Abstract
Transposons make up a sizable portion of most genomes, and most organisms have evolved mechanisms to silence them. In maize, silencing of the Mutator family of transposons is associated with methylation of the terminal inverted repeats (TIRs) surrounding the autonomous element and loss of mudrA expression (the transposase) as well as mudrB (a gene involved in insertional activity). We have previously reported that a mutation that suppresses paramutation in maize, mop1, also hypomethylates Mu1 elements and restores somatic activity to silenced MuDR elements. Here, we describe the progressive reactivation of silenced mudrA after several generations in a mop1 background. In mop1 mutants, the TIRA becomes hypomethylated immediately, but mudrA expression and significant somatic reactivation is not observed until silenced MuDR has been exposed to mop1 for several generations. In subsequent generations, individuals that are heterozygous or wild type for the Mop1 allele continue to exhibit hypomethylation at Mu1 and mudrA TIRs as well as somatic activity and high levels of mudrA expression. Thus, mudrA silencing can be progressively and heritably reversed. Conversely, mudrB expression is never restored, its TIR remains methylated, and new insertions of Mu elements are not observed. These data suggest that mudrA and mudrB silencing may be maintained via distinct mechanisms.
TRANSPOSABLE elements make up at least half of the maize genome (SanMiguel et al. 1996). Given the mutagenic potential of transposons and their ubiquity in plant and animal genomes, it is not surprising that most transposable elements remain quiescent most of the time. Transposon activity is held in check by a well-conserved set of mechanisms that include both post-transcriptional and transcriptional components (Zilberman and Henikoff 2004). Mutants have been identified that reactivate quiescent transposons in a variety of species (Lippman et al. 2003) (reviewed in Okamoto and Hirochika 2001). In plants, loss of DNA methylation is often associated with transposon reactivation (Kato et al. 2003). Arabidopsis MULE (Mutator-like element) class II DNA transposons are reactivated in several recessive homozygous mutants, including DDM1 (DECREASE IN DNA METHYLATION1) (Singer et al. 2001) and MET1 (METHYLTRANSFERASE1) (Kato et al. 2003). Reactivation of Arabidopsis MULEs in a ddm1 mutant background is associated with hypomethylation of terminal inverted repeats (TIRs), expression of the putative transposase, and new insertions (Singer et al. 2001). Hypomethylation is heritable in that it persists even in ddm1/+ progeny outcrossed from a ddm1/ddm1 parent (Lippman et al. 2003). ddm1 mutants also cause reactivation and mobilization of CACTA transposable elements, and, like MULE reactivation, this reactivation is heritable (Kato et al. 2004).
MET1 is a DNA methyltransferase that is also involved in maintaining global CG methylation (Bartee et al. 2001; Kankel et al. 2003). In met1 mutants, MULEs become hypomethylated, and the transposase is expressed (Lippman et al. 2003). CACTA elements in Arabidopsis are strongly reactivated in lines double mutant for MET1 and the DNA methyltransferase gene CMT3 (CHROMOMETHYLASE3), which is involved in maintaining non-CG methylation.
In addition to chromatin-remodeling factors, genes involved in RNAi have been implicated in transposon silencing, both in animals (Aravin et al. 2001; Vastenhouw et al. 2003) and in plants (Lippman and Martienssen 2004). Indeed, the initiation and maintenance of transposon silencing almost certainly involves a complex interaction between RNAi and chromatin modification.
Arabidopsis is an excellent system in which to study the biochemistry and molecular biology of transposon reactivation in plants. The Arabidopsis genome is fully sequenced, and there are a number of mutants that affect transposon activity. However, the transposon systems in Arabidopsis are poorly characterized; the autonomous elements have not been generally identified, and the means by which the transposons were silenced in the first place is not known. In this respect, maize is unique in that it contains several DNA transposon systems that are highly active and that have been extensively characterized.
The Mutator family of transposons is the founding member of the MULE class of elements that are present in plants, fungi, and bacteria (reviewed by (Lisch 2002). All Mutator transposons are characterized by flanking 220-bp TIRs. The Mutator family consists of the autonomous element MuDR and at least nine classes of nonautonomous elements (Bennetzen 1996). MuDR carries two genes, mudrA, which encodes the transposase, and mudrB, a helper gene that is required for transposon integration (Lisch et al. 1995; Raizada and Walbot 2000). The mudrA gene is found in all autonomous MULEs in all species that carry MULEs; the mudrB gene is found only in the genus Zea (Lisch 2002). The MuDR TIRs contain the promoters for both mudrA and mudrB (Raizada et al. 2001). Methylation of sequences within the TIRs of all elements is correlated with Mutator silencing (Chomet et al. 1987, 1991; Martienssen and Baron 1994). A typical maize Mutator active line carries several active MuDR elements as well as up to several hundred nonautonomous elements, and duplication frequencies of these elements can average 100% (Alleman and Freeling 1986).
The large number of active autonomous and nonautonomous elements in the typical Mutator line makes it difficult to perform genetic analysis on the Mutator system. Therefore, we have developed a minimal Mutator line containing a single functional autonomous element, MuDR(p1), at position one (p1) on chromosome 2L, and a single nonautonomous element, Mu1, in the A1 color reporter gene (a1-mum2) (Chomet et al. 1991) (Lisch et al. 1995). This minimal line provides a simple system in which to easily track the excision and duplication events of a single Mutator element. Excision of Mu1 from a1-mum2 results in pigmented spots in the kernel aleurone, which is a direct reflection of transposase activity, as is hypomethylation of Mu1 termini. Also, unlike typical Mu active lines, the minimal line does not exhibit spontaneous silencing, which would interfere with genetic analysis of factors influencing epigenetic regulation of the Mutator system.
We have identified and cloned a single dominant gene, Mu killer (Muk), which heritably and reliably silences the Mutator system via a double-stranded RNA mechanism that targets the 5′ region of mudrA (Slotkin et al. 2005). Muk activity results in silencing of mudrA in the first generation and silencing of mudrB by the next generation. Muk is a variant of MuDR that carries an inverted and duplicated portion of the transposon that includes TIRA and a portion of the mudrA gene, but that lacks mudrB or TIRB. In the presence of Muk, all Mu TIRs become methylated, and there is loss of somatic and germinal activity (Slotkin et al. 2003). Once silenced by Muk, Mutator elements do not become spontaneously reactivated. Thus, Muk can be used as a tool to reliably and heritably silence MuDR elements to study the dynamics of epigenetic Mutator regulation. This, along with the convenience of the minimal Mutator line, makes the maize Mutator system particularly useful for genetic analysis of transposon silencing and reactivation.
Previous work has demonstrated that a mutant that affects paramutation in maize, mediator of paramutation1 (mop1) (Dorweiler et al. 2000), also reverses Mutator methylation and silencing (Lisch et al. 2002). Paramutation is the phenomenon whereby a silenced allele of a particular gene silences an otherwise active allele in trans (Brink et al. 1968). The mop1 mutation prevents establishment of paramutation of the paramutable alleles of b1, r1, and pl1 and increases RNA expression of the paramutagenic b1 and pl1 alleles (Chandler and Stam 2004). The mop1 mutation also reverses Mu1 TIR methylation in Mutator lines silenced by Muk as well as restoring low levels of somatic activity that was lost due to Muk silencing of MuDR (Lisch et al. 2002). While mop1 affects both paramutation and Mutator TIR methylation, unlike DDM1 or MET1 in Arabidopsis, it neither affects global methylation in maize (Dorweiler et al. 2000) nor affects methylation of some other transposable elements, such as those just upstream of the paramutagenic allele of the b1 locus (Lisch et al. 2002).
In a previous study (Lisch et al. 2002) we reported that mop1 hypomethylates Mu1 TIRs and facilitates somatic activity of silenced MuDR elements. Here, we describe in detail the reactivation of silenced MuDR elements after they have been exposed to the mop1 mutation for multiple generations. We find that the TIR adjacent to mudrA, like Mu1 TIRs, is subject to demethylation in a mop1 background. We find that there is a parent-of-origin effect on the maintenance of the reactivated state and that levels of somatic activity become progressively higher in each generation in a mop1 background, with excision frequencies approaching that observed in lines carrying active MuDR elements. We also find that after several generations in a mop1 background, MuDR elements become heritably active even in the absence of the mop1 mutation. Surprisingly, despite high levels of somatic activity, we do not find evidence for new insertions, and we also find that, while mudrA expression is fully restored, mudrB remains silenced, consistent with a previously defined role for mudrB in insertional activity (Lisch et al. 1999). Finally, while TIRA becomes hypomethylated in a mop1 background, TIRB remains methylated. These data suggest that although both mudrA and mudrB are silenced by Muk, there are differences in the means by which that silencing is maintained. The progressive reactivation of silenced mudrA (but not mudrB) in a mop1 background affirms that changes in the epigenetic state can be gradual and cumulative and supports the idea that, over time, the memory of the heterochromatic state can be lost in this mutant background.
MATERIALS AND METHODS
Generation of lines:
The a1-mum2 minimal Mutator line and the a1-mum2 minimal line tester:
The W22-derived minimal Mutator line was previously generated and described (Lisch et al. 1995). It contains one full-length functional MuDR element and one nonautonomous Mutator element, the Mu1 element in the allele a1-mum2 (O'Reilly et al. 1985; Chomet et al. 1991). When an active MuDR element is present, the Mu1 element excises out of a1-mum2 late in somatic development, creating characteristic Mutator spotting in the kernel. When MuDR activity is absent the kernel is uniformly pale. A hemizygous MuDR element on the long arm of chromosome 2 named MuDR(p1) (Chomet et al. 1991) is the single active element in the minimal Mutator line. This element does not become spontaneously epigenetically silenced in the minimal Mutator line. When MuDR(p1) is absent, the minimal line is referred to as the a1-mum2 tester.
Although other MuDR-homologous sequences (hMuDR elements) are present in this (and all) maize background (Chomet et al. 1991; Rudenko and Walbot 2001), these sequences do not appear to contribute to Mutator activity in the minimal line either positively (Chomet et al. 1991; Lisch et al. 1995) or negatively (Slotkin et al. 2003, 2005).
mop1 in the minimal Mutator line:
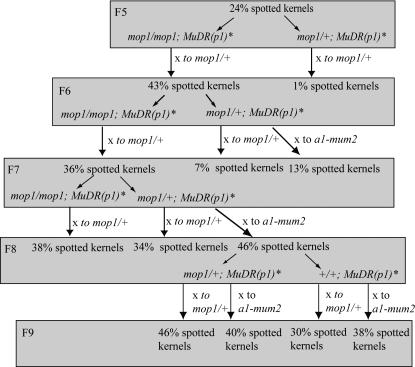
Silenced MuDR(p1) elements were generated by crossing MuDR(p1) to Muk. The resulting progeny were crossed to an a1-mum2 tester and then self-fertilized. Resulting plants were self-fertilized and testcrossed to MuDR(p1) to screen for plants that lacked Muk. Plants that were homozygous for silenced MuDR(p1) [referred to as MuDR(p1)*] and that lacked Muk were then crossed to mop1 homozygotes. The resulting mop1-heterozygous progeny were crossed to a mop1-homozygous tester that carried the a1-mum2 reporter. None of the resulting F1 mutant kernels exhibited somatic mutability. A MuDR(p1)* mop1 mutant progeny of that cross was then self-fertilized. Again, none of the F2 progeny kernels were spotted. An F2 mop1-homozygous progeny plant carrying MuDR(p1)* was then testcrossed to a mop1-heterozygous tester and the resulting ear had 3% spotted kernels. In the next, F3, generation, one exceptional mutant individual carrying MuDR(p1)* gave rise to 23% spotted kernels when testcrossed to a mop1-heterozygous tester. Spotted kernels from this cross were again testcrossed to mop1-heterozygous testers and again, one exceptional F4 mutant individual gave rise to 21% spotted kernels. In the next generation (F5), all mop1-homozygous individuals that carried MuDR(p1)* gave rise to significant numbers of spotted kernels. In this and all subsequent generations the mop1 mutant phenotype correlated well with the presence of large numbers of spotted progeny kernels. A schematic of all generations and crosses beginning with F5 can be found in Figure 1.
Figure 1.
Diagram of the crosses and generations discussed in this study. F5 is the first generation in which a significant percentage of kernel spotting was seen. Each generation indicates the percentage of spotted-kernel progeny produced from the previous generation as well as the genotypes selected for the crosses produced in the next generation. Arrows indicate the testcross.
The mop1/mop1;MuDR(p6)* line:
The mop1/mop1;MuDR(p6)* line was generated in a manner similar to that used to generate the mop1/mop1;MuDR(p1)* line, using in this case a MuDR element at position 6 (p6) that had been previously silenced by Muk and then introgressed into the mop1 mutant background for three generations. MuDR(p6) resulted from the transposition of MuDR(p1) in the minimal line and is therefore a duplicate of that original element in an identical genetic background (the minimal line).
DNA extraction and Southern blotting:
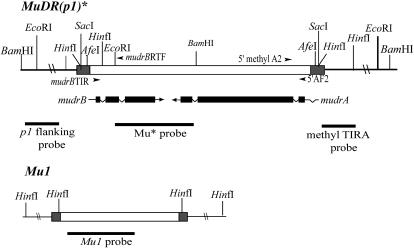
DNA preparation and genomic Southern blotting were performed as previously described (Dorweiler et al. 2000). Maize genomic DNA (10 μg) was digested for >4 hr with an excess of 20 units of restriction enzyme. Mutator restriction sites and probes referred to in this report are shown in Figure 2.
Figure 2.
Representation of MuDR at p1 and Mu1 at a1-mum2. Shaded boxes represent Mutator TIRs; open boxes are Mutator internal sequences that differ between MuDR and Mu1. The mudrA and mudrB exons are solid boxes just below the MuDR element; the lines that angle down represent introns. Arrows indicate the direction of transcription. Restriction sites used in this study are indicated. Probes used in this study are indicated below the transcripts. Primers used in this study are shown as arrowheads.
RNA extraction and RT–PCR analysis of mudrA and mudrB:
RT–PCR of mudrA and mudrB was performed as in (Slotkin et al. 2003). The same oligo(dT)-primed cDNA used in the RT–PCR analysis of the mudrA and mudrB transcripts was also amplified with primers specific for the alanine aminotransferase (aat) transcript to ensure equal starting amounts of RNA. Amplification was performed for 29 cycles using the primers aatF, 5′ ATGGGGTATGGCGAGGAT and aatR, 5′ TTGCACGACGAGCTAAAGACT. Amplification of aat cDNA generates a band of 281 bp, while amplification of the DNA produces a band of 454 bp.
Assay to determine the presence of MuDR(p1):
The presence of a full-length MuDR element was assayed by Southern blots using DNA digested with SacI and probing with any internal region of MuDR (see below for generation of probes). If a full-length MuDR element is present, a fragment of 4684 bp is observed. To determine if the MuDR element was at the p1 position, probing with the methyl TIRA probe that flanks p1 and the TIRA with a HinfI-digested blot (see below) assured the presence of MuDR at p1.
Mutator TIR methylation assay:
Mutator activity is associated with the methylation status of the HinfI restriction site present in all Mu element TIRs (Lisch et al. 1995). Mutator-active individuals have hypomethylated Mu1 TIRs and will produce a 1.3-kb band when digested with HinfI and probed with the internal region of Mu1. Individuals without MuDR or with silenced MuDR elements have hypermethylated Mu1 TIRs that are not digested by the methyl-sensitive HinfI restriction enzyme, producing Mu1 restriction fragments >1.3 kb (Lisch et al. 1995). The exact size of the inactive Mu1 restriction fragment is dependent on the position of the hypermethylated Mu1 element. In the allele a1-mum2, the size of this fragment is 2.1 kb. Additional fragments can also be observed that are the result of hybridization of the Mu1 probe to MRS-A, a maize gene that is homologous to Mu1 (Chandler et al. 1986). The HinfI sites in this gene, which lack Mu TIRs, are not affected by the presence or absence of MuDR.
The methylation and activity status of MuDR(p1) TIR at mudrA (TIRA) can also be assayed by restriction digestion using methyl-sensitive restriction enzyme HinfI. Digestion of TIRA with HinfI produces a 607-bp fragment when the HinfI site in TIRA is hypomethylated, and a larger fragment of 1052 bp when it is methylated. Additional fragments visible on Southern blots represent sequences homologous to MuDR (hMuDRs) that do not contribute either positively or negatively to Mu activity.
The methylation status of TIRA and TIRB can also be assayed using the restriction enzyme AfeI, which is blocked by CG methylation. A double digest of AfeI with BamHI produces a 2552- bp MuDR(p1) fragment when the AfeI site is hypomethylated at TIRA and a 2875-bp fragment when AfeI is methylated using the methyl TIRA probe. An AfeI/BamHI double digest produces a 4-kb fragment when the TIRB is hypomethylated at the AfeI site when using the p1 flanking probe; when the AfeI site is methylated the fragment is ∼7 kb and is difficult to resolve on a blot.
Generation of probes:
Mu1 probe:
The plasmid that carries the probe for the internal region of Mu1 has been previously described (Talbert and Chandler 1988). The Mu1 internal probe is generated by gel isolating an internal AvaI/BstEII fragment.
Internal MuDR probe:
The internal MuDR probe is generated by gel isolating an internal EcoRI/BamHI fragment from the pBMP1.3 plasmid (Chomet et al. 1991).
Methyl TIRA probe:
The methyl TIRA probe was generated by amplification using PCR primers TIRA methyl F, 5′ CGCGCACGAGGAAGGCGTTCT, and TIRA methyl R, 5′ AGCACCCGTCGCTCCACTTCC. The PCR program was as follows: 94° for 2 min, 94° for 30 sec, 63° for 45 sec, and 72° for 40 min, and then repeated for 35 cycles at 72°, with a last step of 10 min. The PCR product was a 52-bp DNA band.
p1 flanking probe:
The p1 flanking probe was generated by the PstI digestion of the pBMP1.3 plasmid. The 800-bp p1-specific fragment hybridizes to a single-copy sequence in the maize genome (Slotkin et al. 2003).
All DNA probes in this report were gel isolated and prepared by the random-priming method using a Prime-It II kit (Stratagene, La Jolla, CA) and 32P-radiolabeled dCTP (Perkin-Elmer, Norwalk, CT). All blots were exposed to a Molecular Dynamics phosphorimaging screen and processed using Adobe Photoshop.
Genotyping for mop1:
mop1 is on chromosome 2 at locus 2.04. The primers umc1259 forward and reverse were used to amplify the SSR linked to mop1 (umc1259 forward: 5′ CTCTTTGGTGGCAGAAGCAGAAT; umc1259 reverse: 5′ TAGCTAAACTTGAGTGCTCTGCCC). umc1259 is tightly linked to the mop1 locus in our minimal lines. When comparing the mop1 mutant phenotype and a linked umc1259-size polymorphism, we have seen crossovers in <1% of individuals analyzed. Additional SSR markers used for mapping included umc1541 forward and reverse (for primer sequences and amplification conditions refer to http://www.maizegdb.org/).
RESULTS
Plants homozygous for mop1 are hypomethylated at mudrA as well as Mu1:
In the absence of transposase, nonautonomous elements such as Mu1 become methylated at the TIRs (Chomet et al. 1991). This methylation is fully reversible when functional MuDR elements are introduced genetically, and it can be reintroduced de novo during development if the transposase is lost due to internal deletions within MuDR (Lisch et al. 1995). Thus, Mu1 TIR methylation appears to be the default state that occurs in the absence of transposase. When otherwise functional MuDR elements are silenced by Muk, their TIRs become methylated as well (Slotkin et al. 2003). Previous work has shown that Mu1 TIRs are hypomethylated in a mop1-homozygous background whether or not a functional MuDR is present, consistent with a role for MOP1 in the default methylation of Mu TIRs that occurs in the absence of the transposase (Lisch et al. 2002).
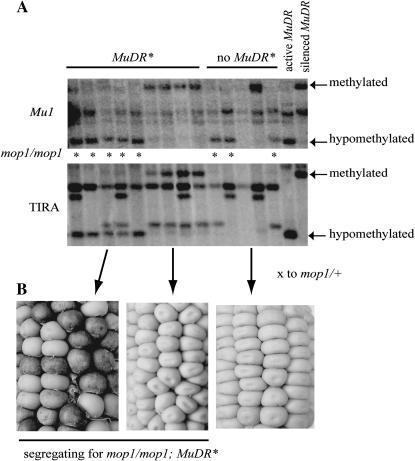
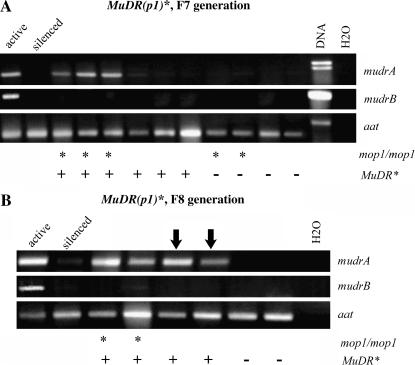
To determine whether the TIRs at MuDR(p1) elements silenced by Muk (designated MuDR(p1)*) also become demethylated in a mop1 background, we examined several families that were segregating for mop1 heterozygotes and homozygotes and for MuDR(p1)*. Muk was no longer present in any of these families, which had carried MuDR(p1)* in a mop1 mutant background for five or six generations. Methylation was assayed by examining Southern blots of DNA digested with HinfI, which is blocked at certain overlapping CG sites. The blots were sequentially probed with an internal fragment of Mu1 and a fragment flanking MuDR(p1) that included sequences adjacent to the TIR flanking mudrA (TIRA) as well as a portion of the TIR (Figure 2). All 43 individuals that genotyped as mop1 homozygous and that contained MuDR(p1)* exhibited hypomethylation at both Mu1 and TIRA. Conversely, all 53 siblings that were mop1 heterozygous and that contained MuDR(p1)* remained methylated at both Mu1 and TIRA (Figure 3A). These data demonstrate that the mop1 mutation reverses the methylation of TIRA as well as Mu1 TIRs.
Figure 3.
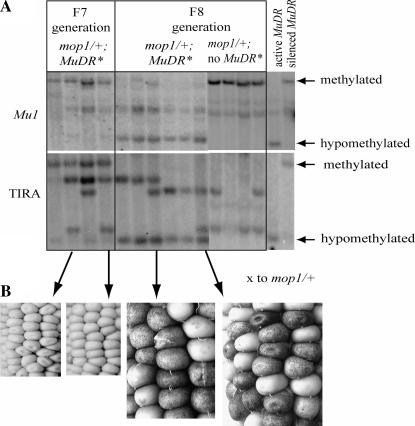
(A) Southern blots of a family from the F7 generation segregating for the mop1 mutation as well as the presence of MuDR(p1)*. The same blot was probed for Mu1 and then stripped and reprobed with TIRA. The genotype of each individual is indicated. Individuals that genotype as mop1 homozygous (“*”) are hypomethylated at both Mu1 and TIRA. Individuals that are mop1 heterozygous are methylated at both Mu1 and TIRA. Individuals that lack MuDR(p1)* are missing fragments corresponding to both methylated and hypomethylated TIRA. Active MuDR(p1) and MuDR(p1)* silenced by Muk are presented as controls. (B) Ear progeny of a cross between indicated individuals represented by the Southern blot by a mop1/+ tester. Individuals that are mop1/mop1;MuDR(p1)* gave rise to ∼36% spotted kernels when crossed to mop1/+. Conversely, individuals that are mop1/+;MuDR(p1)* gave rise to very few spotted kernels when crossed to mop1/+. Individuals lacking MuDR(p1)*, regardless of mop1 phenotype, gave rise to no spotted kernels.
Progressive restoration of high levels of somatic activity of silenced MuDR(p1)* elements in a mop1 mutant background:
Plants silenced by Muk lose somatic activity of Mutator elements, including Mu1 excision from the a1-mum2 reporter allele (Slotkin et al. 2003), resulting in the loss of kernel spotting in the aleurone. In previous work, we observed that mop1-homozygous mutants restored somatic activity to previously silenced MuDR(p1)* elements in a small percentage of kernels (3%) (Lisch et al. 2002). We wanted to know if somatic activity would increase if MuDR(p1)* were carried in a mop1 mutant background for additional generations. After four generations of exposure to the mop1 mutant, a line was derived in which mop1 mutants carrying MuDR(p1)* consistently produced a high frequency of spotted kernels (see materials and methods and Figure 1 for derivation of this line). In one family in the F6 generation, 4 mop1 mutants gave a total of 43% spotted kernels. In contrast, 12 siblings that carried MuDR(p1)* but that were heterozygous for mop1 gave only 1% spotted kernels. None of the progeny of 4 plants that were mop1 mutant but that lacked MuDR(p1)* gave rise to spotted kernels (0/291) (data not shown), confirming that the somatic activity we observed is due to MuDR(p1). A subsequent round of crossing (the F7 generation) gave rise to a similar frequency of spotted kernels (Table 1A); 10 mop1-homozygous mutants gave a total of 36% spotted kernels (360/989). Again, only individuals that were mop1 homozygous and carried MuDR(p1)* gave rise to significant numbers of spotted kernels (Figure 3B); the 17 mop1-heterozygous plants gave rise to only 7% spotted kernels (192/2700) (Table 1B and Figure 3B).
TABLE 1.
Somatic excision activity is restored in an mop1 background
| Crossa | Heavy/medium spotted | Weakly spotted | Pale | Total | Spotted (%) | Heavy/medium spotted (%) |
|---|---|---|---|---|---|---|
| A. mop1/mop1; MuDR(p1)* siblings | ||||||
| 1 | 44 | 33 | 90 | 167 | 46 | 26 |
| 2 | 8 | 0 | 33 | 41 | 20 | 20 |
| 3 | 15 | 0 | 33 | 48 | 31 | 31 |
| 4 | 17 | 3 | 47 | 67 | 30 | 25 |
| 5 | 27 | 10 | 28 | 68 | 57 | 42 |
| 6 | 12 | 21 | 105 | 138 | 24 | 9 |
| 7 | 38 | 30 | 83 | 151 | 45 | 25 |
| 8 | 12 | 20 | 106 | 139 | 24 | 9 |
| 9 | 38 | 29 | 84 | 150 | 45 | 25 |
| 10 | 1 | 0 | 20 | 21 | 5 | 5 |
| Total | 212 | 148 | 629 | 989 | 36 | 21 |
| B. mop1/+; MuDR(p1)* siblings | ||||||
| 1 | 14 | 9 | 164 | 187 | 12 | 7 |
| 2 | 10 | 8 | 104 | 122 | 15 | 8 |
| 3 | 0 | 2 | 104 | 106 | 2 | 0 |
| 4 | 2 | 10 | 178 | 190 | 6 | 1 |
| 5 | 2 | 1 | 146 | 149 | 2 | 1 |
| 6 | 13 | 4 | 35 | 52 | 33 | 25 |
| 7 | 4 | 15 | 140 | 159 | 12 | 3 |
| 8 | 10 | 22 | 170 | 202 | 16 | 5 |
| 9 | 0 | 5 | 251 | 256 | 2 | 0 |
| 10 | 8 | 16 | 77 | 101 | 24 | 8 |
| 11 | 0 | 1 | 16 | 17 | 6 | 0 |
| 12 | 1 | 4 | 216 | 221 | 2 | 0 |
| 13 | 3 | 4 | 204 | 211 | 3 | 1 |
| 14 | 3 | 13 | 173 | 189 | 8 | 2 |
| 15 | 1 | 0 | 130 | 131 | 1 | 1 |
| 16 | 2 | 5 | 200 | 207 | 3 | 1 |
| 17 | 0 | 0 | 200 | 200 | 0 | 0 |
| Total | 73 | 119 | 2508 | 2700 | 7 | 3 |
In the F7 generation mop1/mop1;MuDR(p1)* (A) and mop1/+;MuDR(p1) siblings (B) were crossed to mop1/+ testers.
Although the frequency of spotted kernels was consistently much higher in the F6 and F7 generations than in earlier generations, there was significant variation between individual ears, suggesting a stochastic component to the degree of activity achieved by MuDR in this mutant background. Similarly, there was a range of spotting intensity among individual kernels on a given ear. Despite this variation, the proportion of heavily and medium spotted kernels was clearly much higher in progeny of mop1 mutant plants than in their heterozygous siblings (21% vs. 3%). The frequency of excision in these kernels is indistinguishable from that observed in kernels carrying a fully active MuDR(p1) element.
These results indicate that extended exposure of MuDR(p1)* to the mop1/mop1 background can restore high levels of MuDR(p1)* activity with respect to somatic excisions. However, in these generations mop1 heterozygotes grown from heavily spotted kernels derived from mop1 mutant parents had methylated Mu1 and MuDR TIRs (Figure 3A) and did not transmit a significant number of spotted kernels (Table 1 and Figure 3B), demonstrating that in these generations MuDR(p1)* was not heritably reactivated by mop1.
Somatic excision activity is largely dependent upon the female parental genotype:
We observed that in a mop1/mop1;MuDR(p1)* × mop1/+ cross, each ear had more than the expected 25% spotted kernels, presuming kernel spotting is dependent upon the kernel genotype being mop1 homozygous and carrying MuDR(p1)*. This suggested that at least a subset of the spotted kernels were not mop1 homozygous. This turned out to be the case. Of 135 individuals genotyped, only 41% of the spotted kernels derived from the above cross were mop1 homozygous (data not shown). This suggested that kernel spotting is dependent upon the female parent being mop1 homozygous, and that the genotype of the kernel itself is irrelevant to kernel- spotting intensity.
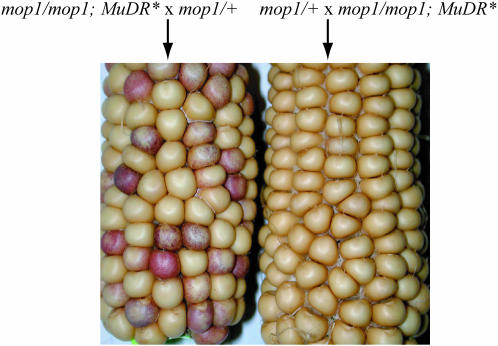
To determine whether or not the genotype of the male parent had any effect on kernel spotting in the progeny, we performed a series of exact reciprocal crosses of F7 plants, whereby mop1/mop1;MuDR(p1)* individuals were crossed to and by mop1/+ plants (Figure 4). When the female parent was mop1/mop1;MuDR(p1)*, 36% of the kernels were spotted, 11% of the total being heavy and medium spotted (Table 2A). In contrast, when the male parent was mop1/mop1;MuDR(p1)*, only 19% of the kernels were spotted, and only 1% of which were heavy or medium spotted (Figure 4 and Table 2A). A subset of the spotted progeny kernels from several reciprocally crossed individuals were genotyped for mop1. As seen previously, 51% of all spotted kernels genotyped from the female cross were mop1/+; the rest were mop1/mop1 (Table 3). Interestingly, almost all (93%) of the spotted kernels from the male cross were mop1 homozygous. We also performed reciprocal crosses of mop1/mop1;MuDR(p1)* to a1-mum2, which is wild type for Mop1. In these crosses the percentage of spotted kernels (31%) (290/921) (Table 2B) produced when mop1/mop1;MuDR(p1)* was female and crossed by an a1-mum2 male was similar to that seen when the female mop1/mop1;MuDR(p1)* was crossed to a mop1/+ male. In the reciprocal cross, there were fewer spotted kernels (12%) (136/1174) (Table 2B), and there were no heavily or medium spotted kernels.
Figure 4.
Ears resulting from an exact reciprocal cross between mop1/mop1;MuDR(p1)* and mop1/+.
TABLE 2.
Somatic activity exhibits a maternal effect in a mop1 background
| A. mop1/mop1; MuDR(p1)* × mop1/+
|
mop1/+ × mop1/mop1; MuDR(p1)*
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Crossa | h/mb | wc | Td | Spotted (%) | h/m (%) | h/mb | wc | Td | Spotted (%) | h/m (%) |
| 1 | 47 | 52 | 203 | 49 | 23 | 1 | 27 | 187 | 15 | 1 |
| 2 | 26 | 49 | 141 | 53 | 18 | 14 | 93 | 355 | 30 | 4 |
| 3 | 7 | 14 | 50 | 42 | 14 | 3 | 74 | 347 | 22 | 1 |
| 4 | 8 | 45 | 231 | 23 | 3 | 0 | 6 | 206 | 3 | 0 |
| 5 | 18 | 24 | 103 | 41 | 17 | 6 | 2 | 204 | 2 | 3 |
| 6 | 1 | 10 | 21 | 52 | 5 | 0 | 26 | 226 | 12 | 0 |
| 7 | 65 | 32 | 196 | 49 | 33 | 2 | 64 | 236 | 28 | 1 |
| 8 | 3 | 38 | 137 | 30 | 2 | 6 | 68 | 260 | 28 | 2 |
| 9 | 11 | 11 | 60 | 37 | 18 | 6 | 58 | 183 | 35 | 3 |
| 10 | 12 | 51 | 155 | 41 | 8 | 3 | 61 | 225 | 28 | 1 |
| 11 | 0 | 5 | 8 | 63 | 0 | 0 | 4 | 204 | 2 | 0 |
| 12 | 2 | 28 | 139 | 22 | 1 | 0 | 1 | 231 | 0 | 0 |
| 13 | 12 | 6 | 133 | 14 | 9 | 2 | 28 | 136 | 22 | 1 |
| 14 | 0 | 42 | 126 | 33 | 0 | 0 | 42 | 199 | 21 | 0 |
| 15 | 2 | 47 | 172 | 28 | 1 | 1 | 6 | 22 | 32 | 5 |
| Total | 214 | 454 | 1875 | 36 | 11 | 44 | 556 | 3221 | 19 | 1 |
| B. mop1/mop1; MuDR(p1)* × a1-mum2
|
a1-mum2 × mop1/mop1; MuDR(p1)*
|
|||||||||
| Crossa | h/mb | wc | Td | Spotted (%) | h/m (%) | h/mb | wc | Td | Spotted (%) | h/m (%) |
| 1 | 4 | 8 | 21 | 57 | 19 | 0 | 14 | 84 | 17 | 0 |
| 2 | 6 | 25 | 70 | 44 | 9 | 0 | 10 | 35 | 29 | 0 |
| 3 | 0 | 4 | 204 | 2 | 0 | 0 | 0 | 200 | 0 | 0 |
| 4 | 7 | 41 | 96 | 50 | 7 | 0 | 6 | 56 | 11 | 0 |
| 5 | 16 | 48 | 143 | 45 | 11 | 0 | 18 | 118 | 15 | 0 |
| 6 | 4 | 11 | 32 | 47 | 13 | 0 | 1 | 81 | 1 | 0 |
| 7 | 5 | 28 | 73 | 45 | 7 | 0 | 0 | 100 | 0 | 0 |
| 8 | 4 | 6 | 20 | 50 | 20 | 0 | 4 | 20 | 20 | 0 |
| 9 | 3 | 0 | 11 | 27 | 27 | 0 | 4 | 104 | 4 | 0 |
| 10 | 9 | 1 | 34 | 29 | 26 | 0 | 26 | 118 | 22 | 0 |
| 11 | 3 | 11 | 23 | 61 | 13 | 0 | 15 | 55 | 27 | 0 |
| 12 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 25 | 0 | 0 |
| 13 | 16 | 30 | 94 | 49 | 17 | 0 | 38 | 178 | 21 | 0 |
| Total | 77 | 213 | 921 | 31 | 8 | 0 | 136 | 1174 | 12 | 0 |
In each pair of crosses (numbered), an individual plant was reciprocally crossed as a female (left) or as a male (right) to an mop1/+ (A) or an a1-mum2 (B) tester.
Heavy/medium spotted kernels;
Weakly spotted kernels; and
Total number of kernels.
TABLE 3.
Frequency of mop1/mop1 mutants among spotted progeny of mop1/mop1;MuDR(p1)* plants
|
mop1/mop1; MuDR(p1)* × mop1/+
|
mop1/+ × mop1/mop1; MuDR(p1)*
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Crossa | m/mb | m/+c | Td | m/m (%) | m/+ (%) | Crossa | m/m | m/+ | T | m/m (%) | m/+ (%) |
| 1 | 15 | 13 | 28 | 54 | 46 | 1 | 12 | 0 | 12 | 100 | 0 |
| 2 | 8 | 5 | 13 | 62 | 38 | 2 | 11 | 0 | 11 | 100 | 0 |
| 3 | 13 | 13 | 26 | 50 | 50 | 3 | 17 | 1 | 18 | 94 | 6 |
| 4 | 8 | 19 | 27 | 30 | 70 | 4 | 14 | 4 | 18 | 78 | 22 |
| Total | 44 | 50 | 94 | 49 | 51 | Total | 54 | 5 | 59 | 93 | 7 |
Genotype of a subset of spotted kernels from reciprocal crosses.
mop1/mop1.
mop1/+;
Total embryos from spotted kernels genotyped for mop1.
Together, these data suggest that when the female parent is mop1/mop1;MuDR(p1)*, kernel- spotting intensity is independent of the kernel genotype; conversely, when the male parent is mop1/mop1;MuDR(p1)*, kernel-spotting intensity is largely dependent upon the kernel genotype.
MuDR(p1)* becomes heritably reactivated after multiple generations in a mop1 background:
To comprehensively examine the cumulative effects of mop1 on MuDR(p1)* in the same environment, three generations of kernels (F6, F7, and F8) were planted simultaneously in the summer of 2003. In all generations, mop1 homozygotes and heterozygotes carrying reactivated MuDR(p1)* were crossed as female to a1-mum2 (wild type for Mop1) and/or mop1/+. We found that in the F6 and F7 generations, as noted above, all mop1/+;MuDR(p1)* progeny plants were methylated at Mu1 or TIRA (Table 4). When testcrossed, these plants gave rise to few or no spotted kernels (Table 5 and Figure 5). Surprisingly, in the next (F8) generation, all mop1/+;MuDR(p1)* plants grown from spotted kernels (50 of 50) were hypomethylated at TIRA (Table 4). Of these 50 mop1/+;MuDR(p1)* plants that were hypomethylated at TIRA, roughly half (29) were also hypomethylated at Mu1; the remaining 21 were not (Table 4). When Mu1-hypomethylated mop1/+;MuDR(p1)* (F8) plants were crossed to either a-1mum2 or mop1/+ (resulting in the F9 generation), they gave rise to an average of 47% spotted kernels (Table 6), matching the percentages found in cases where the parent had been mop1/mop1;MuDR(p1)* . In contrast, sibling plants that were methylated at Mu1 (but were hypomethylated at TIRA) gave rise to only 3% (80 of 2286) spotted kernels. These data suggest that in the F8 generation, although there was a heritable effect on TIRA methylation in all mop1-heterozygous progeny that showed high levels of somatic activity in the aleurone, only a subset of those progeny were active enough to hypomethylate Mu1 in the embryo, and it was those individuals that went on to transmit continued somatic activity in a subsequent generation.
TABLE 4.
Hypomethylation of mop1/+ progeny of mutant individuals over several generations
| Cross | Hypo TIRAa | Hypo Mu1b | Meth Mu1c | Total TIRA | Hypo TIRA (%) | Hypo Mu1 (%) | Meth Mu1 (%) |
|---|---|---|---|---|---|---|---|
| F6 | 0 | 0 | 0 | 75 | 0 | 0 | 0 |
| F7 | 0 | 0 | 0 | 27 | 0 | 0 | 0 |
| F8 | 50 | 29 | 21 | 50 | 100 | 58 | 42 |
Number of mop1/+ progeny plants with hypomethylation at TIRA among heavily spotted mop1/+ progeny plants.
Of plants that were hypomethylated at TIRA, number that were also hypomethylated at Mu1.
Of plants that were hypomethylated at TIRA, number that were methylated at Mu1.
TABLE 5.
MuDR(p1)* activity becomes heritable after several generations in an mop1 background
| Crossa | Heavy/medium spotted | Weakly spotted | Pale | Total | Spotted (%) | Heavy/medium spottedb (%) | No. of crosses |
|---|---|---|---|---|---|---|---|
| A. Crosses by a mop1/+ testers | |||||||
| F6mop1/mop1 | 241 | 64 | 411 | 716 | 43 | 34 | 4 |
| F6mop1/+ | 6 | 13 | 1276 | 1295 | 1 | 0 | 12 |
| F7mop1/mop1 | 212 | 148 | 627 | 987 | 36 | 21 | 10 |
| F7mop1/+ | 73 | 119 | 2508 | 2700 | 7 | 3 | 17 |
| F8mop1/mop1 | 124 | 31 | 248 | 403 | 38 | 31 | 5 |
| F8mop1/+ | 644 | 272 | 1813 | 2729 | 34 | 24 | 17 |
| F9mop1/+ | 504 | 179 | 799 | 1482 | 46 | 34 | 5 |
| B. Crosses to a1-mum2 testers | |||||||
| F7mop1/mop1 | 734 | 177 | 1028 | 1939 | 47 | 38 | 15 |
| F7mop1/+ | 108 | 105 | 1416 | 1629 | 13 | 7 | 11 |
| F8mop1/mop1 | 126 | 71 | 191 | 388 | 51 | 32 | 3 |
| F8mop1/+ | 260 | 98 | 411 | 770 | 46 | 34 | 6 |
| F9mop1/+ | 342 | 164 | 789 | 1285 | 39 | 26 | 7 |
Generation (FX) and genotype of the female plants carrying MuDR(p1)* crossed to the indicated male genotypes; all individuals from a particular generation are siblings.
Percentage of heavy/medium spotted of total kernels.
Figure 5.
(A) Mu1 and TIRA Southern blots of individuals from both F7 and F8 generations. All individuals are mop1/+. Note that in the F7 generation, all individuals are methylated at both Mu1 and TIRA. In the F8 generation in this figure, all individuals that are mop1/+;MuDR(p1)* are hypomethylated at Mu1 and TIRA, indicating that MuDR(P1)* has been heritably reactivated in this generation. Individuals lacking MuDR(p1)* are methylated at Mu1 and lack the methylated or the hypomethylated TIRA fragments. (B) Ear progeny of a cross between indicated individuals represented by the Southern blot to a mop1/+ tester. mop1/+ individuals from the F7 generation gave rise to few or no spotted-kernel progeny when outcrossed to a mop1/+ tester. However, Mu1-hypomethylated mop1/+;MuDR(p1)* individuals from the F8 generation gave rise to ∼46% spotted kernels when outcrossed to mop1/+.
TABLE 6.
Persistence of somatic activity even in the absence of the mop1 mutation: Mu1 methylation of mop1/;+MuDR(p1)* individuals, F8 generation, and progeny
| ha | Cross to | Spotted | Total | Spotted (%) | ma | Cross to | Spotted | Total | Spotted (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | mop1/+ | 80 | 184 | 43 | 1 | mop1/+ | 3 | 123 | 2 |
| 2 | mop1/+ | 48 | 99 | 48 | 2 | mop1/+ | 1 | 112 | 1 |
| 3 | mop1/+ | 108 | 207 | 52 | 3 | a1-mum2 | 4 | 204 | 2 |
| 4 | a1-mum2 | 58 | 123 | 47 | 4 | mop1/+ | 3 | 158 | 2 |
| 5 | mop1/+ | 178 | 334 | 53 | 5 | a1-mum2 | 6 | 192 | 3 |
| 6 | mop1/+ | 66 | 222 | 30 | 6 | a1-mum2 | 7 | 207 | 3 |
| 7 | mop1/+ | 127 | 258 | 49 | 7 | mop1/+ | 1 | 121 | 1 |
| 8 | mop1/+ | 96 | 172 | 56 | 8 | mop1/+ | 2 | 202 | 1 |
| 9 | a1-mum2 | 120 | 256 | 47 | 9 | a1-mum2 | 4 | 203 | 2 |
| 10 | a1-mum2 | 46 | 92 | 50 | 10 | a1-mum2 | 14 | 214 | 7 |
| 11 | mop1/+ | 96 | 263 | 37 | 11 | mop1/+ | 27 | 227 | 12 |
| 12 | a1-mum2 | 168 | 327 | 51 | 12 | mop1/+ | 5 | 186 | 3 |
| 13 | a1-mum2 | 144 | 280 | 51 | 13 | mop1/+ | 3 | 137 | 4 |
| Total | 1335 | 2817 | 47 | Total | 80 | 2286 | 3 |
As indicated in Table 4, all plants were hypomethylated at TIRA, but only about half were also hypomethylated at Mu1. h, plant had hypomethylated Mu1 element; m, plant had methylated Mu1 element. Plants were crossed by either a1-mum2 or mop1/+ testers as indicated.
Plants grown from the F8 generation kernels that were spotted.
One family in the F9 generation that was segregating for both mop1/+ and homozygous wild- type individuals, was testcrossed by mop1/+ or to a1-mum2 (Table 7). MuDR activity continued to transmit in the resulting ears. Notably, one ear, derived from a cross between a homozygous wild-type plant and an a1-mum2 tester segregated 38% for spotted kernels, demonstrating that the somatic activity of MuDR(p1)* persisted even in the absence of even one copy of the mop1 mutant allele.
TABLE 7.
Crosses from one family of the F9 generation segregating for wild type
| Crossa | h/mb | wc | Pale | Td | Spotted (%) | h/m (%) | Kernels (% wt) |
|---|---|---|---|---|---|---|---|
| mop/+; MuDR(p1)* × a1-mum2 | 2 | 6 | 93 | 101 | 8 | 2 | 75 |
| mop/+; MuDR(p1)* × a1-mum2 | 62 | 48 | 145 | 255 | 43 | 24 | 75 |
| mop/+; MuDR(p1)* × a1-mum2 | 7 | 12 | 140 | 159 | 12 | 4 | 75 |
| +/+; MuDR(p1)* × mop1/+ | 1 | 10 | 102 | 113 | 10 | 1 | 75 |
| +/+; MuDR(p1)* × mop1/+ | 45 | 39 | 68 | 152 | 55 | 30 | 75 |
| +/+; MuDR(p1)* × a1-mum2 | 38 | 14 | 84 | 136 | 38 | 28 | 100 |
Plants in this generation were either heterozygous for mop1 or homozygous for Mop1.
Heavy/medium spotted kernels.
Weakly spotted kernels; and
Total number of kernels.
We wanted to know whether the hypomethylation of Mu1 in mop1/+;MuDR(p1)* individuals was the result of a generic effect on Mu1 due to its long-term exposure to mop1 (because mop1 has an effect on Mu1 independent of MuDR activity) or whether it was the result of reactivated MuDR(p1)* transposase (which can hypomethylate Mu1 elements independent of mop1). To test this, we examined siblings in the F8 generation that were mop1/+ but that lacked MuDR(p1)*. Mu1 was methylated in all (20 of 20) of these individuals (data not shown), suggesting that MOP1 was present and competent to mediate default methylation of TIRs but that this process is prevented in plants that carry reactivated MuDR(p1)* due to activity of the transposase in sibling plants that carried heritably reactivated MuDR elements.
mudrA but not mudrB expression is restored in mop1-reactivated MuDR(p1)*:
When silenced by Muk, MuDR(p1)* loses expression of polyadenylated and nonpolyadenylated mudrA and polyadenylated mudrB transcript. By the next generation, both mudrA and mudrB become transcriptionally silenced, even in the absence of Muk (Slotkin et al. 2003). We wanted to see whether or not the mop1 mutation was able to restore expression of these genes. In the F6 and F7 generations, plants that were mop1/mop1;MuDR(p1)* expressed polyadenylated mudrA, but mop1/+;MuDR(p1)* sibling plants did not, nor did plants that were mop1 mutant but that lacked MuDR(p1)* (Figure 6A). By the F8 generation however, mudrA expression was maintained in mop1/+;MuDR(p1)* individuals that are also hypomethylated at Mu1 (Figure 6B) and that transmitted significant numbers of spotted progeny kernels when testcrossed (Table 6). However, polyadenylated mudrB expression was not restored in a mop1 mutant background in any individuals, even in later generations in which MuDR(p1)* activity had become independent of the mop1 mutant.
Figure 6.
mudrA and mudrB expression. (A) RT–PCR of both mudrA and mudrB in the F7 generation. Individuals that are mop1/mop1;MuDR(p1)* express mudrA, but mop1/+;MuDR(p1)* individuals and those that lack MuDR(p1)* do not express mudrA. However, mop1/mop1;MuDR(p1)* individuals do not express mudrB. (B) RT–PCR of mudrA and mudrB in the F8 generation. mop1/+;MuDR(p1)* as well as mop1/mop1;MuDR(p1)* individuals now express mudrA, in conjunction with the heritable reactivation of MuDR(p1)* in this later generation. Once again, mudrB is still not expressed in any individual, not even mop1 homozygotes. aat is the cDNA control.
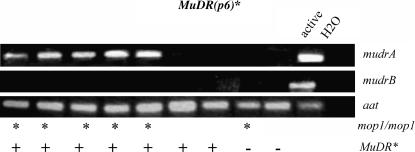
To determine whether the differential reactivation of mudrA and mudrB expression in a mop1 background was due to a position effect of MuDR* at p1, we examined mudrA and mudrB expression at another silenced MuDR locus, position 6 (p6). MuDR(p6)* had been introgressed into a mop1 background for at least three generations and exhibited a small percentage of kernel spotting, around 2% (data not shown). We found that mop1/mop1;MuDR(p6)* individuals exhibited mudrA but not mudrB expression (Figure 7), just as was seen with MuDR(p1)*. This suggests that the differential reactivation of mudrA and mudrB is a generic effect of the mop1 mutation and not due to a position effect on MuDR.
Figure 7.
mudrA and mudrB expression at another MuDR position (MuDR(p6)*). Individuals that are mop1/mop1;MuDR(p6)* express mudrA but not mudrB, similar to what is observed in reactivated MuDR(p1)*.
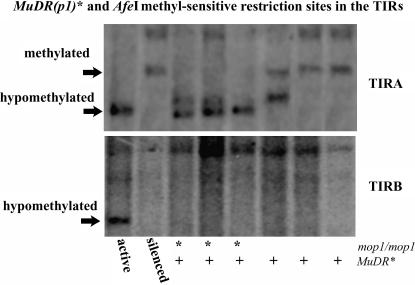
We wished to discover the methylation status of TIRB vs. TIRA to see if there was a methylation correlate with the differential mudrA and mudrB expression in a mop1 background. Unfortunately our primary methylation assay, the HinfI digest, did not produce conclusive data as the fragment sizes around mudrB were too small to resolve on a Southern blot. Therefore, we looked to another restriction site, AfeI, which is found in both TIRA and TIRB and that is blocked by CG methylation. We digested DNA from a family segregating for mop1 and MuDR(p1)* with AfeI and BamHI (which cuts once within MuDR and which is not methyl sensitive) and then probed the blot with the methyl TIRA probe (as mentioned previously) and subsequently with a p1 flanking probe (Figure 8). We found that in individuals that were mop1/mop1;MuDR(p1)* the TIRA was hypomethylated, but the TIRB remained methylated, indicating that at the AfeI site there is a difference in methylation between TIRA and TIRB.
Figure 8.
Methyation analysis of TIRA vs. TIRB. Shown is a Southern blot depicting individuals carrying MuDR(p1)* and that are mop1/mop1 or mop1/+. Individuals were digested with AfeI (a methyl-sensitive enzyme that cuts once within each TIR) and BamHI (not methyl sensitive and that cuts once within the MuDR element as well as outside each TIR). The Southern blot was probed first with the methyl TIRA probe and then stripped and reprobed with the p1 flanking probe. mop1/mop1;MuDR(p1)* individuals are hypomethylated at TIRA but are not hypomethylated at TIRB. Active MuDR is hypomethylated at both TIRA and TIRB. Silenced MuDR and mop1/+;MuDR(p1)* individuals are hypomethylated at neither TIR.
New transpositions are not observed in a mop1 background:
One of the effects of Mutator silencing is the loss of new Mu element transpositions. In an active Mutator minimal line, new transpositions by Mu1 and MuDR(p1) occur a rate of 10–20% transpositions per generation (Lisch et al. 1995). When new MuDR(p1) transpositions occur, the resulting ears exhibit a higher percentage of spotted kernels than that expected from an ear segregating for only MuDR(p1) (50% expected spotted kernels in a cross MuDR(p1)/− × −/−). We wanted to see if the mop1 mutation restored transposition of either MuDR(p1)* or Mu1 in silenced Mutator lines. To test this, we examined Southern blots for the appearance of unique fragments consistent with new transposition events, and we looked for ears that segregated for significantly more than 50% spotted kernels. HinfI digests of mop1-heterozygous progeny of mop1 mutant plants were probed with Mu1, and BamHI and EcoRI digests were probed with an internal fragment of MuDR. If full transpositional activity were restored, we would expect that between 10 and 20% of the individuals seen would exhibit new transpositions of either Mu1 or MuDR(p1)*. No new insertions of Mu1 were observed in 437 progeny of mop1/mop1 plants that carried reactivated MuDR(p1)*. The same was true for the 119 progeny examined by Southern blot for evidence of new insertions of MuDR. In addition, of the 932 ears we examined that were derived from mop1-homozygous plants carrying reactivated MuDR(p1)*, we have not detected any with a percentage of spotted kernels significantly greater than 50%. This suggests that while the mop1 mutant can reactivate Mutator somatic activity, it fails to reactivate transpositional activity either in cis (MuDR insertions) or in trans (Mu1 insertions). Because mudrB regulates transposition, and because in a mop1 mutant mudrB remains silenced, the lack of transposition in a mop1 mutant is further evidence for the relationship between transpositional activity and mudrB activity.
DISCUSSION
Unlike the immediate and heritable reactivation of silenced transposons in a ddm1 mutant background (Lippman et al. 2003; Kato et al. 2004), reactivation of MuDR elements in a mop1 mutant background occurs only gradually and stochastically. Only after multiple generations of exposure to the mop1 mutation do we observe evidence of heritable activity of silenced MuDR elements in the absence of the mutation. This process is reminiscent of the gradual appearance of epimutations, such as fwa, after several generations in a ddm1 mutant background (Kakutani et al. 1996; Soppe et al. 2000). Interestingly, the promoter and upstream portion of the FWA transcript are composed of a transposable element (Lippman et al. 2004). These data suggest that the effects of missing one component of the silencing machinery (MOP1 in this case, DDM1 in Arabidopsis) can be cumulative over time, perhaps because the loss of one component results in destabilization of a complex, which in turn leads to the loss of additional components and subsequent additional destabilization. Since a number of factors involved in the construction of stabilized chromatin appear to be mutually reinforcing (i.e., DNA methylation and histone modification (Johnson et al. 2002; Soppe et al. 2002), it is reasonable to assume that, although the loss of one component may not have an immediate effect of gene activity, the absence of reinforcement of the silenced state could eventually result in destabilization of the silencing chromatin. Several other mutations that affect both paramutable alleles and Mu element methylation have been identified (Lisch and Hollick, unpublished data). It will be interesting to see how efficiently double mutants reactivate silenced MuDR elements.
The absence of both new insertions and mudrB expression in our reactivated lines is consistent with earlier data suggesting a role for mudrB in Mu element transposition (Lisch et al. 1999) (Raizada and Walbot 2000). Like our reactivated MuDR elements, deletion derivatives that lack mudrB, as well as transgenes that express only mudrA, can condition only excisions. Differential reactivation of two genes on the same transposon has not been observed previously. It suggests that the silenced state of these two genes differs in some way. Either mudrB is simply more deeply silenced than mudrA or the silenced state of the two genes is qualitatively different. There is some evidence that the latter may be the case, since TIRA becomes hypomethylated in a mop1 background but TIRB does not. Further, although both genes are silenced after exposure to Muk, their mode of silencing appears to be different. Muk is an inverted duplicated version of MuDR that lacks the mudrB gene. It produces a long inverted repeat transcript that is homologous to the mudrA gene that produces small (23 and 26 nt) mudrA-homologous RNA molecules that are amplified when Muk silences MuDR elements. No such RNA molecules homologous to mudrB are observed (Slotkin et al. 2005). The mudrA gene is transcriptionally silenced by the immature ear of F1 plants carrying both MuDR and Muk. In contrast, the mudrB gene remains transcriptionally active in these same F1 immature ears, and it is only in the next generation that mudrB becomes transcriptionally silenced (Slotkin et al. 2003). This process requires that mudrB be in cis to mudrA; when a deletion derivative of MuDR(p1) that carries only mudrB is placed in trans to MuDR(p1), it is not silenced in the presence of Muk (Slotkin et al. 2005). Thus, mudrB silencing is mediated via mudrA silencing, most likely via a distinct pathway that involves a signal that spreads in cis from mudrA to mudrB. If this is the case, then the chromatin at silenced mudrB may well be qualitatively different from that at mudrA.
The identity of mop1 remains mysterious, so we can only speculate as to the mechanism of MuDR reactivation. The fact that this mutation affects both paramutation and MuDR activity but neither global methylation nor methylation of some transposon sequences upstream of the maize paramutagenic b1 allele implies a relatively restricted (or partially redundant) role in maize gene silencing. This is reinforced by our observation that only one of the two genes encoded by MuDR is reactivated in a mop1 background. The relative specificity of the mop1 mutation may be because the Mop1 gene is qualitatively different from mutations in Arabidopsis that affect transposon activity, or because the more global functions of those genes have been partitioned among multiple genes in maize. This possibility is supported by the existence of multiple mutations that affect both Mutator methylation and paramutation (Hollick and Chandler 2001; Lisch and Hollick, unpublished data).
An understanding of the differences between mudrA and mudrB silencing and resulting differences in chromatin at the mudrA and mudrB promoters at various stages of reactivation, should shed light on the relationship between the means by which genes are silenced and the nature of the silenced state once achieved. The evidence to date suggests that the initiation and maintenance of silencing is far more complex than simple “on” and “off” states. History, position, context, and timing may all play a role in these processes. The cumulative reactivation of mudrA in a mop1 background suggests that changes in chromatin memory through several rounds of meiosis can be gradual, and that there are intermediate chromatin states between silenced and active chromatin that may in themselves have unique regulatory consequences and interesting implications for our understanding of heritable changes in the chromatin state.
References
- Alleman, M., and M. Freeling, 1986. The Mu transposable elements of maize: evidence for transposition and copy number regulation during development. Genetics 112: 107–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravin, A. A., N. M. Naumova, A. V. Tulin, V. V. Vagin, Y. M. Rozovsky et al., 2001. Double-stranded RNA-mediated silencing of genomic tandem repeats and transposable elements in the D-melanogaster germline. Curr. Biol. 11: 1017–1027. [DOI] [PubMed] [Google Scholar]
- Bartee, L., F. Malagnac and J. Bender, 2001. Arabidopsis cmt3 chromomethylase mutations block non-CG methylation and silencing of an endogenous gene. Genes Dev. 15: 1753–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennetzen, J. L., 1996. The Mutator transposable element system of maize. Curr. Top. Microbiol. Immunol. 204: 195–229. [DOI] [PubMed] [Google Scholar]
- Brink, R. A., E. D. Styles and J. D. Axtell, 1968. Paramutation: directed genetic change. Paramutation occurs in somatic cells and heritably alters the functional state of a locus. Science 159: 161–170. [DOI] [PubMed] [Google Scholar]
- Chandler, V., C. Rivin and V. Walbot, 1986. Stable nonmutator stocks of maize have sequences homologous to the Mu1 transposable element. Genetics 114: 1007–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler, V. L., and M. Stam, 2004. Chromatin conversations: mechanisms and implications of paramutation. Nat. Rev. Genet. 5: 532–544. [DOI] [PubMed] [Google Scholar]
- Chomet, P., D. Lisch, K. J. Hardeman, V. L. Chandler and M. Freeling, 1991. Identification of a regulatory transposon that controls the Mutator transposable element system in maize. Genetics 129: 261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomet, P. S., S. Wessler and S. L. Dellaporta, 1987. Inactivation of the maize transposable element Activator (Ac) is associated with its DNA modification. EMBO J. 6: 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorweiler, J. E., C. C. Carey, K. M. Kubo, J. B. Hollick, J. L. Kermicle et al., 2000. mediator of paramutation1 is required for establishment and maintenance of paramutation at multiple maize loci. Plant Cell 12: 2101–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollick, J. B., and V. L. Chandler, 2001. Genetic factors required to maintain repression of a paramutagenic maize pl1 allele. Genetics 157: 369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, L., X. Cao and S. Jacobsen, 2002. Interplay between two epigenetic marks. DNA methylation and histone H3 lysine 9 methylation. Curr. Biol. 12: 1360–1367. [DOI] [PubMed] [Google Scholar]
- Kakutani, T., J. A. Jeddeloh, S. K. Flowers, K. Munakata and E. J. Richards, 1996. Developmental abnormalities and epimutations associated with DNA hypomethylation mutations. Proc. Natl. Acad. Sci. USA 93: 12406–12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kankel, M. W., D. E. Ramsey, T. L. Stokes, S. K. Flowers, J. R. Haag et al., 2003. Arabidopsis MET1 cytosine methyltransferase mutants. Genetics 163: 1109–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato, M., A. Miura, J. Bender, S. E. Jacobsen and T. Kakutani, 2003. Role of CG and non-CG methylation in immobilization of transposons in Arabidopsis. Curr. Biol. 13: 421–426. [DOI] [PubMed] [Google Scholar]
- Kato, M., K. Takashima and T. Kakutani, 2004. Epigenetic control of CACTA transposon mobility in Arabidopsis thaliana. Genetics 168: 961–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippman, Z., and R. Martienssen, 2004. The role of RNA interference in heterochromatic silencing. Nature 431: 364–370. [DOI] [PubMed] [Google Scholar]
- Lippman, Z., B. May, C. Yordan, T. Singer and R. Martienssen, 2003. Distinct mechanisms determine transposon inheritance and methylation via small interfering RNA and histone modification. PLoS Biol. 1: E67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippman, Z., A. V. Gendrel, M. Black, M. W. Vaughn, N. Dedhia et al., 2004. Role of transposable elements in heterochromatin and epigenetic control. Nature 430: 471–476. [DOI] [PubMed] [Google Scholar]
- Lisch, D., 2002. Mutator transposons. Trends Plant Sci. 7: 498–504. [DOI] [PubMed] [Google Scholar]
- Lisch, D., P. Chomet and M. Freeling, 1995. Genetic characterization of the Mutator system in maize: behavior and regulation of Mu transposons in a minimal line. Genetics 139: 1777–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisch, D., L. Girard, M. Donlin and M. Freeling, 1999. Functional analysis of deletion derivatives of the maize transposon MuDR delineates roles for the MURA and MURB proteins. Genetics 151: 331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisch, D., C. C. Carey, J. E. Dorweiler and V. L. Chandler, 2002. A mutation that prevents paramutation in maize also reverses Mutator transposon methylation and silencing. Proc. Natl. Acad. Sci. USA 99: 6130–6135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martienssen, R., and A. Baron, 1994. Coordinate suppression of mutations caused by Robertson's mutator transposons in maize. Genetics 136: 1157–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly, C., N. S. Shepherd, A. Pereira, Z. Schwarz-Sommer, I. Bertram et al., 1985. Molecular cloning the A1 locus of Zea mays using the transposable elements En and Mu. EMBO J. 4: 591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto, H., and H. Hirochika, 2001. Silencing of transposable elements in plants. Trends Plant Sci. 6: 527–534. [DOI] [PubMed] [Google Scholar]
- Raizada, M. N., and V. Walbot, 2000. The late developmental pattern of Mu transposon excision is conferred by a cauliflower mosaic virus 35S-driven MURA cDNA in transgenic maize. Plant Cell 12: 5–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raizada, M. N., K. V. Brewer and V. Walbot, 2001. A maize MuDR transposon promoter shows limited autoregulation. Mol. Genet. and Genomics 265: 82–94. [DOI] [PubMed] [Google Scholar]
- Rudenko, G. N., and V. Walbot, 2001. Expression and post-transcriptional regulation of maize transposable element MuDR and its derivatives. Plant Cell 13: 553–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SanMiguel, P., A. Tikhonov, Y. K. Jin, N. Motchoulskaia, D. Zakharov et al., 1996. Nested retrotransposons in the intergenic regions of the maize genome. Science 274: 765–768. [DOI] [PubMed] [Google Scholar]
- Singer, T., C. Yordan and R. A. Martienssen, 2001. Robertson's Mutator transposons in A. thaliana are regulated by the chromatin-remodeling gene Decrease in DNA Methylation (DDM1). Genes Dev. 15: 591–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin, R. K., M. Freeling and D. Lisch, 2003. Mu killer causes the heritable inactivation of the Mutator family of transposable elements in Zea mays. Genetics 165: 781–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin, R. K., M. Freeling and D. Lisch, 2005. Heritable transposon silencing initiated by a naturally occurring transposon inverted duplication. Nat. Genet. 37: 641–644. [DOI] [PubMed] [Google Scholar]
- Soppe, W. J., S. E. Jacobsen, C. Alonso-Blanco, J. P. Jackson, T. Kakutani et al., 2000. The late flowering phenotype of fwa mutants is caused by gain-of-function epigenetic alleles of a homeodomain gene. Mol. Cell 6: 791–802. [DOI] [PubMed] [Google Scholar]
- Soppe, W. J., Z. Jasencakova, A. Houben, T. Kakutani, A. Meister et al., 2002. DNA methylation controls histone H3 lysine 9 methylation and heterochromatin assembly in Arabidopsis. EMBO J. 21: 6549–6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbert, L. E., and V. L. Chandler, 1988. Characterization of a highly conserved sequence related to mutator transposable elements in maize. Mol. Biol. Evol. 5: 519–529. [DOI] [PubMed] [Google Scholar]
- Vastenhouw, N. L., S. E. J. Fischer, V. J. P. Robert, K. L. Thijssen, A. G. Fraser et al., 2003. A genome-wide screen identifies 27 genes involved in transposon silencing in C-elegans. Curr. Biol. 13: 1311–1316. [DOI] [PubMed] [Google Scholar]
- Zilberman, D., and S. Henikoff, 2004. Silencing of transposons in plant genomes: kick them when they're down. Genome Biol. 5: 249. [DOI] [PMC free article] [PubMed] [Google Scholar]