Abstract
Mutual trophic interactions between contiguous habitats have remained poorly understood despite their potential significance for community maintenance in ecological landscapes. In a deciduous forest and stream ecotone, aquatic insect emergence peaked around spring, when terrestrial invertebrate biomass was low. In contrast, terrestrial invertebrate input to the stream occurred primarily during summer, when aquatic invertebrate biomass was nearly at its lowest. Such reciprocal, across-habitat prey flux alternately subsidized both forest birds and stream fishes, accounting for 25.6% and 44.0% of the annual total energy budget of the bird and fish assemblages, respectively. Seasonal contrasts between allochthonous prey supply and in situ prey biomass determine the importance of reciprocal subsidies.
Keywords: forest–stream ecotone, allochthonous prey flux
A regional landscape is a heterogeneous collection of habitats that differ in various environmental properties, namely medium, temperature, light, nutrients, productivity, and species composition. Apparently discrete food webs developing in local habitats can be, however, tightly linked by an energy flux across the interface between the habitats. In each habitat, across-habitat transfers of materials or organisms often exert critical influences on communities (1, 2). Theoretical considerations of such trophic interactions have proposed that energy generally flows from more to less productive habitats and provides significant subsidies to recipient systems only (2–5). Most empirical studies have dealt only with such one-sided subsidies (1, 2). However, productivity in a local habitat generally fluctuates seasonally, and on other time scales. Peak productivities in juxtaposed habitats can be asynchronous. If decoupled fluctuations reverse the productivity gradient and the direction of energy transfer between contiguous habitats, local communities in both habitats could be subsidized reciprocally and alternately.
Reciprocal subsidies may be important between forest and streams in northern temperate latitudes, where two principal determinants of primary productivity, temperature and light, change dramatically with season. Forest and stream food webs are widely viewed as energetically coupled, with considerable edge-mediated effects (5). Inputs of particulate organic matter from riparian forests represent an important energy source of stream production (6, 7). Accidental inputs of terrestrial invertebrates, in particular, are known to be a major prey category directly available for stream consumers such as fish (8–12). Conversely, previous studies have often argued that riparian forests generally support greater species diversity and population abundance of terrestrial consumers than adjacent upland habitats (13, 14), although the mechanisms responsible for this edge effect have remained poorly understood (5). Riparian consumers, e.g., birds, bats, and spiders, may benefit from energy transfers gained from aquatic insects emerged from streams (15–17). Net flux direction, however, can change seasonally. Terrestrial plant productivity peaks in summer, and decreases with the approach of winter in forests, as air temperatures drop. In contrast, streams can experience relatively stable water temperatures throughout the year (see Fig. 1A). Especially in temperate deciduous forest, stream productivity is highest during the forest defoliation period (autumn and spring) and declines in summer as streams become more shaded by riparian canopies (18). These seasonal asynchronies in productivity maxima between streams and adjacent forests could set the stage for reciprocal subsidies to both terrestrial and aquatic predators in many temperate ecosystems. Here we present empirical evidence for such reciprocal subsidies to forest birds and stream fishes by allochthonous prey flux across a habitat interface.
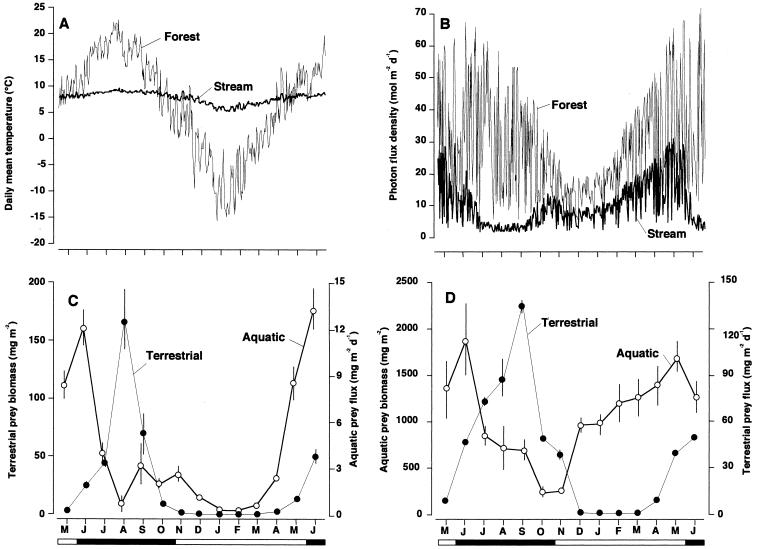
Figure 1.
Contrasts in seasonal dynamics of physical environmental variables and prey invertebrates between a forest and stream in northern Japan. Temperatures were recorded every hour in both stream and forest with automatic thermometers (Optic StowAway, Onset Inc., Bourne, MA). Light conditions were recorded every hour just above the stream surface and forest canopies with automatic photon flux density meters (LI-250, Li-Cor, Lincoln, NE). (A) Daily mean temperatures of forest and stream. (B) Daily cumulative photon flux densities at the forest canopy and stream surfaces. (C) Terrestrial prey biomass on tree foliage and aquatic prey flux to forest. Both biomass (P < 0.0001) and flux (P < 0.0001) differed significantly among months (by one-way ANOVA on log10-transformed data for both). (D) Aquatic prey biomass and terrestrial prey flux to stream. Both biomass (P < 0.0001) and flux (P < 0.0001) differed significant among months (by one-way ANOVA on log10-transformed data). Black and white circles in C and D represent mean values (±1 SEM) for terrestrial and aquatic prey, respectively. Black and white portions of horizontal bars at bottom of figures indicate leafing and defoliation periods, respectively.
Study Site.
Field studies, involving ≈3,500 person-hours, were conducted in a forest plot [200 m wide × 500 m long, 10 hectare (ha) area] along the Horonai Stream in the Tomakomai Experimental Forest (TOEF; 42° 43′ N, 141° 36′ E), Hokkaido, Japan, during the 14 months from May 1997 to June 1998. The forest was dominated by deciduous tree species oak (Quercus crispula), cherry (Prunus sargentii), and ash (Fraxinus mandshurica), and 30 other canopy species. There is no clear boundary between riparian and upland forest types. In the forest, most tree species break bud in early June and shed their leaves in mid October. In this small, cold-spring-fed stream [15.4 km2 in drainage area, 14 km long, 2–5 m wide, gradient <1%], discharge remained stable (0.25 m2⋅s−1 on average), with major flood disturbance rarely occurring. No scouring floods occurred during the study period. Approximately 97% of the entire width of the study reach was shaded by the forest canopy during the leafing period (Fig. 1B). Although mean monthly air temperatures fluctuated considerably (Fig. 1A), water temperatures in a study reach (3.5 m wide × 1.2 km long, 4,200 m2 area) running across the plot usually remained stable throughout the year (Fig. 1A).
Consumers.
In the forest, insectivorous birds included four year-round residents (the great tit Parus major, the marsh tit Parus palustris, the nuthatch Sitta europaea, and the pigmy woodpecker Dendrocopos kizuki), five summer residents (the brown flycatcher Muscicapa latirostris, the pale-legged willow warbler Phylloscopus tenellipes, the narcissus flycatcher Ficedula narcissina, the crowned willow warbler Phylloscopus occipitalis, and the black-faced bunting Emberiza spodocephala), and a winter resident wren (Troglodytes troglodytes). Nine other species were also observed. All of the summer and winter birds arrived at the study area in May and October and left in August and March, respectively. The stream was inhabited by four water-column salmonids, including rainbow trout (Oncorhynchus mykiss), white-spotted char (Salvelinus malma), Dolly Varden (Salvelinus malma), and masu salmon (Oncorhynchus masou). These salmonids, and a benthic sculpin (Cottus nozawae), were all fluvial insectivores.
Methods
Prey Abundance.
The biomass of invertebrate prey was surveyed in both the forest plot and stream reach every month. Terrestrial invertebrates on the foliage (>2 m high) were collected quantitatively from 40 oak, eight linden (Tilia japonica), cherry, painted maple (Acer mono), Japanese maple (Acer palmatum var. matsumurae), and lilac (Syringa reticulata) trees chosen haphazardly every month (19). A branch was held onto a tray (80 × 80 cm area) and beaten repeatedly, and the arthropods which dropped onto the tray were collected. Leaves were carefully checked, and remaining invertebrates, including leaf rollers, were collected. Mean diameter at the base of the branch was 2.8 ± 0.7 cm (n = 80). The biomass of stream invertebrates was estimated from samples collected from twelve haphazardly chosen locations in the study reach (20). The stream invertebrates were collected by a 225-μm mesh Surber net sampler (25 × 25 cm quadrat, 1 m net length). In addition, across-habitat prey fluxes, including both the accidental inputs of terrestrial invertebrates to and the emergence of aquatic insects from the study reach, were simultaneously estimated. Emerging aquatic prey were collected daily with 18 square-pyramid emergence traps (1 × 1 m area, 1 m high, made of 0.5 mm mesh) set across the study reach at a height of 1 cm above the stream surface. The traps were deployed concurrently for four days twice a month, with their bases located just above the water surface. The daily input of terrestrial prey was estimated from samples collected by 15 pan traps (1 × 1 m area, 15 cm deep) set in a current of the stream. The traps were filled with water plus 2–3 drops of surfactant, and set across the entire length of the study reach. The pan contents were sieved with 250-μm mesh after 1-weeks deployment. Beating, Surber net, emergence, and pan trap samples were preserved in 70% ethanol and sorted into terrestrial and aquatic invertebrates, both of which were identified to order. The biomass of each order (measured as wet mass and dry mass) was subsequently estimated (cf. 10). Aquatic insects in the pan traps and beating samples, and terrestrial invertebrates in the emergence traps, all of which contributed only a minor portion (<5%) of the total biomass in each, were excluded from all of the analyses.
Consumers.
Monthly changes in prey composition were examined for the dominant bird and fish species. To make the bird observations, 20-m square grids were established with colored markers placed on the forest floor. Observers walked along the grid on a systematic basis during a 5-h period immediately after dawn everyday (excepting rainy days) during the study period, and directly observed bird foraging to record prey categories. Prey types were classified into terrestrial preys including invertebrates and plant materials, and aquatic invertebrate preys. When an individual was encountered, it was followed for as long as possible (within 40 m of the original location); we were able to identify prey categories for 7,200 (53.6%; 114 for each species in each month on average) of a total of 13,433 observations. The stomach contents of 1,409 individual fish (20 individuals of each species each month on average) were also examined. Fishes were captured during a 5-h period immediately after dawn by using a backpack electrofishing unit (Model 12, Smith-Root, Inc., Vancouver, WA). Fish diets were collected by using a stomach pumping method (12) and samples were preserved in 70% ethanol. The biomass of stream fishes was estimated by the three-pass removal method (21) in four subreaches (50 m long, 3.5 m wide, on average, 175 m2 area) in the study reach in May, August, and November 1997, and February and May 1998. The total fish biomass ranged between 963.3 and 1265.5 g (wet mass) per 175 m2 over the five seasons. The bird abundance excluding the nestlings was also surveyed by a mapping method (22). The total abundance of 10 dominant bird species examined ranged between 36 and 94 in a 10 ha plot over the study period (five seasons).
Energy Flows.
The annual contribution of allochthonous prey (i.e., aquatic and terrestrial prey for birds and fishes, respectively) to the total energy budget was estimated for each assemblage as well as for individual species, by using the data obtained from May 1997 to April 1998. For each bird species, the daily energy demand (E) was estimated from E = 4.2 BM (0.61) kj (where BM = body mass g; ref. 23), which was then multiplied by the frequency of aquatic prey foraging to total observations so as to estimate their energy contribution. The energy value of prey was taken as being equal for terrestrial and aquatic prey, because of the considerable overlap in individual dry mass and calorific value (Nakano and Murakami, unpublished data). Fish daily ration (R: mg per 100 mg dry mass fish d−1) was estimated from the following [salmonids, r = e−1.857 + 0.196T, R2 = 0.71, P < 0.0001, n = 32; sculpin, r = e−2.89 + 0.212T, R2 = 0.881, P = 0.006, n = 8, where T = monthly mean temperature; ref. 24]. The salmonid species were taken as being equal. Subsequently, their monthly consumption rate of terrestrial prey was obtained by multiplying the estimated ration by the mean proportion of the terrestrial invertebrates (by mass) in the total stomach contents for each species. All values were converted into an area-based, annual unit (cf. 25), taking seasonal changes in the abundance of bird and the biomass of fishes into consideration.
Results and Discussion
The seasonal dynamics of the allochthonous prey supply differed considerably from the in situ prey biomass in both the forest and the stream (Fig. 1 C and D). In the forest, terrestrial prey biomass during the leafing period was much greater than that during defoliation periods, peaking in mid summer (August) and being nearly zero during winter (December to March; Fig. 1C). Although aquatic prey flux to the forest was greatest around bud breaking (May to June; Fig. 1C), a low level of emergence was observed continuously, even during winter. In contrast, aquatic prey biomass during the defoliation period was much greater than that during the leafing period (Fig. 1D). Terrestrial prey flux to the stream was proportional to biomass of terrestrial prey (r = 0.92, P < 0.0001). Although aquatic prey flux to the forest was not correlated with biomass of aquatic prey in the stream (r = 0.029, P = 0.92), the flux peaked simultaneously with benthic biomass in June.
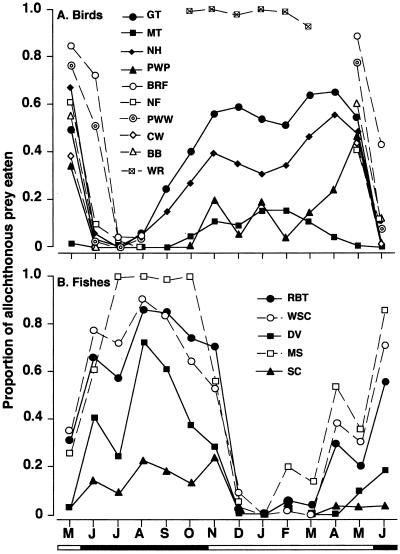
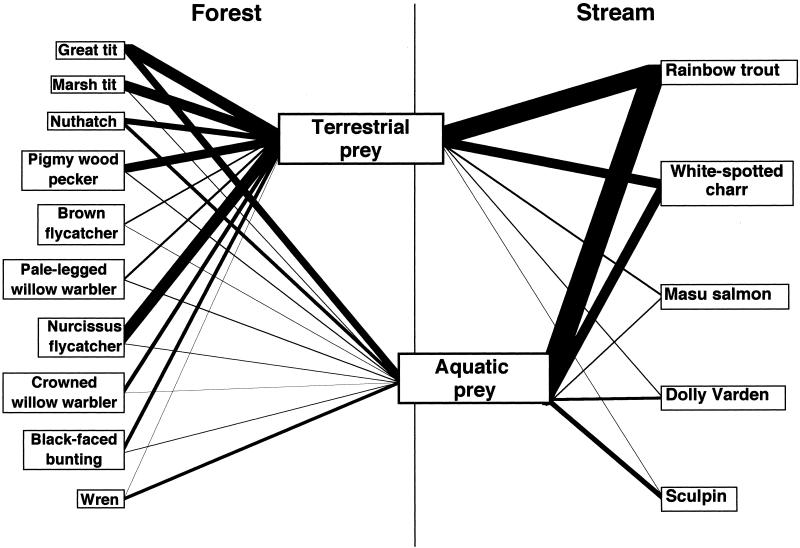
The aquatic prey provided significant seasonal subsidies to forest birds during the defoliation period, accounting for 50–90% of the monthly energy budget in half of the species (Fig. 2A). In both resident great tit and nuthatch, intake rates of aquatic prey were much higher during the defoliation period than during the leafing periods. During winter, both species foraged intensively on emerged aquatic prey while searching along the stream banks. However, aquatic prey accounted for only a small portion of the resource in other resident birds, such as marsh tit and pigmy woodpecker. The seasonal change in aquatic prey contribution was negatively related to terrestrial prey biomass for great tit, marsh tit, and nuthatch, but not for pigmy woodpecker (Table 1). The contribution of aquatic prey to the annual, total resource budget was estimated as 38.6% for great tit, 31.9% for nuthatch, 7.4% for marsh tit, and 9.5% for pigmy woodpecker (Fig. 3).
Figure 2.
Contrast in seasonal dynamics of allochthonous prey contribution to forest birds and stream fishes. (A) Proportion by frequency of aquatic prey foraging to the total observations in each forest bird species. The contribution of aquatic prey differed significantly both among months and species in both year-round and summer-resident birds (P < 0.01 for all by Friedman test). GT, great tit; MT, marsh tit; NH, nuthatch; PWP, pigmy woodpecker; BRF, brown flycatcher; NF, narcissus flycatcher; PWW, pale-legged willow warbler; CW, crowned willow warbler; BB, black-faced bunting; WR, wren. Black and white horizontal bars at the bottom of the figure indicate observation periods for winter and summer birds, respectively. (B) Proportion by dry mass of terrestrial prey to the total diets in stream fish species. The proportion differed significantly among both seasons and species (P < 0.0001 for both by Friedman test). RBT, rainbow trout; WSC, white-spotted char; DV, Dolly Varden; MS, masu salmon; SC, freshwater sculpin. Black and white portions of horizontal bars at bottom of figure indicate leafing and defoliation periods.
Table 1.
Results of stepwise multiple linear regressions of monthly variations in allochthonous prey contribution to the diets of forest bird and stream fish species on allochthonous prey flux and in situ prey biomass
| Species | Source | Standardized coefficient | R2 |
|---|---|---|---|
| Bird | |||
| Great tit | Terrestrial prey biomass | −0.74* | 0.55* |
| Marsh tit | Terrestrial prey biomass | −0.84* | 0.70* |
| Nuthatch | Terrestrial prey biomass | −0.59* | 0.35* |
| Pigmy woodpecker | Terrestrial prey biomass | — | <0.01 |
| Fish | |||
| Rainbow trout | Terrestrial prey flux | 0.86* | 0.89* |
| Aquatic prey biomass | −0.20 | ||
| White-spotted char | Terrestrial prey flux | 0.95* | 0.90* |
| Dolly Varden | Terrestrial prey flux | 0.85* | 0.72* |
| Masu salmon | Terrestrial prey flux | 0.83* | 0.69* |
| Freshwater sculpin | Terrestrial prey flux | 0.79* | 0.85* |
| Aquatic prey biomass | −0.30* |
*, Significant, P < 0.05.
Figure 3.
Food web linkage across a forest–stream interface representing predator subsidies by allochthonous, invertebrate prey flux. Relative contributions of terrestrial and aquatic prey to the annual total resource budget of each species were represented by line thickness. Annual resource budget of each species was represented as a value proportional to the total assemblage-based budgets, separately for the bird and fish assemblages.
Even within the short residence of summer birds, drastic changes were found in their aquatic prey use, with significant variation among the species (Fig. 2A). In all of the species, the contribution decreased dramatically with bud break, and remained continually low until the birds departed in August. A higher aquatic prey contribution was observed again during the periods characterized by smaller terrestrial prey biomass, but not with greater aquatic prey flux. The total contribution of aquatic prey was estimated as 31.8% in brown flycatcher, 35.0% in pale-legged willow warbler, 15.5% in narcissus flycatcher, 9.4% in crowned willow warbler, and 20.5% in black-faced bunting, during their four-month stay (Fig. 3). The winter wren was largely dependent on aquatic prey, which contributed 98.2% of the six-month resource budget. This species was frequently observed to catch the aquatic prey from the stream channel as well as on the banks. Pooling of data for all of the bird species showed aquatic prey as accounting for 25.6% of the annual total energy demand of the entire bird assemblage (402,607 kj per 10 ha y−1).
Similarly, allochthonous fluxes of terrestrial insect prey also subsidized stream fishes, the flux contributing greatly (60–100%) to the food resource, especially, of the three water-column-dwelling fishes during the leafing periods (Fig. 2B). For all of the fishes, the proportion of terrestrial prey in the diets during leafing was much greater than that during defoliation, although it varied among the species. Terrestrial prey input was a better predictor of seasonal change in terrestrial prey contribution to fish diet than aquatic prey biomass (Table 1). In all of the fish species, the greater the terrestrial prey flux, the greater its contribution, with minor influences of aquatic prey biomass in rainbow trout and sculpin. The contribution of terrestrial prey to an annual resource budget was estimated as 46.3% in rainbow trout, 50.5% in white-spotted char, 22.6% in Dolly Varden, 56.7% in masu salmon, and 12.0% in sculpin (Fig. 3). When all of the species were pooled, terrestrial prey input was estimated as contributing to 44.0% of the annual, total resource budget of the stream fish assemblage (531,421 kj per 4,200 m2⋅y−1).
Both forest birds and stream fishes were reciprocally subsidized by invertebrate prey flux across the habitat interface. Such allochthonous prey fluxes could be a critical factor in maintaining both the forest bird and stream fish assemblages simply because they greatly augmented the resource budgets of the assemblages annually as well as seasonally. The most important element of these reciprocal subsidies, however, was the seasonal asynchrony in the dynamics of in situ prey biomass and allochthonous prey supply. The stream supplied great energy transfers to the riparian forest, primarily when terrestrial prey biomass was relatively low. Because the birds exploited aquatic prey intensively when the terrestrial resource was scarce, seasonal asynchrony could be a principal factor controlling the subsidy efficiency of aquatic prey flux. Although great terrestrial prey inputs occurred when the aquatic resource was scarce, terrestrial prey contribution to fish diets was more closely linked with their flux per se than with the in situ resource level. This may indicate greater availability of terrestrial prey than aquatic prey (12).
The reciprocal prey flux across a forest–stream interface could have indirect but significant influences on food web dynamics by altering the top-down effects of subsidized predators in both habitats, as pointed out by both recent theoretical (4) and experimental studies (11, 26). The importance of understanding such ecological functions produced by habitat linkages is growing because human interference is rapidly altering the spatial patterns of habitat interfaces on a broad scale. Well developed overhanging vegetation in a riparian forest, for instance, can enhance the input of terrestrial invertebrates (27). Furthermore, meandering and/or braided stream channels can be expected to increase the supply of emerging aquatic insects per unit area of forest. However, increasing destruction of stream banks by various human activities, e.g., riparian deforestation, channelization, or road construction, can seriously impair reciprocal energy exchange. The importance of reciprocal resource subsidies between habitats indicates that the loss or degradation of one habitat may have more detrimental effects on neighboring communities than we have previously recognized.
Acknowledgments
We thank M. Higashi, M. Power, G. Takimoto, and T. Iwata for comments, and Y. Kawaguchi, H. Mitsuhashi, K. Motomori, H. Urabe, H. Asano, and K. Ono for logistical support. Financial support was provided by the Japanese Ministry of Education, Science, Sports, and Culture (09NP1501 and 11440224).
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
See commentary on page 14.
References
- 1.Polis G A, Holt R D, Menge B A, Winemiller K O. In: Food Webs. Polis G A, Winemiller K O, editors. New York: Chapman & Hall; 1996. pp. 435–460. [Google Scholar]
- 2.Polis G A, Anderson W B, Holt R D. Annu Rev Ecol Syst. 1997;28:289–316. [Google Scholar]
- 3.DeAngelis D L. Ecology. 1980;61:764–771. [Google Scholar]
- 4.Huxel G R, McCann K. Am Nat. 1998;152:460–469. doi: 10.1086/286182. [DOI] [PubMed] [Google Scholar]
- 5.Fagan W F, Cantrell R S, Cosner C. Am Nat. 1999;153:165–182. doi: 10.1086/303162. [DOI] [PubMed] [Google Scholar]
- 6.Vannote R L, Minshall G W, Cummins K W. Can J Fish Aquat Sci. 1980;37:130–137. [Google Scholar]
- 7.Naiman R J, Décamps H. Annu Rev Ecol Syst. 1997;28:621–658. [Google Scholar]
- 8.Cloe W W, Garman G C. Freshwater Biol. 1995;36:105–114. [Google Scholar]
- 9.Wipfli M S. Can J Fish Aquat Sci. 1997;54:1259–1269. [Google Scholar]
- 10.Nakano S, Fausch K D, Kitano S. J Anim Ecol. 1999;68:1079–1092. [Google Scholar]
- 11.Nakano S, Miyasaka H, Kuhara N. Ecology. 1999;80:2435–2441. [Google Scholar]
- 12.Nakano S, Kawaguchi Y, Taniguchi Y, Miyasaka H, Shibata Y, Urabe H, Kuhara N. Ecol Res. 1999;14:351–360. [Google Scholar]
- 13.Knopf F L, Samson F B. Conserv Biol. 1994;8:669–676. [Google Scholar]
- 14.McGarigal K, McComb W C. J Wildl Manage. 1992;56:10–23. [Google Scholar]
- 15.Jackson J K, Fisher S G. Ecology. 1986;67:629–638. [Google Scholar]
- 16.Gray L J. Am Midl Nat. 1992;129:288–300. [Google Scholar]
- 17.Power M E. In: Linking species and ecosystems. Jones C G, Lawton J H, editors. New York: Chapman & Hall; 1995. pp. 52–60. [Google Scholar]
- 18.Sumner W T, Fisher S G. Freshwater Biol. 1979;9:205–212. [Google Scholar]
- 19.Murakami M. Ecol Res. 1998;13:73–82. [Google Scholar]
- 20.Miyasaka H, Nakano S. Oecologia. 1999;118:99–106. doi: 10.1007/s004420050707. [DOI] [PubMed] [Google Scholar]
- 21.Urabe H, Nakano S. Ecol Res. 1999;14:341–349. [Google Scholar]
- 22.Kendeigh S C. Ecol Monogr. 1944;14:67–106. [Google Scholar]
- 23.Walsberg K C. Avian Biol. 1983;7:161–220. [Google Scholar]
- 24.Kawaguchi Y. Ph.D. thesis. Japan: Niigata University; 2000. [Google Scholar]
- 25.Cummins K W, Wuycheck J C. Mitt Int Ver Theor Angew Limnol. 1971;18:1–158. [Google Scholar]
- 26.Murakami M, Nakano S. Proc R Soc Lond Ser B. 2000;267:1597–1601. doi: 10.1098/rspb.2000.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edward E D, Huryn A D. Hydrobiologia. 1996;337:151–159. [Google Scholar]