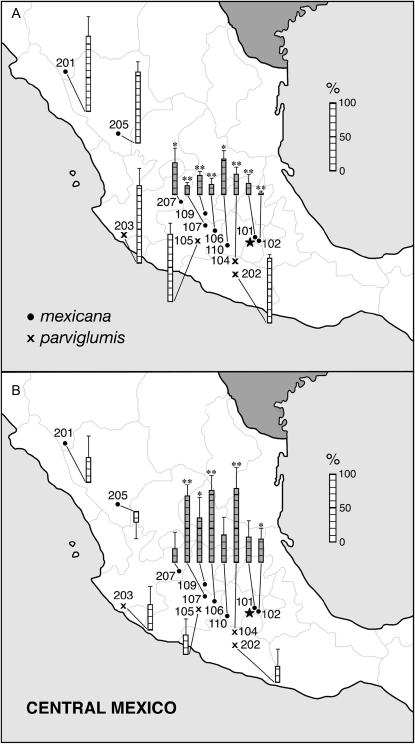
To assess the compatibility of teosinte pistils and maize pollen, emasculated plants of 13 teosinte populations were wind pollinated with Ga1-m tcb1 maize (inbred W22). Use of a Ga1-m tcb1 pollinator served to match the allelic composition of maize populations indigenous to the region of Mexico where the teosintes were collected (Kermicle et al. 2005), thereby assuring compatibility for the Ga1-s vs. ga1 system. Seed (filled fruitcases) produced in this cross relative to when these populations were interpollinated with other teosintes belonging to the same subspecies estimates incompatibility due to genes other than Ga1-s. Eight of the 13 populations produced significantly fewer seed when pollinated with maize than with teosinte, ranging down to 2.3% (Figure 1A).
Figure 1.
Seed set on emasculated plants of 13 annual teosinte populations pollinated with maize. Results expressed relative to when teosinte pollinates itself (± SE). Open bars, tcb1 and Tcb1-m teosinte populations; shaded bars, teosintes containing Tcb1-s. (A) Ga1-m tcb1 maize pollinator. (B) Increase in seed set due to introgressing Tcb1-s into the maize pollinator, that is, the difference between Ga1-m tcb1 and Ga1-m Tcb1-s as pollinators. *0.05 > P > 0.01, **P < 0.01, based on one-tailed t-tests.
Genotyping three plants of each teosinte population with respect to alleles of the tcb1 locus revealed a definite association with maize crossability. All eight that crossed with reduced efficiency contained Tcb1-s, whereas the five that crossed well were tcb1 tcb1 (four populations) or contained the neutral allele Tcb1-m (one population) (Table 3). Tcb1-s was present in each of the three test plants in four of the eight populations containing it and polymorphic with Tcb1-m in four. No Tcb1-s tcb1 heterozygous plant was observed and only one Tcb1-m tcb1 heterozygote, in collection 109.
TABLE 3.
Tcb1 composition of three plants in each of 13 teosinte populations
| Teosinte
|
Fraction of tcb1-maize/teosinte hybrids producing >50% seed set in crosses to Tcb1-s/− femalesa
|
tcb1 allele present in maize backcross lineage
|
||||||
|---|---|---|---|---|---|---|---|---|
| Collection | Ssp. | Habitat | ||||||
| 101 | mexicana | Weedy | 6/6 | 10/10 | NT | s | s | s |
| 102 | mexicana | Weedy | 7/7 | 8/9 | 6/6 | m | s | s |
| 104 | parviglumis | Wild | 7/7 | 7/7 | 7/7 | s | s | s |
| 105 | parviglumis | Wild | 0/7 | 0/6 | 0/7 | t | t | t |
| 106 | mexicana | Weedy | 8/8 | 8/8 | 8/8 | m | s | s |
| 107 | mexicana | Weedy | 7/7 | 8/8 | 7/8 | s | s | s |
| 109 | mexicana | Ruderal/weedy | 7/14 | 8/8 | 8/8 | m | m | s |
| 110 | mexicana | Weedy | 7/8 | 15/17 | 8/8 | NT | m | s |
| 201 | mexicana | Ruderal/weedy | 2/12 | 0/7 | 0/7 | t | t | t |
| 202 | parviglumis | Wild | 3/8 (0/8b) | 0/8 | 4/8 (0/8b) | t | t | t |
| 203 | parviglumis | Wild | 8/8 | 8/8 | 8/8 | m | m | m |
| 205 | mexicana | Ruderal/weedy | 0/8 | 0/7 | 0/8 | t | t | t |
| 207 | mexicana | Ruderal/weedy | 6/6 | 6/6 | 6/6 | s | s | s |
s, pistil barrier and pollen competence to surmount the barrier; m, pistil barrier absent but pollen competent; t, lacking both pistil barrier and pollen competence; NT, not tested.
Results for three teosinte plants given in the same order as the three columns to the right.
Crosses onto Tcb1-s Tcb1-s. Heterozygous Tcb1-s tcb1 test plants used for all other entries.
To be an effective barrier in preventing maize from pollinating teosinte, the sympatric maize populations should be tcb1, not Tcb1-s or Tcb1-m. Composition of three plants each of the 12 sympatric and parapatric maize populations (Table 1) was uniformly tcb1 tcb1, like all midwestern United States dent sources tested to date (data not given).
Tcb1 restoration of compatibility:
Parallel tests in which emasculated teosinte plants were wind pollinated with a W22 Ga1-m strain also carrying Tcb1-s estimates the extent to which teosinte and maize incompatibility is attributable to Tcb1-s. Seed set was improved in each of the eight Ga1-s-containing populations (Figure 1B). Despite low statistical power due to the limited number of plants tested, the increase was significant or highly so in five (one-tailed t-test). Of the remaining three Tcb1-s populations, Co1lection 110 was polymorphic for Tcb1-m, and gave an intermediate value of 52.3% when pollinated with Tcb1-m tcb in the previous test. Although not a significant increase, the level of compatibility when pollinated with Ga1-m Tcb1-s reached 92.7% (sum of Figure 1, A and B). The same was not true of the remaining two Tcb1-s populations, which gave final compatibility values of 56.4 (collection 101) and 69.0% (collection 207). They are candidates for presence of additional pollen-pistil compatibility factors; likewise with collection 102, which, although significantly restored by Tcb1-s, reached a final compatibility level of only 36.9%. The other four Tcb1-s-containing populations having significant restoration values reached final compatibility levels of ≥93.8%.
Are the pollen and pistil actions of Tcb1-s due to more than one gene?
Efficiency of reproductive isolation based on pollen-pistil recognition is a function of how regularly the pistil barrier and the competence of pollen to surmount that barrier are inherited together. Joint inheritance could be based on a single gene or on separate, closely linked genes. To test whether pistil and pollen effects could be separated by recombination, Tcb1-s tcb1 plants also heterozygous for the flanking markers glossy leaf-7 (2 cM distal) and brown midrib-3 (1 cM proximal) were crossed onto gl7 tcb1 bm3 testers. Of 183 gl7-bm3 recombinant progeny among 8263 total, 87 possessed both pollen and pistil Tcb1-s function as evaluated through reciprocal crosses with a Tcb1-s tester. Ninety-six had neither (Table 4). The observation that none possessed only pollen or only pistil function is consistent with a single gene having both pollen and pistil actions, or with separate genes so closely linked as not to be separated in these tests. This outcome leaves unexplained the relation of Tcb1-s to the pollen-action-only allele Tcb1-m present in some teosinte populations, and the origin of Tcb1-m from Tcb1-s detected in the course of other experiments (Kermicle and Allen 1990).
TABLE 4.
Presence and absence of Tcb1-s among recombinants in the gl7-bm3 region
| Kernel class
|
Testcross population
|
Recombinant class
|
No. of plantsa
|
|
|---|---|---|---|---|
| Tcb1-s | tcb1 | |||
| Su su | 4179 | gl7 Bm3 | 65 | 46 |
| su su | 4084 | Gl7 bm3 | 22 | 50 |
Test plants are progeny of F1 gl7 tcb1 bm3 su1-B/Gl7 Tcb1-s Bm3 Su1 backcrossed to the multiple recessive strain
All 183 recombinants in the gl7-bm3 region had either both pollen and pistil Tcb1 function or neither. A deficit in the Gl7-bm3 recombinant classes relative to gl7-Bm3 ones is attributed to incomplete penetrance of the nonglossy (Gl) phenotype in seedlings grown from the su su class of kernels.
Reduced pollen transmission of Tcb1 in crosses to maize:
Pollen carrying the introgressed Tcb1-s segment fertilizes tcb1 tcb1 maize less efficiently than tcb1 pollen. This reduction could be significant in preventing Tcb1-s from becoming established in open-pollinated maize landraces that grow sympatrically with teosinte. If so, the gene or genes responsible for reduced transmission are expected to be closely linked with Tcb1-s. To test linkage, representative parental and selected recombinant individuals from 1950 of the Su su testcross progeny reported in Table 4 were tested for Su:su transmission in a second generation testcross onto 3 su su plants (inbred 195A). The starchy (Su1 su1) vs. sugary (su1 su1) kernel phenotype is efficient for obtaining large numbers of progeny and, at 6 MU from tcb1, its use underestimates any effect of Tcb1-s on pollen transmission only moderately. The Su1 su1 class for three parentally marked, Gl7 Tcb1-s Bm3 control chromosomes averaged from 35.3 to 41.3%. Thirteen gl7 Tcb1-s Bm3 recombinants, including all 10 that had >47% Su su on the basis of the single-ear, first-generation test, ranged from 36.7 to 44.4% Su su. The 19 gl7 tcb1 Bm3 recombinants in this sample ranged from 47.3 to 52.5% Su su. A lack of overlap between the Tcb-s and tcb1 groups indicates failure to separate reduced pollen transmission from Tcb1 itself. Thus not only does Tcb1-s confer a barrier to prevent teosinte from being fertilized by maize, but also it or tightly linked genes could retard the introduction of Tcb1-s from teosinte into maize.
Teosinte and maize differ markedly in length of the style a pollen tube must transverse to effect fertilization. The husk leaves of maize enclose the entire female inflorescence (developing cob) and in lines such as inbred W22 extend well beyond it whereas the female inflorescence of teosinte is only partly covered, typically leaving the distal fruitcases exposed. To test whether style length would influence competition between classes of pollen, the apical ear shoots in one lot of inbred W22 su su plants were trimmed ∼1 cm distal to the tip of the developing cob, and silks then allowed to exsert beyond that point. Ear shoots in a second group of sibling plants were left untrimmed. Single collections of pollen from 12 Tcb1-s Su/tcb1 su plants were divided between pairs of trimmed and untrimmed shoots. Each of the 12 pairs produced more Su su kernels from the trimmed than from the untrimmed ear, averaging 46.5 and 42.3%, respectively (P < 0.001; paired t-test). Hence trimming the shoots, a common practice before making hand crosses, reduced the competitive disadvantage of Tcb1-s on maize pistils by more than one half.
DISCUSSION
Annual Mexican teosinte comprises Zea mays subspecies parviglumis, which grows wild, and subspecies mexicana, which includes two main ecotypes. One, denoted ruderal/weedy, thrives in disturbed habitats including cultivated fields. The second grows exclusively in cultivated fields, usually with maize, as an obligate weed (Sanchez-G. and Ruiz-C. 1997). The exclusively weedy populations resemble the local maize in general plant type and maturity (Wilkes 1967), yet crossing is markedly assortative. The present findings examine the role of the crossing barrier gene Tcb1-s in facilitating adaptation of these taxa to the same habitat. Pistils carrying Tcb1-s are unreceptive to pollen carrying the tcb1 allele of this locus (Evans and Kermicle 2001). Tcb1-s predominated in the five weedy populations tested, was present in one population and one other plant of four weedy/ruderal populations, and was found in one of four parviglumis populations, all classified as wild, indicating an association of Tcb1-s with ecology of the teosinte populations.
Disruptive selection and reproductive isolation:
Previous studies identified various counterselective forces acting on weedy teosinte and maize (Wilkes 1967; Mangelsdorf 1974; Sanchez-G. and Ruiz-C. 1977). As a cultigen, maize is favored by human activity, such as by selective planting and weeding. As a weed, teosinte is subject to natural selection, including protection of the seed by a tough fruitcase and dispersal by shattering. Yet, postzygotic isolation by genetic means is relatively weak. Their karyotypes are similar (Beadle 1932; Kato-Y 1977), pollen fertility of F1 hybrids is high, generally >95% (Wilkes 1967), and the level of recombination in the hybrid is similar to that in maize (Emerson and Beadle 1932), with the particular level dependent upon the parent lines used to produce the F1 (Doebley and Stec 1993). However, hybrids are poorly adapted either as a weed or as a crop plant. For example, their seed do not shatter but neither are they readily accessible as grain. Despite disruptive selection, hybridization provides a bridge for limited introgression of teosinte sequences into maize (Matsuoka et al. 2002), and of maize sequences into some ssp. mexicana populations (Fukunaga et al. 2005). Maladaption of hybrids and early backcross generation plants evidently is such that gene exchange can occur, yet without the minor component, teosinte, being extinguished by genetic assimilation. The present findings suggest that assortative fertilization abets postzygotic ecological isolation in maintaining this balance.
The abetting of reproductive isolation by pollen-pistil compatibility factors such as Tcb1-s need not involve the classic reinforcement sequence. Typically, prezygotic isolation, such as that found between sympatric populations of Drosophila subspecies (Ehrman 1965; Noor 1995), is considered to come into play subsequent to nascent postzygotic isolation. Alternatively, gratuitous sexual-incompatibility genes may preexist in local populations. Such seems to be the case presently within spp. parviglumis where the Teloloapan population contains Tcb1-s although this population grows wild, occurring only incidentally as a weed in maize fields. In this circumstance, Tcb1-s may not increase the fitness of plants carrying it, but be maintained at high frequency as a “selfish” gene. Similarly, Tcb1-s may have swept some populations of spp. mexicana preceding the introduction of maize into Central Mexico. Perhaps only those populations containing Tcb1-s or similar factors resisted being extinguished by the strongly favored maize. Prezygotic isolation of this sort is preemptive rather than reactive.
Association of Tcb1-s with the weedy ecotype of ssp. mexicana populations does not preclude the involvement of other pollen-pistil factors in preventing teosinte from being fertilized by maize. For two of the five weedy, Tcb1-s-containing populations, incorporation of Tcb1-s together with Ga1-m into the maize pollinator restored compatibility only partially (Figure 1B). Other major pollen-pistil compatibility barriers that isolate these two populations from maize are not expected to include Ga1-s, since the maize counterparts carry the cross-neutral allele Ga1-m, which fertilizes plants containing Ga1-s. Curiously, the distribution of Ga1-s nevertheless appears to be associated with weediness (Kermicle et al. 2005). These workers found Ga1-s pollen to have a slight advantage over Ga1-m on Ga1-s-containing pistils and suggest further that Ga1-s might play a supporting role by strengthening the action of other compatibility genes.
Crossability between teosinte and maize as measured in this study likely overestimates the level of natural hybridization. Providing a continuous supply of foreign pollen to the exclusion of conspecific pollen, as was done here, maximizes the opportunity for crossing. In a natural setting, foreign pollen may be available only during part of the flowering season. Even then, it typically competes poorly with conspecific pollen, often effecting fertilization only in absence of the latter (numerous reports beginning with the extensive studies of J. G. Kölreuter, 1763 et seq., as discussed by Mayr 1986). Hence, even those teosinte populations that yielded an appreciable set of seed when pollinated exclusively with maize (Figure 1A) may produce few hybrids under natural conditions.
For reproductive isolation to be effective, hybridization by crossing in both directions must be considered. The present evidence bears to fertilization of maize by teosinte pollen only in that, on tcb1 tcb1 pistils, Tcb1-s pollen is transmitted less frequently than tcb1. Although this disadvantage may be sufficient to prevent introgression of Tcb1-s into maize, it seems insufficient to prevent substantial fertilization of maize by teosinte pollen, with eventual introgression of genes not closely linked with Tcb1-s. Other genes that prevent teosinte from being fertilized by maize pollen likely exist. Within maize, five genes in addition to Ga1-s have been identified that when present in pistils cause a preference for pollen carrying the same allele (Nelson 1993). In rice, a comprehensive segregation QTL analysis of a Japonica × Indica hybrid identified 15 gametophyte-based distortions from F2 Mendelian expectation (Harushima et al. 2001). Such genes often affect only the pollen or the embryo sac, causing distortion only when the cross is made in one direction. Nevertheless, genes having unidirectional effects could act in combination to constitute a synthetic, bidirectional barrier.
Is Tcb1 a single gene?
Three properties are attributed to the Tcb1-s locus: a pistil barrier to pollen not containing Tcb1, competence of pollen to surmount this barrier, and reduced success of Tcb1 pollen in fertilizing tcb1 tcb1 pistils. Attempts to separate these properties by recombination were unsuccessful. This outcome should be considered in light of the uneven distribution of crossovers in the maize genome. Almost all recombination is concentrated within the low-copy sequences with little if any within repetitive classes (Fu et al. 2002), which constitute a majority of the DNA (San Miguel and Bennetzen 1998). A typical, single-copy maize gene encompasses 0.1 cM, that is, undergoes 1 crossover/1000 gametic chromosomes. Accordingly, if a single-copy gene lies between the pistil barrier and pollen competence functions of Tcb1, a number of recombinants would have been expected among the 8000 testcross progeny analyzed. This reasoning assumes regular homology between the teosinte segment carrying Tcb1 and the gl7 tcb1 bm3 segment in the maize tester stock. However, local heterologies exist even between maize strains (Fu and Dooner 2002). The possibility of heterology cannot be discounted completely even though the overall frequency of recombination between gl7 and bm3 was not reduced in this combination relative to the frequency within a maize background (Evans and Kermicle 2001). The same arguments apply to lack of separation of incompatibility from reduced Tcb1-s pollen transmission on tcb1 tcb1 pistils. The negative evidence is less strong in this case with only 1950 chromosomes tested. Whether these compatibility relationships are governed by a single gene or by an assemblage of genes, it will be interesting to learn whether they extend deep into the phylogenetic history of Zea, such as the gene complexes governing self-incompatibility in other genera (Newbigin 1996; Boyes et al. 1997).
Acknowledgments
I thank Suketoshi Taba for assistance in assembling the Mexican Zea material, Jesus Sanchez-G. for advice in classifying the teosinte collections according to habitat, and David Baum, John Doebley, and Millard Susman for suggestions concerning the manuscript. Research was supported by U.S. Department of Agriculture National Research Initiative award 35301-13314.
References
- Asher, P. D., and S. J. Peloquin, 1968. Pollen tube growth and incompatibility following intra- and inter-specific pollinations in Lilium longiflorum. Am. J. Bot. 55: 1230–1234. [Google Scholar]
- Beadle, G. W., 1932. Studies of Euchlaena and its hybrids with Zea. I. chromosome behavior in E. mexicana and its hybrids with Z. mays. Zeitschr. Abst. Vererb. 62: 291–304. [Google Scholar]
- Bernacchi, D., and S. D. Tanksley, 1997. An interspecific backcross of Lycopersion esculentum × L. hirsutum: linkage analysis and a QTL study of sexual compatibility factors and floral traits. Genetics 147: 861–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyes, D. C., M. E. Nasrallah, J. Vrebalov and J. B. Nasrallah, 1997. The self-compatibility (S) haplotypes of Brassica contain highly divergent and rearranged sequences of ancient origin. Plant Cell 9: 237–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Nettancourt, D., 2001. Incompatibility and Incongruity in Wild and Cultivated Plants, Ed. 2. Springer, Berlin.
- Doebley, J., and A. Stec, 1993. Inheritance of the morphological differences between maize and teosinte: comparison of results for two F2 populations. Genetics 134: 559–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrman, L., 1965. Direct observation of sexual isolation between allopatric and between sympatric strains of different Drosophila paulistorum races. Evolution 19: 459–464. [Google Scholar]
- Emerson, R. A., and G. W. Beadle, 1932. Studies of Euchlaena and its hybrids with Zea. II. Crossing over between the chromosomes of Euchlaena and those of Zea. Zeitschr. Abst. Vererb. 62: 305–315. [Google Scholar]
- Evans, M. M. S., and J. L. Kermicle, 2001. Teosinte crossing barrier 1, a locus governing hybridization of teosinte with maize. Theor. Appl. Genet. 103: 259–265. [Google Scholar]
- Fu, H., and H. K. Dooner, 2002. Intraspecific violation of genetic colinearity and its implications for maize. Proc. Natl. Acad. Sci. USA 99: 9573–9578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, H., Z. Zheng and H. K. Dooner, 2002. Recombination rates between adjacent genic and retrotransposon regions in maize vary by 2 orders of magnitude. Proc. Natl. Acad. Sci. USA 99: 1082–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga, K., J. Hill, Y. Vigouroux, Y. Matsuoka, J. Sanchez-G. et al., 2005. Genetic diversity and population structure of teosinte. Genetics 169: 2241–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harushima, Y., M. Nakagahra, M. Yano, T. Sasaki and N. Kurata, 2001. A genome-wide survey of reproductive barriers in an intraspecific hybrid. Genetics 159: 883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao, T-H., and T. Tsukamoto, 2004. The molecular and genetic basis of S-RNase-based self-incompatibility. Plant Cell 16: S72–S83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato-Y, T. A., 1997. Review of introgression between maize and teosinte, pp. 44–53 in Gene Flow Among Maize Landraces, Improved Maize Varieties and Teosinte: Implications for Transgenic Maize, edited by J. A. Serratos, M. C. Willcox and F. Castillo. CIMMYT, Mexico City.
- Kermicle, J. L., and J. O. Allen, 1990. Cross incompatibility between maize and teosinte. Maydica 35: 399–408. [Google Scholar]
- Kermicle, J. L., S. Taba and M. M. S. Evans, 2005. The gametophyte-1 locus and reproductive isolation among Zea mays subspecies. Maydica (in press).
- Lewis, D., and L. K. Crowe, 1958. Unilateral incompatibility in flowering plants. Heredity 12: 233–256. [Google Scholar]
- Liedl, B. E., S. McCormick and M. A. Mutschler, 1996. Unilateral incongruity in crosses involving Lycopersicon penellii and L. esculentum is distinct from self-incompatibility in expression, timing and location. Sex Plant Reprod. 9: 299–308. [Google Scholar]
- Mangelsdorf, P. C., 1974. Corn: Its Origin, Evolution and Improvement. Belknap Press/Harvard University Press. Cambridge, MA.
- Matsuoka, Y., Y. Vigourous, M. M. Boodman, G. J. Sanchez, E. Buckler et al., 2002. A single domestication for maize shown by multilocus microsatellite genotyping. Proc. Natl. Acad. Sci. USA 99: 6080–6084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr, E., 1986. Joseph Gottlieb Kölreuter's contributions to biology. Osiris 2: 135–176. [Google Scholar]
- Murfett, J., T. J. Strabala, D. M. Zurek, B. Mou, B. Beecher et al., 1996. S RNase and interspecific pollen rejection in the genus Nicotiana: multiple pollen-rejection pathways contribute to unilateral incompatibility between self-incompatible and self-compatible species. Plant Cell 8: 943–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasralla, J. B., 2002. Recognition and rejection of self in plant reproduction. Science 296: 305–308. [DOI] [PubMed] [Google Scholar]
- Nelson, O. E., 1993. The gametophyte factors of maize, pp. 496–503 in The Maize Handbook, edited by M. Freeling and V. Walbot. Springer-Verlag, New York/Berlin/Heidelberg, Germany.
- Newbigin, E., 1996. The evolution of self-incompatibility: a molecular voyeur's perspective. Sex Plant Reprod. 9: 357–361. [Google Scholar]
- Noor, M. A., 1995. Speciation driven by natural selection in Drosophila. Nature 375: 674–675. [DOI] [PubMed] [Google Scholar]
- Sanchez-G., J. J., and J. A. Ruiz-C., 1997. Teosinte distribution in Mexico, pp. 18–35 in Gene Flow Among Maize Landraces, Improved Maize Varieties and Teosinte: Implications for Transgenic Maize, edited by J. A. Serratos, M. C. Willcox and F. Castillo. CIMMYT, Mexico, D.F.
- SanMiguel, P., and J. L. Bennetzen, 1998. Evidence that a recent increase in maize genome size was caused by the massive amplification of intergene retrotransposons. Ann. Bot. 82: 37–44. [Google Scholar]
- Skogsmyr, I., and A. Lankinen, 2002. Sexual selection: an evolutionary force in plants? Biol. Rev. 77: 537–562. [DOI] [PubMed] [Google Scholar]
- Swanson, R., A. F. Edlund and D. Preuss, 2004. Species specificity in pollen-pistil interactions. Annu. Rev. Genetics 38: 739–818. [DOI] [PubMed] [Google Scholar]
- Wilkes, H. G., 1967. Teosinte: The Closest Relative of Maize. Bussey Institute, Harvard University, Cambridge, MA.
- Wilkes, H. G., 1977. Hybridization of maize in Mexico and Guatemala and the improvement of maize. Econ. Bot. 31: 254–293. [Google Scholar]