Abstract
Mutations affecting ion channels and neuronal membrane excitability have been identified in Drosophila as well as in other organisms and characterized for their acute effects on behavior and neuronal function. However, the long-term effect of these perturbations on the maintenance of neuronal viability has not been studied in detail. Here we perform an initial survey of mutations affecting Na+ channels and K+ channels in Drosophila to investigate their effects on life span and neuronal viability as a function of age. We find that mutations that decrease membrane excitability as well as those that increase excitability can trigger neurodegeneration to varying degrees. Results of double-mutant interactions with dominant Na+/K+ ATPase mutations, which themselves cause severe neurodegeneration, suggest that excitotoxicity owing to hyperexcitability is insufficient to explain the resultant phenotype. Although the exact mechanisms remain unclear, our results suggest that there is an important link between maintenance of proper neuronal signaling and maintenance of long-term neuronal viability. Disruption of these signaling mechanisms in any of a variety of ways increases the incidence of neurodegeneration.
GENETIC factors have been implicated in virtually every neurological disease, including degenerative disorders such as Alzheimer's, Huntington's, and Parkinson's diseases. Although several genes have been linked to neurodegenerative diseases, including those encoding α-synuclein, huntingtin, tau, APP, and parkin, the molecular mechanisms mediating neuronal loss remain largely unknown (Bonini and Fortini 2003; Driscoll and Gerstbrein 2003; Shulman et al. 2003). To elucidate the underlying molecular pathways, Drosophila has been used successfully to model various neurodegenerative conditions, including tauopathy, polyglutamine repeat diseases, Parkinson's disease, and Alzheimer's disease (reviewed by Bonini and Fortini 2003). In complementary studies, forward genetic approaches have been used to identify genes required for age-dependent maintenance of neuronal viability (Buchanan and Benzer 1993; Kretzschmar et al. 1997; Min and Benzer 1997, 1999; Palladino et al. 2002; Troulinaki and Tavernarakis 2005).
In similar screens, we found that mutations in ATPα, the gene encoding the α-subunit of the Na+/K+ ATPase, which cause stress-sensitive seizures, temperature-sensitive paralysis, and reduced life span, also cause pronounced neurodegeneration in the central nervous system (Schubiger et al. 1994; Palladino et al. 2002, 2003). Mutations of the ATPase β2 subunit in mice also cause neurodegeneration (Magyar et al. 1994; Molthagen et al. 1996). Pharmacological disruption of the Na+/K+ ATPase has been shown to cause membrane depolarization resulting in increased intracellular calcium, ultimately leading to necrosis (Chatterjee and Roy 1965; Lees et al. 1990). In addition, depletion of intracellular K+ levels, due to pharmacological impairment of pump activity, has been linked to apoptosis (Beauvais et al. 1995; Bortner et al. 1997; Yu et al. 1997). These results suggest that perturbations in membrane excitability and ion homeostasis may be important triggers for neuronal death. To investigate the significance of altered membrane excitability and ion homeostasis on neuronal viability, we utilized the existing set of mutations in Drosophila affecting voltage-gated Na+ and K+ channels alone and in combination with Na+/K+ ATPase mutations to examine their effect on life span and neuronal maintenance.
We found that many ion channel mutations are associated with an elevated occurrence of neurodegeneration. Mutations affecting eag or Sh K+ channels alone or in double-mutant combinations result in neuronal hyperexcitability but do not trigger striking neurodegeneration even though the adult life span of the double mutant is much reduced. In contrast, mutations in the sei K+ channel gene exhibit normal life spans but manifest significant neurodegeneration in the central nervous system (CNS). Mutations in para, which encodes a voltage-activated Na+ channel, decrease excitability but confer neurodegeneration, indicating that decreased neuronal activity can also impair neuronal viability. We examined double mutants of ATPα in combination with various ion channel mutations to test whether the severe neurodegeneration seen in ATPα mutants resulted from hyperexcitability-induced excitoxicity. The results of the genetic interactions that we observed suggest that mechanisms other than or in addition to excitotoxicity underlie neurodegeneration in ATPα mutants. Our results focus attention on the existence of an important link between maintenance of proper neuronal signaling and maintenance of neural viability. Molecular and physiological characterization of the relevant mechanisms should have important implications for understanding a broad array of disorders.
MATERIALS AND METHODS
Fly strains:
Fly stocks were cultured on cornmeal–molasses agar media at 22°–29°. ATPα alleles DTS1, DTS2, 2206, and DTS1R1 were used and are described elsewhere (Schubiger et al. 1994; Palladino et al. 2003). Other strains used in this study include parats1, parats115, parast109, paralk5, parald34, mlenapts (napts1), Sh133, eag1, seits1, seits2, elav3A, EKO E323, and HS-GAL4. Controls for genetic modifiers of excitability were performed on Sh133 in several genetic backgrounds, including w1118 and CS, with all lines exhibiting similar and strong leg shaking upon exposure to ether. Unless otherwise stated, wild type and control refer to Canton-S, which is the original background for many of these mutants.
ATPα immunohistochemistry:
Goat monoclonal antibody (mAB) against the chicken Na+/K+ ATPase α (a5) was obtained from the Iowa hybridoma bank. Immunohistochemistry was performed on dissected third instar larvae to visualize Na+/K+ ATPase α localization in the motor axon bundles using standard procedures (Bellen and Budnik 2000). The a5 antibody was used (1:100) in PBTX on 4% paraformaldehyde fixed specimens. Dissected animals were uniquely tagged and processed together in the same microcentrifuge tube. Anti-goat fluorescein conjugate was used 1:500 as a secondary antibody. Identical imaging parameters were used to visualize fluorescein localization on a Zeiss confocal microscope. ImageJ was used to compare the fluorescence intensity in motor axon bundles.
Life-span analysis:
Life spans were measured at 29° according to standard protocols (Kretzschmar et al. 1997; Lin et al. 1998; Min and Benzer 1999; Kapahi et al. 2004) as previously described (Palladino et al. 2002, 2003). In brief, newly eclosed animals were separated by sex, placed in vials (<21/vial), and transferred daily to fresh vials, and the number of surviving flies in each vial was recorded. Animals that were lost or removed for analysis were subtracted from the total population in calculations. The age in days at which only 50% of the original population still survived (50% survivorship) was compared among different genotypes using the Mann-Whitney test.
Histology:
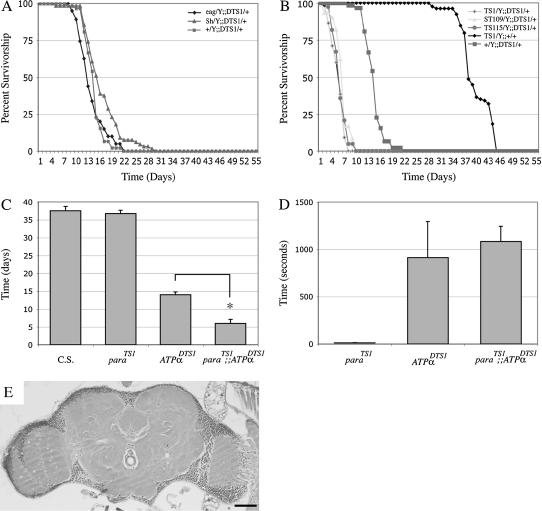
Histological analyses were performed as previously described (Palladino et al. 2002, 2003). Briefly, heads and bodies from adult flies were dissected and placed in freshly prepared Carnoy's fixative at room temperature for 1–2 days and then washed with 70% ethanol and processed into paraffin. Heads were embedded to obtain frontal sections. Serial 4-μm sections were stained with hematoxylin and eosin and examined under a light microscope (n > 10 for each genotype). The degree of neuropathology present for each genotype was determined by the examination of the frequency of vacuolar pathology in serial sections, according to the following rating scale. A score of 0 was assigned to brains exhibiting no gross neuropathology or a single small vacuolar structure (<12 μm in diameter), similar to aged wild type. Brain tissue presenting with small sporadic individual vacuolar structures (<12 μm in diameter) in multiple sections of a brain was given a score of 1. More frequent small individual vacuolar structures (<12 μm in diameter) appearing in most sections of each brain was scored as a 2. Heads exhibiting widespread small vacuolar pathology (<15 μm in diameter) affecting the majority of sections or large vacuolar or clustering vacuolar structures (15–20 μm in diameter) were assigned a value of 3. Brain tissue with numerous (>100) vacuolar structures 10–20 μm in diameter in individual sections and/or clustering vacuolar structures affecting an area >500 μm2 were given a score of 4. A score of 5 indicates that a large proportion of brain tissue is missing (>40%).
RESULTS
Neuropathology in K+ channel mutants:
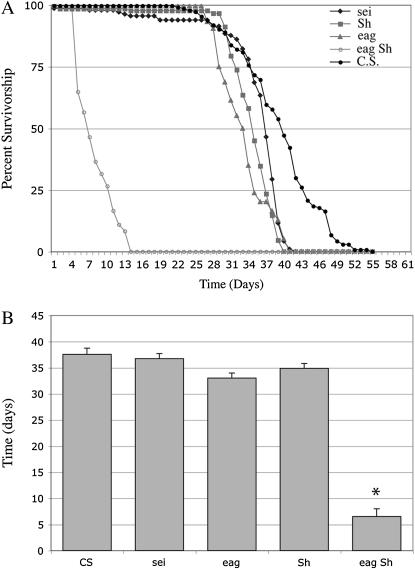
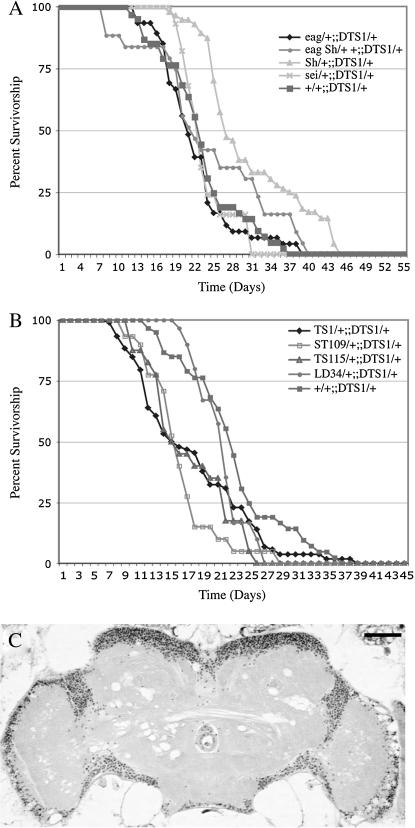
Mutations in a number of genes encoding various K+ channel subunits have been isolated in Drosophila. In general, loss of K+ channel function leads to neuronal hyperexcitability (Ganetzky and Wu 1986; Noebels 2003). Repeated or even single seizures have been reported to produce neuronal damage in mammals (Sutula et al. 2003). Thus, it was of interest to determine whether K+ channel mutants exhibit enhanced susceptibility to neurodegeneration. For these studies we examined mutant alleles of eag, Sh, and sei, which have been well characterized behaviorally and electrophysiologically. eag and Sh cause leg shaking under ether anesthesia (Kaplan and Trout 1969; Jan et al. 1977; Wu et al. 1983), whereas sei causes convulsive seizures when the mutants are placed at 37°–40° (Jackson et al. 1984). eag and Sh interact genetically in double mutants to produce flies that shake and have severe motor defects under all conditions owing to extreme hyperexcitability (Ganetzky and Wu 1983). As severe neurodegeneration is often associated with a strongly reduced life span (Buchanan and Benzer 1993; Kretzschmar et al. 1997; Palladino et al. 2002), we first determined the life span of eag, Sh, and sei mutants as well as eag Sh double mutants (Figure 1, Table 1). Only eag Sh double mutants, which become severely uncoordinated with age, consistent with previous observations (Ganetzky and Wu 1983), showed significantly shortened life span (7 days ± 2.1, P < 0.001).
Figure 1.
Life spans of individual mutant K+ channel strains are normal. Survival was determined daily for populations of various genotypes (5–10 vials/genotype). (A) Life spans for sei (diamonds), Sh (squares), and eag (triangles) K+ channel mutant strains. (B) No significant alteration in median age (T50% survivorship) was observed for K+ channel mutants individually. eag Sh double mutants are severely uncoordinated and have significantly reduced life spans (open circles; *P < 0.001). Wild-type control strain is Canton-S (CS; solid circles). Each point represents mean survival ±SEM.
TABLE 1.
Viability and neuropathology of various ion channel mutants
| 50% ± SD | Pathology | 50% ± SD | Pathology | |
|---|---|---|---|---|
| Genotype | +/+ | +/+ | DTS1/+ | DTS1/+ |
| +/Y | 38 ± 4.4 | 0/1 | 14 ± 0.8 | 4 |
| eag/Y | 33 ± 1.7 | 1 | 14 ± 2.1 | 3/4 |
| Sh/Y | 35 ± 2.6 | 1 | 16 ± 3.4 | 3 |
| eag Sh/Y | 7 ± 2.1 | 0 | 7 ± 0.7 | NA |
| seits1/seits1 | 37 ± 0.5 | 2 | NA | NA |
| napts1/napts1 | 27 ± 1.9 | 1 | NA | NA |
| parats1/Y | 37 ± 1.5 | 1/2 | 6 ± 1.1 | 0/1 |
| +/+ | 38 ± 4.4 | 0/1 | 23 ± 3.4 | 4 |
| eag/+ | 47 ± 0.7 | NA | 21 ± 1.4 | NA |
| Sh/+ | 39 ± 16.2 | NA | 31 ± 6.1 | NA |
| eag Sh/+ | 36 ± 4.2 | NA | 24 ± 3.5 | 3/4 |
| seits1/+ | 39 ± 2.4 | NA | 23 ± 2.1 | 4 |
| parats1/+ | 48 ± 3.3 | 2 | 16 ± 5.3 | 4 |
Viability is assessed as median age (50%). Error is standard deviation (SD). Genotype in column heading represents the third chromosome. DTS1 indicates ATPαDTS1. Neuropathology was scored 0 (none) to 5 (marked).
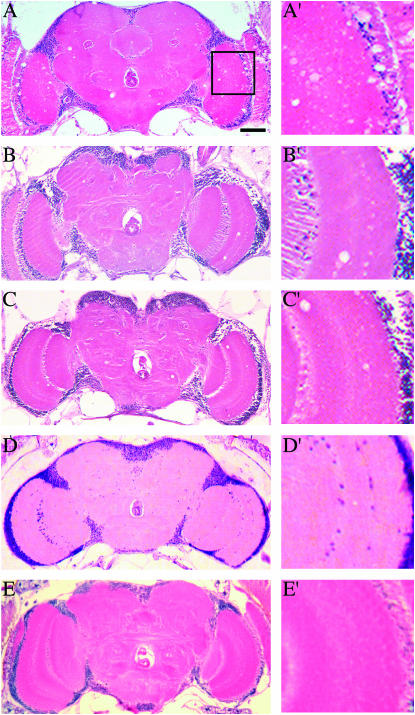
Histological analysis of the CNS at 50% survivorship for each mutant strain revealed mild neurodegeneration in these mutants (Table 1). Vacuolar pathology in the central brain as well as in the optic lobes was detected to varying degrees in each of these mutants but was rarely seen in age-matched controls (Figure 2). Both sei alleles examined, seits1 (Figure 2A) and seits2 (data not shown), display substantial pathology, whereas eag Sh double mutants exhibited no significant neurodegeneration at 50% survivorship (7 days; Figure 2D).
Figure 2.
Neurodegeneration in K+ channel mutant animals. Frontal sections of mutants aged to median life span are shown. (A) At day 37 seits1 mutants exhibit significant pathology presented as sporadic vacuoles throughout the central nervous system. The region designated by the square is enlarged at the right as A′–E′. Micrographs showing intermittent brain pathology in eag (B) and Sh (C) mutants at 33 and 35 days, respectively. (D) No gross pathology was ever observed in eag Sh double mutants, which die within 1 week. (E) Aged CS animals display little or no pathology. Bar, 50 μm.
To determine if repeated seizures exacerbate the pathology observed in sei mutants, seizures were evoked daily by exposure to 40° for 3 min. For all sei alleles tested, this treatment results in seizures that persist for up to a minute before the mutants become completely paralyzed. Wild-type flies are unaffected by this treatment. The seizure phenotype of both seits1 and seits2 progresses with age and becomes more severe with daily exposure to the restrictive temperature. However, wild-type control animals began to exhibit some locomotor impairment after ∼25 days of daily exposure to 40° that included temperature-dependent incoordination and inactivity with occasional partial paralysis. Daily induction of seizures did not result in significant changes in recovery time, seizure behavior, or life span when compared with controls. Although we observed no simple correlation with the frequency of seizure activity, our results show that loss or impairment of K+ channel function can result in elevated levels of neurodegeneration.
Shortened life span and neurodegeneration in Na+ channel mutants:
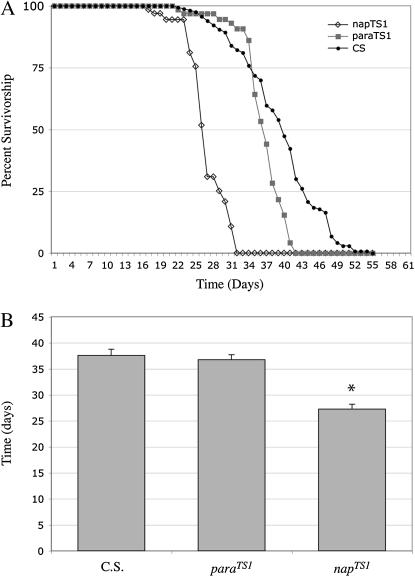
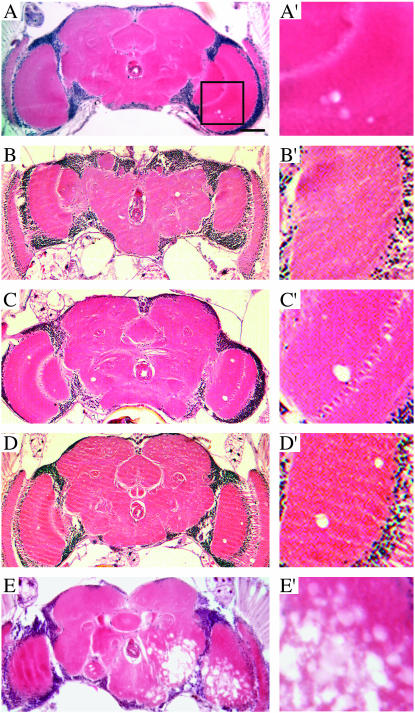
To examine the effects of decreased electrical activity on neuronal viability, we asked whether mutations that reduce Na+ channel activity or expression decrease life span and cause increased neurodegeneration. para and mlenapts mutations disrupt voltage-dependent Na+ channel function and result in reduced neuronal activity (Suzuki et al. 1971; Siddiqi and Benzer 1976; Wu et al. 1978; Wu and Ganetzky 1980; Loughney et al. 1989; Budnik et al. 1990; Kernan et al. 1991; Reenan et al. 2000). No significant changes in median life span were observed in the para mutants examined (Figure 3, Table 1; parats1 T50% = 37 days, parats1/ts115 T50% = 46 days, parast109/ld34 T50% = 39 days) (see also Palladino et al. 2002). However, the mlenapts strain exhibits significantly reduced life span (Figure 3; T50% = 27 days, P < 0.001) as well as mild neuropathology with multiple regions of vacuolization present in most brain sections (Figure 4A). Varying degrees of neurodegeneration were observed in the allelic series of para mutants (Figure 4). The pathology was similar to that observed in K+ channel mutants with the appearance of sporadic vacuolization in the neuropil. However, in comparison with K+ channel mutants, neurodegeneration in para mutants exhibited greater penetrance and a more severe manifestation with a higher density of vacuolization in each brain section (Figure 4, B–D). Neurodegeneration in para mutants was scored as in Table 1 (histo-pathology score: parats1 = 1/2, parast109/ld34 = 2, and parats1/ts115 = 1/3). The degree of neurodegeneration, measured at the midpoint of their respective survival curves, appears to increase with the life span of each para allele, suggesting that the mechanism underlying this pathology has a critical component that depends on absolute time. These results show that Na+ channel mutations known to reduce neuronal excitability are associated with age-dependent neurodegeneration, which is even more severe than that associated with the K+ channel mutants examined. Thus, impaired neuronal excitability as well as increased neuronal excitability can trigger neurodegeneration.
Figure 3.
mlenapts mutant animals exhibit shortened life spans. (A) Survivorship values for mlenapts (open diamonds) and parats1 (solid squares) are plotted with Canton-S as wild-type control (solid circles). (B) No significant change in life span was observed in any para mutant strain examined, including parats1 shown here (Palladino et al. 2002). A significant reduction in life span was found in the mlenapts strain (T50% = 27 days, P < 0.001). Each point represents mean survival ±SEM.
Figure 4.
Na+ channel mutants exhibit age-dependent neuropathology. (A) mlenapts animals at 50% survivorship display mild neurodegeneration. Varying degrees of pathology are observed in para alleles, ranging from single sporadic vacuoles in neighboring brain sections to numerous holes per section. The region designated by the square is enlarged at the right as A′–E′. Sections from parats1 (B), parats1/parats115 (C), and parast109/Df(1)LD34 (D) heads are shown, which were collected at the median age for each genotype (∼38 days). (E) An uncommon brain from a parats1 animal with sporadic massive neurodegeneration. Bar, 50 μm.
Ion channel mutations can exacerbate early adult lethality in ATPα mutants:
We previously found that mutations in the Na+/K+ ATPase result in severe neurodegeneration and early adult lethality (Palladino et al. 2003). Electrophysiological studies showed that these mutations also cause neuronal hyperexcitability perhaps by destabilizing the membrane resting potential. These results raise the possibility that an excitotoxic mechanism is responsible for neurodegeneration in these mutants. However, the precise mechanism leading to loss of neuronal tissue in these mutants remains unclear because integrity of septate junctions might also be disrupted (Genova and Fehon 2003; Paul et al. 2003). Metabolic processes could also be impaired in ATPα mutants through increased ATPase activity (Palladino et al. 2003). Therefore, we tested whether altered neuronal excitability was the primary cause of neurodegeneration in ATPα mutants by examining their phenotypic interactions in combination with ion channel mutations. Thus, if hyperexcitability-associated excitoxicity were a key factor in the mechanism of neurodegeneration and life-span reduction in ATPα mutants, we would expect that K+ channel mutations would further enhance these phenotypes. However, as measured by life-span duration, none of the K+ channel mutants examined increased phenotypic severity in double-mutant combinations with ATPα compared with the single mutants (Figure 5A, Table 1). Furthermore, even in eag Sh; ATPαDTS1 triple mutants there was no significant reduction in life span compared with eag Sh controls.
Figure 5.
para mutants exhibit shortened life spans in the presence of dominant ATPα mutations. Analysis of the presence of ion channel mutations on ATPα mutant life spans was performed. Introduction of ion channel mutations resulted in significant interactions only with ATPα dominant mutations. (A) No genetic interactions were observed with K+ channels based on life-span analysis. The presence of eag and/or Sh did not significantly alter any ATPα mutant life spans. (B) Combination of para mutations with dominant ATPα alleles resulted in a striking reduction of survivorship. parats1/Y;;ATPαDTS1/+ (diamonds), parast109/Y;;ATPαDTS1/+ (triangles), and parats115/Y;;ATPαDTS1/+ (circles) animals die in ∼1 week, while +/+;;ATPαDTS1/+ (solid squares) and parats1/Y;CyO/+ (solid diamonds) controls have a T50% of 14 and 38 days, respectively. Each point represents mean survival ±SEM. (C) Median age of survivorship for parats1 and ATPαDTS1 mutant animals. (D) Mean time to recovery after 3 min at 38°. parats mutants display immediate recovery from paralysis upon return to permissive temperature while both ATPαDTS1 and parats1/Y;;ATPαDTS1/+ mutant animals require at least several minutes (P < 0.001). (E) A representative section from a parats1/Y;;ATPαDTS1/+ mutant animal, which exhibits mild to no neurodegeneration. Bar, 50 μm.
Conversely, we generated double-mutant combinations between para and ATPα alleles to test if a reduction in excitatory channels could suppress the neuropathological phenotypes of ATPα mutants. Surprisingly, ATPαDTS1 and ATPαDTS2 exhibited a striking reduction in life span in double-mutant combinations with various para alleles (Figure 5B, Table 1). We did not observe the same effect with the recessive ATPα2206 and ATPαDTS1R1 alleles in combination with para mutations (data not shown). Thus, the dominant ATPα alleles appear to be more severe than ATPα null alleles when heterozygous, suggesting a dominant negative mode of action. Although parats1, parats115, and parast109 all display essentially normal life spans individually, the median life span drops to <1 week in combination with ATPαDTS1 (Figure 5B; P < 0.001 for each) or ATPαDTS2 (data not shown). Despite the greatly reduced life span of parats/Y; ATPαDTS1/+ mutants, we observed only minimal neuropathology at 50% survivorship (Figure 5C; Table 1), similar to age-matched ATPαDTS1/+ controls. This finding suggests that overt neurodegeneration is not the cause of this unexpectedly early death, which may instead reflect some other type of severe neural impairment in the double mutants.
Behavioral phenotypes of the double mutants further indicated that the mechanism of paralysis in ATPα mutants also involves a component that does not appear to depend on neuronal activity. parats1; ATPαDTS adults become instantly paralyzed upon exposure to 38°, as do parats1 mutants alone, consistent with the block of Na+-mediated action potentials (Suzuki et al. 1971; Wu and Ganetzky 1980; Loughney et al. 1989; O'Dowd et al. 1989). Upon being returned to 22° following a 3-min exposure to 38°, parats1 mutants recovery instantly. In contrast, parats1; ATPαDTS adults do not recover instantly but require several minutes to regain normal activity, similar to ATPαDTS mutants alone (Figure 5D). Thus, the mechanisms mediating temperature-sensitive seizures and paralysis in ATPαDTS mutants appear to be epistatic to the rapid paralysis of parats mutants. Because neuronal activity is presumably blocked in parats mutants at elevated temperature, the slow recovery of double mutants suggests that the temperature-sensitive mechanism of ATPαDTS is not strictly dependent on neuronal electrical activity, which, for example, could contribute to further disruption of ionic gradients when Na+/K+ ATPase activity is impaired. The difference in para and ATPαDTS temperature-sensitive mechanisms becomes more revealing upon prolonged exposure to 38°. Following 15 min of paralysis at 38°, parats animals quickly recover upon returning to 22°. However, when ATPαDTS mutants are exposed to 38° for the same length of time >60% never recover and survivors require >1 hr before exhibiting any movement. Thus, it appears that at elevated temperatures parats mutants simply cease neuronal activity while ATPαDTS mutants cease neuronal activity and initiate a toxic mechanism, possibly disruption of ion homeostasis.
To test if these interactions are simply due to the summation of less viable phenotypes, we also examined various ATPα mutants with heterozygosity for several of these recessive ion channel mutations. Animals heterozygous for various channel mutations also revealed significant interactions with ATPα mutants (Figure 6; Table 1). ATPαDTS1 mutant females heterozygous for K+ channel mutations did not show a difference in adult viability from single-mutant control animals (Figure 6A), consistent with the results that we obtained from our analysis of double mutants where the K+ channel mutations were hemizygous (Figure 5A). Analysis of heterozygosity for para alleles in combination with ATPα mutations gave different results, depending on the particular allele. parats1, parats115, and parast109 all exhibit shorter life spans when heterozygous in an ATPαDTS genetic background (Figure 6B). Surprisingly, heterozygosity for the null allele, paralk5, or the deficiency Df(1)lD34 do not shorten either the ATPαDTS1 or the ATPαDTS2 life span. The differences in these para heterozygotes may result from modifiers in these genetic backgrounds. Histological analyses of parats1/+; ATPαDTS1/+ animals show an increase in the severity of spongiform neuropathology compared with ATPαDTS1/+ age-matched controls (Figure 6C). Behavioral testing of double-mutant animals revealed no significant changes in temperature sensitivity (data not shown). Thus, although their behavioral phenotypes are somewhat distinct, a link between the mechanisms underlying para and ATPα mutant pathologies seems to exist.
Figure 6.
Animals heterozygous for ion channel mutations interaction with ATPαDTS1. (A) Heterozygous sei/+, eag/+, Sh/+, and eag Sh/+ + do not show any interaction with ATPα mutants. (B) Heterozygous para allele combinations ts1/+ (diamonds), st109/+ (open squares), and ts115/+ (triangles) with ATPαDTS1/+ show a significant reduction in life span (P < 0.05). Surprisingly, the para deficiency Df(1)LD34 (circles) did not have any affect on ATPαDTS1 life span. (C) parats1/+;;ATPαDTS1/+ brain slice at day 16 shows more severe pathology than age-matched parats1 or ATPαDTS1 alone. Each point represents mean survival ±SEM. Bar, 50 μm.
Normal expression and localization of sodium pump in dominant ATPα mutants:
Na+/K+ ATPases have a fundamental cellular role in establishing and regulating ionic gradients, which is especially important in neurons. Although defects in ion homeostasis could alter neural activity and lead to intracellular concentrations of ions that are toxic (e.g., Na+, K+, and, secondarily, Ca2+), protein trafficking defects that disrupt the endoplasmic reticulum (ER) secretory pathway and other cellular functions could also result in the observed neurodegeneration. Furthermore, a mutation in a vertebrate Na+/K+ ATPase that is similar to the dominant ATPα mutations in Drosophila has been shown to cause defects in processing of the mutant protein, resulting in reduced expression overall (Arguello et al. 1999).
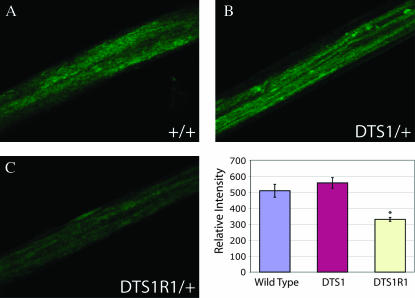
If the ATPαDTS mutations act in a dominant negative manner affecting the secretion of the tetramer, there could be a significant reduction in Na+/K+ ATPase trafficking to the membrane. To test if protein expression is reduced through aberrant trafficking or maturation, we performed immunohistochemical analysis. We saw no significant intracellular Na+/K+ ATPase α immunoreactivity and no colocalization with PDI∷GFP (an ER marker; Bobinnec et al. 2003) in vivo (data not shown). It is possible that the ATPαDTS mutations do cause inefficient secretion of the protein but the proteins are rapidly degraded or their intracellular immunoreactivity is poor. To test this possibility, we quantified ATPα protein localized to nerve bundles in ATPαDTS1 and wild-type animals. There was no reduction of ATPα immunoreactivity in motor neuron bundles in ATPαDTS1 compared with wild-type animals (Figure 7). To assess the sensitivity of our assay, we also examined ATPαDTS1R1 animals (heterozygous null mutation) for ATPα protein expression in nerve bundles. These mutants did show a significant reduction of ATPα protein (∼35%, P < 0.001), demonstrating that our assay is sufficiently sensitive to detect changes and the basis for ATPα haplo-insufficiency phenotypes. These data argue strongly against a simple protein-trafficking defect in dominant ATPα mutants and suggest that the protein dysfunction is at the membrane and likely compromises ion homeostasis.
Figure 7.
ATPα immunolocalization in axon bundles. Immunohistochemistry with anti-Na+/K+ ATPase α mAB and a fluorescence-conjugated secondary antibody was performed on larvae of the indicated genotypes. Confocal microscopy was performed under identical conditions and image acquisition parameters for all genotypes. Representative images of motor axon bundles are shown for (A) wild type, (B) ATPαDTS1/+, and (C) ATPαDTS1R1/+. ImageJ software was used for intensity analysis. Wild type had an average intensity of 509.11 ± 40.45, ATPαDTS1 had an average intensity of 558.01 ± 33.40, and ATPαDTS1R1 had an average intensity of 330.17 ± 12.28 (error given is SEM). Protein expression was not different between ATPαDTS1 and control flies, whereas ATPαDTS1R1 flies demonstrated a significant 35% reduction in ATPα expression (P < 0.001, indicated by an asterisk). N = 14 motor neurons evaluated from three independent animals.
Early lethality in dominant ATPα mutants may not result from excitotoxicity alone:
Using another method to test whether the neuropathological phenotypes in ATPα mutants are due to hyperexcitability, we asked whether these phenotypes could be suppressed by expressing the inhibitory electrical knockout (EKO) K+ channels (White et al. 2001) in these flies. Expression of the UAS–EKO E323 strain was driven in ATPαDTS1 flies using the second chromosome heat-shock (HS) and third chromosome elav–GAL4 drivers. The HS–GAL4 driver is weakly expressed in all tissues at the constant temperature of 29° and the elav3A line expresses GAL4 at moderate levels throughout the nervous system. Use of neuronal drivers with greater levels of expression were lethal due to severe neuronal silencing (White et al. 2001; data not shown). We observed no significant change in life span of ATPαDTS1 when EKO was expressed under the control of either driver (26 ± 7 days; P = 0.438). An identified mechanism for excitotoxicity in vertebrates leading to neuronal death is prolonged activation of the N-methyl-d-asparate (NMDA) glutamate receptor (Schanne et al. 1979; Siesjo and Bengtsson 1989; Choi and Rothman 1990). MK801 is an antagonist of the NMDA receptor and a strong blocker of excitotoxicity (Foster et al. 1987; Foster and Wong 1987). NMDA receptors have been shown to be expressed in the adult fly brain (Ultsch et al. 1993; Chiang et al. 2002) and MK801 has been demonstrated to block central pattern generators in Drosophila larvae (Cattaert and Birman 2001). We fed various concentrations of the drug in a yeast paste (Cattaert and Birman 2001) to ATPαDTS mutants. None of the concentrations administered (0, 0.1, 0.5, and 1 mg/ml) were successful in significantly extending the life span of ATPαDTS1 mutants (data not shown). Consistent with what we observed using Na+ channel mutations to limit excitability in ATPα mutants, the results of these experiments suggest a distinct mechanism, independent from increased excitability or excitotoxicity, which may be contributing to the early death of ATPαDTS1 mutants.
DISCUSSION
Ion channel mutants exhibit progressive neuron loss:
Although a number of ion channel mutations exist in Drosophila and other organisms that perturb neuronal signaling mechanisms in a variety of ways, there are no systematic studies on how these functional impairments affect neuronal viability. In this study, we examined a collection of Drosophila mutants with altered membrane excitability to determine if they had an increased incidence of neurodegeneration. Mutations in eag, Sh, and sei K+ channels all cause increased neuronal excitability (Ganetzky and Wu 1982a, 1983; Jackson et al. 1984; Elkins and Ganetzky 1990). Although we did not find any striking reduction in adult life span in any of these mutants, we did commonly observe varying degrees of neurodegeneration. Among these mutants, neurodegeneration was most severe in sei. At elevated temperatures, sei mutants display substantially increased and continuous neural activity in the flight motor pathway that parallels their behavioral seizures (Kasbekar et al. 1987; Elkins and Ganetzky 1990). Sh and eag mutations have different effects on this same circuit (Engel and Wu 1992). Conversely, Sh and eag have profound effects on motor neuron activity at the larval neuromuscular junction, whereas sei looks normal (Jan et al. 1977; Ganetzky and Wu 1982b, 1983, 1986). The different neuropathological consequences of these mutants thus may be directly related to the precise ways in which neuronal activity and/or synaptic transmission are perturbed in each mutant. These mechanisms could be strongly dependent on important differences in levels of expression and subcellular localization of the different K+ channel types. Sh5, not used in our studies, was previously reported to be short lived at 25° (Trout and Kaplan 1970), and recent analysis revealed that numerous Sh mutations caused striking reductions in normal sleep and displayed shortened life spans when outcrossed to remove accumulated modifiers (Cirelli et al. 2005). It is possible that unidentified modifiers in the backgrounds of some of the mutants that we have examined reduce the severity of the neurodegenerative phenotypes, although we observed no changes in the life span of outcrossed Sh133 mutants. Another possible complication is that individual neurons express a large number of different K+ channel subtypes, some of which may share substantial functional overlap (Ganetzky and Wu 1983; Sutherland et al. 1999; Misonou and Trimmer 2004). As a consequence, eag Sh double mutants show striking synergistic effects on neuronal hyperexcitability in comparison with either single mutant. In addition, eag Sh double mutants are severely uncoordinated under all conditions and die within a week of eclosion, although they exhibit no detectable neurodegeneration during their shortened life spans. If there is a time-dependent component to the onset and progression of neurodegeneration, it is possible that these extremely hyperexcitable double mutants die from other causes too quickly to manifest severe neuropathological lesions.
Although K+ channel mutations lead to hyperexcitability, the neurodegeneration observed in these mutants might be a consequence of neuronal dysfunction, rather than excitotoxicity per se. Consistent with this idea, we have found that mutations affecting Na+ channels, which decrease neuronal membrane excitability, also cause neurodegeneration. In fact, the severity of the neurodegenerative phenotypes in Na+ channel mutants appears to be greater than that for K+ channel mutants. The fact that the neuropathological phenotypes reported here do not correlate with simple changes in membrane excitability or with current models of excitotoxicity is intriguing. We observed neurodegeneration not only for mutations of para, which encodes voltage-gated Na+ channels, but also for the mlenapts mutation, which reduces expression of para-encoded Na+ channels through a post-transcriptional splicing defect (Reenan et al 2000). The more striking neurodegeneration observed in mlenapts corresponds with a more severe reduction in neuronal activity at permissive temperatures (Ganetzky and Wu 1982a; Ganetzky 1984).
The effect of mlenapts on life span and neurodegeneration appears to be more severe than that of para mutants themselves, even though the temperature-dependent paralysis and conduction block in mlenapts is mediated primarily through reduction in para expression (Wu et al. 1978; Kernan et al. 1991; Reenan et al. 2000). Despite these similarities, as only a single allele was assayed here, background effects cannot be discounted. Nonetheless, because of its role in post-transcriptional processing and editing of mRNA, it is likely that the mlenapts mutation also affects the expression of other, as yet unidentified, proteins in addition to Na+ channels. Similarly, recent studies have shown that numerous nervous system transcripts undergo RNA editing by dADAR (Hoopengardner et al. 2003) and mutations in dADAR result in striking age-dependent neurodegeneration (Palladino et al. 2000). Our finding that mutations in a variety of individual ion channel genes can produce neurodegeneration suggests that the more severe neuropathology observed in dADAR mutants may result from cumulative neuronal dysfunction owing to defects in RNA editing of transcripts for multiple different ion channels and other proteins required for neurotransmission.
Mechanisms of neurodegeneration in ATPα mutants:
Establishment and maintenance of the ionic gradients that produce a normal membrane resting potential are essential for proper neural function and are critically dependent on the activity of the Na+/K+ ATPase. Indeed, most of the ATP expended by neurons is used to fuel the Na+/K+ pump. Not surprisingly, mutations affecting the activity of the Na+/K+ ATPase cause profound electrophysiological and behavioral perturbations. These mutations provide another window for examining the relationship between neuronal activity and neuronal viability. We previously found that a 50% reduction in ATPα expression (heterozygosity for a null allele) results in shortened life span, conditional paralysis, and neurodegeneration (Palladino et al. 2003). Heterozygotes for dominant mutations of ATPα have similar but more severe phenotypes. Both the null mutations and the dominant mutations cause lethality when homozygous (Palladino et al. 2003; Paul et al. 2003). Although the residues altered in the dominant mutations have been implicated in maturation of the protein (Arguello et al. 1999), we found no significant change in protein levels or localization in ATPαDTS1 mutant nerves. Thus, overt defects in trafficking or localization of the Na+/K+ pump do not account for the dominant phenotypes of ATPαDTS mutants.
Instead, our data support the idea that both the dominant and recessive ATPα mutations cause loss of Na+/K+ ATPase function that alters ion homeostasis with resultant defects in membrane excitability and neuronal signaling. Na+/K+ ATPase activity is the primary means of K+ uptake in neurons. Its failure leads to depletion of intracellular K+ and accumulation of intracellular Na+ and Ca2+ (Archibald and White 1974; DiPolo and Beauge 1991; Xiao et al. 2002). Low intracellular K+ and high intracellular Ca2+ have been linked to apoptotic- and necrotic-mediated cell death, respectively (Hughes et al. 1997; Hughes and Cidlowski 1999; Yu and Choi 2000). Ouabain, a specific antagonist of Na+/K+ ATPase activity, also causes dose-dependent hyperexcitability and neural degeneration (Bignami and Palladini 1966; Lees and Leong 1994, 1995, 1996) similar to the effects of ATPα mutants. To account for the more severe phenotype associated with the dominant ATPα mutations in comparison with haplo-insufficiency, we propose that the dominant mutations represent antimorphic alleles that interfere with the activity of the normal polypeptide. Thus, the residual Na+/K+ ATPase activity in heterozygotes would be even <50%. Although this interpretation is consistent with all of the available data, we cannot rule out the possibility that the dominant ATPα mutations cause a novel gain-of-function that is responsible for the more severe neurodegeneration caused by these mutations.
As inhibition of the Na+/K+ ATPase results in depolarization of the membrane potential (Spuler et al. 1988), induces convulsions, and can result in excitotoxic neuronal death (Lewin 1970; Lorenzo 1970; Lees and Leong 1994), we tested the idea that the neuronal hyperexcitability caused by ATPα mutants is directly responsible for neurodegeneration, perhaps through an excitotoxic mechanism. If this were the case, we expected the phenotype to become more severe in combination with K+ channel mutations that further increase membrane excitability and to be suppressed in combination with Na+ channel mutations that decrease membrane excitability. However, neither Sh nor eag mutations enhanced neurodegeneration caused by dominant or recessive ATPα mutations, suggesting that increased neuronal excitability by itself is probably not the direct cause of neurodegeneration. Nonetheless, since we have examined only the effect of a small subset of all K+ channels, it is possible that eliminating other K+ channels, depending on their pattern of expression and subcellular localization, would lead to substantially greater neurodegeneration in combination with ATPα mutations.
Numerous studies have demonstrated that Na+ channel inhibition protects against excitotoxicity (reviewed in Spedding and Lepagnol 1995; Obrenovitch 1997; Hemmings 2004). Thus, if ATPα mutants were causing excitotoxic-dependent neurodegeneration, we would expect that para mutations, which decrease neuronal excitability (Wu and Ganetzky 1980; Loughney et al. 1989; Stern et al. 1990), would partially rescue the phenotype. Instead, we found that dominant ATPα mutations in combination with para mutations resulted in flies that were uncoordinated, quickly manifested overt locomotor impairment, and had significantly reduced life spans. Although the life span of the double mutants was greatly reduced compared with ATPαDTS mutants alone, no significant neuropathology was observed. Possibly, these double mutants die too soon for significant degeneration to develop. This result suggests that the early death of the double mutants is related to severe neural dysfunction, which may also trigger neurodegeneration, but neurodegeneration itself is not the cause of death. In any case, the interaction is different from what would be expected according to a simple excitotoxic model. The basis for the strong interaction between para and ATPα mutations is still unknown. One possibility is that ATPαDTS mutations produce a partial depolarization that inactivates Na+ channels, similar to the mechanism occurring in periodic paralyses (Cannon 2000) and further reducing excitability in parats mutants. However, para-like reduced excitability cannot be the only component mediating dominant ATPα temperature sensitivity as prolonged exposures to nonpermissive paralysis-inducing temperatures can be lethal for ATPαDTS1 animals while parats mutants recover quickly.
Decreases in K+ levels and increases in Ca2+ levels have been strongly linked to apoptotic and necrotic pathways, respectively. Both pathways have been associated with disruption of Na+/K+ ATPase function (Chatterjee and Roy 1965; Lees et al. 1990; Beauvais et al. 1995; Bortner et al. 1997; Yu et al. 1997), which recently has been found to induce “hybrid death” with concurrent signs of apoptosis and necrosis in the same cells (Xiao et al. 2002; Yu 2003). Lack of protection from introducing Na+ channel mutations and lack of aggravation from removing K+ channels, combined with failure of EKO expression and MK801 administration to rescue early lethality in ATPα alleles, all suggest that excitotoxicity is not the sole cause of the mutant pathology. This may be due to a “hybrid death” mechanism present in our mutants where, in the absence of excitotoxic-induced necrosis, decreased K+ levels may still induce apoptosis. We cannot rule out the possibility that both apoptotic and necrotic mechanisms act in parallel to produce neurodegeneration in the ATPα mutants. Our Na+ channel results suggest that multiple processes resulting in neuronal dysfunction and death and possibly activating interrelated injury cascades may be at work in these mutants (Stys 2005).
As mutations in ion channels and Na+/K+ ATPase α-subunits have been linked to a variety of cardiovascular and neuronal disorders (Jewell et al. 1992; Lingrel 1992; Renkawek et al. 1992; Lees 1993; Calandriello et al. 1995; James et al. 1999; Hubner and Jentsch 2002; Kullmann 2002; De Fusco et al. 2003; Vanmolkot et al. 2003), including rapid-onset dystonia with parkinsonism (de Carvalho Aguiar et al. 2004), characterizing the precise mechanisms underlying these pathologies is essential. Our data suggest that there may be specific “quality control” pathways monitoring the state of neuronal activity. Not only may these pathways initiate cell death upon reduction of K+ concentrations, but also specific mechanisms that act as checkpoints to distinguish normal activity from aberrant activity for a given neuronal type and subcellular domain may exist. These results provide the first evidence for neurodegeneration in animals containing mutations affecting either voltage-gated K+ or Na+ channels. Our data also suggest that excitotoxicity is not the sole mechanism inducing the massive neuronal death observed in animals containing dominant mutations in the Na+/K+ ATPase. These findings suggest that neuronal viability requires proper maintenance of neuronal signaling, which can be disrupted by either increasing or decreasing membrane excitability. Understanding the mechanisms initiating these degenerative pathways may ultimately have important therapeutic applications for prevention of neuronal death resulting from prolonged disruption of ionic concentrations under periods of ischemia, hypoxia, hypoglycemia, and trauma.
Acknowledgments
We are grateful to Haig Keshishian and Benjamin White for sharing the EKO flies; Chiara Cirelli, Daniel Bushey, and Giulio Tononi for the outcrossed Sh133 lines; Robert Kreber for his technical assistance; Miranda Sarachine for assistance with immunochemistry; the University of Pittsburgh Center for the Environmental Basis of Human Disease for support; and Simon Watkins and the University of Pittsburgh Center for Biologic Imaging for access to microscopes and histology equipment. T.F. is supported by National Institutes of Health (NIH) grant NS44722 and B.G. is funded by NIH grant NS15390.
References
- Archibald, J. T., and T. D. White, 1974. Rapid reversal of internal Na+ and K+ contents of synaptosomes by ouabain. Nature 252(5484): 595–596. [DOI] [PubMed] [Google Scholar]
- Arguello, J. M., J. Whitis and J. B. Lingrel, 1999. Alanine scanning mutagenesis of oxygen-containing amino acids in the transmembrane region of the Na,K-ATPase. Arch. Biochem. Biophys. 367(2): 341–347. [DOI] [PubMed] [Google Scholar]
- Beauvais, F., L. Michel and L. Dubertret, 1995. Human eosinophils in culture undergo a striking and rapid shrinkage during apoptosis. Role of K+ channels. J. Leukoc. Biol. 57(6): 851–855. [DOI] [PubMed] [Google Scholar]
- Bellen, H. J., and V. Budnik, 2000. The neuromuscular junction, pp. 175–200 in Drosophila Protocols, edited by W. Sullivan, M. Ashburner and R. Scott Hawley. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Bignami, A., and G. Palladini, 1966. Experimentally produced cerebral status spongiosus and continuous pseudorhythmic electroencephalographic discharges with a membrane-ATPase inhibitor in the rat. Nature 209(21): 413–414. [DOI] [PubMed] [Google Scholar]
- Bobinnec, Y., C. Marcaillou, X. Morin and A. Debec, 2003. Dynamics of the endoplasmic reticulum during early development of Drosophila melanogaster. Cell Motil. Cytoskeleton 54(3): 217–225. [DOI] [PubMed] [Google Scholar]
- Bonini, N. M., and M. E. Fortini, 2003. Human neurodegenerative disease modeling using Drosophila. Annu. Rev. Neurosci. 26: 627–656. [DOI] [PubMed] [Google Scholar]
- Bortner, C. D., F. M. Hughes, Jr. and J. A. Cidlowski, 1997. A primary role for K+ and Na+ efflux in the activation of apoptosis. J. Biol. Chem. 272(51): 32436–32442. [DOI] [PubMed] [Google Scholar]
- Buchanan, R. L., and S. Benzer, 1993. Defective glia in the Drosophila brain degeneration mutant drop-dead. Neuron 10(5): 839–850. [DOI] [PubMed]
- Budnik, V., Y. Zhong and C. F. Wu, 1990. Morphological plasticity of motor axons in Drosophila mutants with altered excitability. J. Neurosci. 10(11): 3754–3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calandriello, L., R. Curini, E. M. Pennisi and G. Palladini, 1995. Spongy state (status spongiosus) and inhibition of Na,K-ATPase: a pathogenetic theory. Med. Hypotheses 44(3): 173–178. [DOI] [PubMed] [Google Scholar]
- Cannon, S. C., 2000. Spectrum of sodium channel disturbances in the nondystrophic myotonias and periodic paralyses. Kidney Int. 57: 772–779. [DOI] [PubMed] [Google Scholar]
- Cattaert, D., and S. Birman, 2001. Blockade of the central generator of locomotor rhythm by noncompetitive NMDA receptor antagonists in Drosophila larvae. J. Neurobiol. 48(1): 58–73. [DOI] [PubMed] [Google Scholar]
- Chatterjee, M. L., and A. R. Roy, 1965. Toxic effects of ouabain on the isolated heart of reserpinised rabbit. Bull. Calcutta Sch. Trop. Med. 13(2): 54–57. [PubMed] [Google Scholar]
- Chiang, A. S., W. Y. Lin, H. P. Liu, M. A. Pszczolkowski, T. F. Fu et al., 2002. Insect NMDA receptors mediate juvenile hormone biosynthesis. Proc. Natl. Acad. Sci. USA 99(1): 37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, D. W., and S. M. Rothman, 1990. The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annu. Rev. Neurosci. 13: 171–182. [DOI] [PubMed] [Google Scholar]
- Cirelli, C., D. Bushey, S. Hill, R. Huber, R. Kreber et al., 2005. Reduced sleep in Drosophila Shaker mutants. Nature 434(7037): 1087–1092. [DOI] [PubMed] [Google Scholar]
- de Carvalho Aguiar, P., K. J. Sweadner, J. T. Penniston, J. Zaremba, L. Liu et al., 2004. Mutations in the Na+/K+-ATPase alpha3 gene ATP1A3 are associated with rapid-onset dystonia parkinsonism. Neuron 43(2): 169–175. [DOI] [PubMed] [Google Scholar]
- De Fusco, M., R. Marconi, L. Silvestri, L. Atorino, L. Rampoldi et al., 2003. Haploinsufficiency of ATP1A2 encoding the NA+/K+ pump alpha2 subunit associated with familial hemiplegic migraine type 2. Nat. Genet. 33(2): 192–196. [DOI] [PubMed] [Google Scholar]
- DiPolo, R., and L. Beauge, 1991. Regulation of Na-Ca exchange. An overview. Ann. NY Acad. Sci. 639: 100–111. [DOI] [PubMed] [Google Scholar]
- Driscoll, M., and B. Gerstbrein, 2003. Dying for a cause: invertebrate genetics takes on human neurodegeneration. Nat. Rev. Genet 4(3): 181–194. [DOI] [PubMed] [Google Scholar]
- Elkins, T., and B. Ganetzky, 1990. Conduction in the giant nerve fiber pathway in temperature-sensitive paralytic mutants of Drosophila. J. Neurogenet. 6(4): 207–219. [DOI] [PubMed] [Google Scholar]
- Engel, J. E., and C. F. Wu, 1992. Interactions of membrane excitability mutations affecting potassium and sodium currents in the flight and giant fiber escape systems of Drosophila. J. Comp. Physiol. A 171: 93–104. [DOI] [PubMed] [Google Scholar]
- Foster, A. C., and E. H. Wong, 1987. The novel anticonvulsant MK-801 binds to the activated state of the N-methyl-D-aspartate receptor in rat brain. Br. J. Pharmacol. 91(2): 403–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster, A. C., R. Gill, J. A. Kemp and G. N. Woodruff, 1987. Systemic administration of MK-801 prevents N-methyl-D-aspartate-induced neuronal degeneration in rat brain. Neurosci. Lett. 76(3): 307–311. [DOI] [PubMed] [Google Scholar]
- Ganetzky, B., 1984. Genetic studies of membrane excitability in Drosophila: lethal interaction between two temperature-sensitive paralytic mutations. Genetics 108: 897–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganetzky, B., and C. F. Wu, 1982. a Drosophila mutants with opposing effects on nerve excitability: genetic and spatial interactions in repetitive firing. J. Neurophysiol. 47(3): 501–514. [DOI] [PubMed] [Google Scholar]
- Ganetzky, B., and C. F. Wu, 1982. b Indirect suppression involving behavioral mutants with altered nerve excitability in Drosophila melanogaster. Genetics 100: 597–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganetzky, B., and C. F. Wu, 1983. Neurogenetic analysis of potassium currents in Drosophila: synergistic effects on neuromuscular transmission in double mutants. J. Neurogenet. 1(1): 17–28. [DOI] [PubMed] [Google Scholar]
- Ganetzky, B., and C. F. Wu, 1986. Neurogenetics of membrane excitability in Drosophila. Annu. Rev. Genet. 20: 13–44. [DOI] [PubMed] [Google Scholar]
- Genova, J. L., and R. G. Fehon, 2003. Neuroglian, gliotactin, and the Na+/K+ ATPase are essential for septate junction function in Drosophila. J. Cell Biol. 161(5): 979–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmings, H. C., Jr., 2004. Neuroprotection by Na+ channel blockade. J. Neurosurg. Anesthesiol. 16(1): 100–101. [DOI] [PubMed] [Google Scholar]
- Hoopengardner, B., T. Bhalla, C. Staber and R. Reenan, 2003. Nervous system targets of RNA editing identified by comparative genomics. Science 301(5634): 832–836. [DOI] [PubMed] [Google Scholar]
- Hubner, C. A., and T. J. Jentsch, 2002. Ion channel diseases. Hum. Mol. Genet. 11(20): 2435–2445. [DOI] [PubMed] [Google Scholar]
- Hughes, F. M., Jr., and J. A. Cidlowski, 1999. Potassium is a critical regulator of apoptotic enzymes in vitro and in vivo. Adv. Enzyme Regul. 39: 157–171. [DOI] [PubMed] [Google Scholar]
- Hughes, F. M., Jr., C. D. Bortner, G. D. Purdy and J. A. Cidlowski, 1997. Intracellular K+ suppresses the activation of apoptosis in lymphocytes. J. Biol. Chem. 272(48): 30567–30576. [DOI] [PubMed] [Google Scholar]
- Jackson, F. R., S. D. Wilson, G. R. Strichartz and L. M. Hall, 1984. Two types of mutants affecting voltage-sensitive sodium channels in Drosophila melanogaster. Nature 308(5955): 189–191. [DOI] [PubMed] [Google Scholar]
- James, P. F., I. L. Grupp, G. Grupp, A. L. Woo, G. R. Askew et al., 1999. Identification of a specific role for the Na,K-ATPase alpha 2 isoform as a regulator of calcium in the heart. Mol. Cell 3(5): 555–563. [DOI] [PubMed] [Google Scholar]
- Jan, Y. N., L. Y. Jan and M. J. Dennis, 1977. Two mutations of synaptic transmission in Drosophila. Proc. R. Soc. Lond. B Biol. Sci. 198(1130): 87–108. [DOI] [PubMed] [Google Scholar]
- Jewell, E. A., O. I. Shamraj and J. B. Lingrel, 1992. Isoforms of the alpha subunit of Na,K-ATPase and their significance. Acta Physiol. Scand. Suppl 607: 161–169. [PubMed] [Google Scholar]
- Kapahi, P., B. M. Zid, T. Harper, D. Koslover, V. Sapin et al., 2004. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr. Biol. 14: 885–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan, W. D., and W. E. Trout, III, 1969. The behavior of four neurological mutants of Drosophila. Genetics 61: 399–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasbekar, D. P., J. C. Nelson and L. M. Hall, 1987. Enhancer of seizure: a new genetic locus in Drosophila melanogaster defined by interactions with temperature-sensitive paralytic mutations. Genetics 116: 423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernan, M. J., M. I. Kuroda, R. Kreber, B. S. Baker and B. Ganetzky, 1991. napts, a mutation affecting sodium channel activity in Drosophila, is an allele of mle, a regulator of X chromosome transcription. Cell 66(5): 949–959. [DOI] [PubMed] [Google Scholar]
- Kretzschmar, D., G. Hasan, S. Sharma, M. Heisenberg and S. Benzer, 1997. The swiss cheese mutant causes glial hyperwrapping and brain degeneration in Drosophila. J. Neurosci. 17(19): 7425–7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann, D. M., 2002. The neuronal channelopathies. Brain 125: 1177–1195. [DOI] [PubMed] [Google Scholar]
- Lees, G. J., 1993. Contributory mechanisms in the causation of neurodegenerative disorders. Neuroscience 54(2): 287–322. [DOI] [PubMed] [Google Scholar]
- Lees, G. J., and W. Leong, 1994. Brain lesions induced by specific and non-specific inhibitors of sodium-potassium ATPase. Brain Res. 649(1–2): 225–233. [DOI] [PubMed] [Google Scholar]
- Lees, G. J., and W. Leong, 1995. The sodium-potassium ATPase inhibitor ouabain is neurotoxic in the rat substantia nigra and striatum. Neurosci. Lett. 188(2): 113–116. [DOI] [PubMed] [Google Scholar]
- Lees, G. J., and W. Leong, 1996. Interactions between excitotoxins and the Na+/K+-ATPase inhibitor ouabain in causing neuronal lesions in the rat hippocampus. Brain Res. 714(1–2): 145–155. [DOI] [PubMed] [Google Scholar]
- Lees, G. J., A. Lehmann, M. Sandberg and A. Hamberger, 1990. The neurotoxicity of ouabain, a sodium-potassium ATPase inhibitor, in the rat hippocampus. Neurosci. Lett. 120(2): 159–162. [DOI] [PubMed] [Google Scholar]
- Lewin, E., 1970. Epileptogenic foci induced with ouabain. Electroencephalogr. Clin. Neurophysiol. 29(4): 402–403. [DOI] [PubMed] [Google Scholar]
- Lin, Y. J., L. Seroude and S. Benzer, 1998. Extended life-span and stress resistance in the Drosophila mutant methuselah. Science 282: 943–946. [DOI] [PubMed] [Google Scholar]
- Lingrel, J. B., 1992. Na,K-ATPase: isoform structure, function, and expression. J. Bioenerg. Biomembr. 24(3): 263–270. [DOI] [PubMed] [Google Scholar]
- Lorenzo, A. S., 1970. Experimental study of seizure mechanisms. Electroencephalogr. Clin. Neurophysiol. 28(4): 417–418. [PubMed] [Google Scholar]
- Loughney, K., R. Kreber and B. Ganetzky, 1989. Molecular analysis of the para locus, a sodium channel gene in Drosophila. Cell 58(6): 1143–1154. [DOI] [PubMed] [Google Scholar]
- Magyar, J. P., U. Bartsch, Z. Q. Wang, N. Howells, A. Aguzzi et al., 1994. Degeneration of neural cells in the central nervous system of mice deficient in the gene for the adhesion molecule on Glia, the beta 2 subunit of murine Na,K-ATPase. J. Cell Biol. 127(3): 835–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min, K. T., and S. Benzer, 1997. Spongecake and eggroll: two hereditary diseases in Drosophila resemble patterns of human brain degeneration. Curr. Biol. 7(11): 885–888. [DOI] [PubMed] [Google Scholar]
- Min, K. T., and S. Benzer, 1999. Preventing neurodegeneration in the Drosophila mutant bubblegum. Science 284(5422): 1985–1988. [DOI] [PubMed] [Google Scholar]
- Misonou, H., and J. S. Trimmer, 2004. Determinants of voltage-gated potassium channel surface expression and localization in mammalian neurons. Crit. Rev. Biochem. Mol. Biol. 39(3): 125–145. [DOI] [PubMed] [Google Scholar]
- Molthagen, M., M. Schachner and U. Bartsch, 1996. Apoptotic cell death of photoreceptor cells in mice deficient for the adhesion molecule on glia (AMOG, the beta 2-subunit of the Na, K-ATPase). J. Neurocytol. 25(4): 243–255. [DOI] [PubMed] [Google Scholar]
- Noebels, J. L., 2003. The biology of epilepsy genes. Annu. Rev. Neurosci. 26: 599–625. [DOI] [PubMed] [Google Scholar]
- O'Dowd, D. K., S. E. Germeraad and R. W. Aldrich, 1989. Alterations in the expression and gating of Drosophila sodium channels by mutations in the para gene. Neuron 2(4): 1301–1311. [DOI] [PubMed] [Google Scholar]
- Obrenovitch, T. P., 1997. Sodium and potassium channel modulators: their role in neuroprotection. Int. Rev. Neurobiol. 40: 109–135. [DOI] [PubMed] [Google Scholar]
- Palladino, M. J., L. P. Keegan, M. A. O'Connell and R. A. Reenan, 2000. A-to-I pre-mRNA editing in Drosophila is primarily involved in adult nervous system function and integrity. Cell 102(4): 437–449. [DOI] [PubMed] [Google Scholar]
- Palladino, M. J., T. J. Hadley and B. Ganetzky, 2002. Temperature-sensitive paralytic mutants are enriched for those causing neurodegeneration in Drosophila. Genetics 161: 1197–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palladino, M. J., J. E. Bower, R. Kreber and B. Ganetzky, 2003. Neural dysfunction and neurodegeneration in Drosophila Na+/K+ ATPase alpha subunit mutants. J. Neurosci. 23(4): 1276–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul, S. M., M. Ternet, P. M. Salvaterra and G. J. Beitel, 2003. The Na+/K+ ATPase is required for septate junction function and epithelial tube-size control in the Drosophila tracheal system. Development 130(20): 4963–4974. [DOI] [PubMed] [Google Scholar]
- Reenan, R. A., C. J. Hanrahan and B. Ganetzky, 2000. The mle(napts) RNA helicase mutation in Drosophila results in a splicing catastrophe of the para Na+ channel transcript in a region of RNA editing. Neuron 25(1): 139–149. [DOI] [PubMed] [Google Scholar]
- Renkawek, K., W. O. Renier, J. J. de Pont, O. J. Vogels and F. J. Gabreels, 1992. Neonatal status convulsivus, spongiform encephalopathy, and low activity of Na+/K(+)-ATPase in the brain. Epilepsia 33(1): 58–64. [DOI] [PubMed] [Google Scholar]
- Schanne, F. A., A. B. Kane, E. E. Young and J. L. Farber, 1979. Calcium dependence of toxic cell death: a final common pathway. Science 206(4419): 700–702. [DOI] [PubMed] [Google Scholar]
- Schubiger, M., Y. Feng, D. M. Fambrough and J. Palka, 1994. A mutation of the Drosophila sodium pump alpha subunit gene results in bang-sensitive paralysis. Neuron 12(2): 373–381. [DOI] [PubMed] [Google Scholar]
- Shulman, J. M., L. M. Shulman, W. J. Weiner and M. B. Feany, 2003. From fruit fly to bedside: translating lessons from Drosophila models of neurodegenerative disease. Curr. Opin. Neurol. 16(4): 443–449. [DOI] [PubMed] [Google Scholar]
- Siddiqi, O., and S. Benzer, 1976. Neurophysiological defects in temperature-sensitive paralytic mutants of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 73(9): 3253–3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siesjo, B. K., and F. Bengtsson, 1989. Calcium fluxes, calcium antagonists, and calcium-related pathology in brain ischemia, hypoglycemia, and spreading depression: a unifying hypothesis. J. Cereb. Blood Flow Metab. 9(2): 127–140. [DOI] [PubMed] [Google Scholar]
- Spedding, M., and J. Lepagnol, 1995. Pharmacology of sodium and calcium channel modulation in neurons: implications for neuroprotection. Biochem. Soc. Trans. 23(3): 633–636. [DOI] [PubMed] [Google Scholar]
- Spuler, A., W. Endres and P. Grafe, 1988. Glucose depletion hyperpolarizes guinea pig hippocampal neurons by an increase in potassium conductance. Exp. Neurol. 100(1): 248–252. [DOI] [PubMed] [Google Scholar]
- Stern, M, R Kreber and B Ganetzky, 1990. Dosage effects of a Drosophila sodium channel gene on behavior and axonal excitability. Genetics 124: 133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stys, P. K., 2005. General mechanisms of axonal damage and its prevention. J. Neurol. Sci. 233: 3–13. [DOI] [PubMed] [Google Scholar]
- Sutherland, M. L., S. H. Williams, R. Abedi, P. A. Overbeek, P. J. Pfaffinger et al., 1999. Overexpression of a Shaker-type potassium channel in mammalian central nervous system dysregulates native potassium channel gene expression. Proc. Natl. Acad. Sci. USA 96(5): 2451–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutula, T. P., J. Hagen and A. Pitkanen, 2003. Do epileptic seizures damage the brain? Curr. Opin. Neurol. 16(2): 189–195. [DOI] [PubMed] [Google Scholar]
- Suzuki, D. T., T. Grigliatti and R. Williamson, 1971. Temperature-sensitive mutations in Drosophila melanogaster. VII. A mutation (para-ts) causing reversible adult paralysis. Proc. Natl. Acad. Sci. USA 68(5): 890–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troulinaki, K., and N. Tavernarakis, 2005. Neurodegenerative conditions associated with ageing: A molecular interplay? Mech. Ageing Dev. 126(1): 23–33. [DOI] [PubMed] [Google Scholar]
- Trout, W. E., and W. D. Kaplan, 1970. A relation between longevity, metabolic rate, and activity in shaker mutants of Drosophila melanogaster. Exp. Gerontol. 5(1): 83–92. [DOI] [PubMed] [Google Scholar]
- Ultsch, A., C. M. Schuster, B. Laube, H. Betz and B. Schmitt, 1993. Glutamate receptors of Drosophila melanogaster. Primary structure of a putative NMDA receptor protein expressed in the head of the adult fly. FEBS Lett. 324(2): 171–177. [DOI] [PubMed] [Google Scholar]
- Vanmolkot, K. R., E. E Kors, J. J. Hottenga, G. M. Terwindt, J. Haan et al., 2003. Novel mutations in the Na+, K+-ATPase pump gene ATP1A2 associated with familial hemiplegic migraine and benign familial infantile convulsions. Ann. Neurol. 54(3): 360–366. [DOI] [PubMed] [Google Scholar]
- White, B. H., T. P. Osterwalder, K. S. Yoon, W. J. Joiner, M. D. Whim et al., 2001. Targeted attenuation of electrical activity in Drosophila using a genetically modified K(+) channel. Neuron 31(5): 699–711. [DOI] [PubMed] [Google Scholar]
- Wu, C. F., and B. Ganetzky, 1980. Genetic alteration of nerve membrane excitability in temperature-sensitive paralytic mutants of Drosophila melanogaster. Nature 286(5775): 814–816. [DOI] [PubMed] [Google Scholar]
- Wu, C. F., B. Ganetzky, L. Y. Jan and Y. N. Jan, 1978. A Drosophila mutant with a temperature-sensitive block in nerve conduction. Proc. Natl. Acad. Sci. USA 75(8): 4047–4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, C. F., B. Ganetzky, F. N. Haugland and A. X. Liu, 1983. Potassium currents in Drosophila: different components affected by mutations of two genes. Science 220(4601): 1076–1078. [DOI] [PubMed] [Google Scholar]
- Xiao, A. Y., L. Wei, S. Xia, S. Rothman and S. P. Yu, 2002. Ionic mechanism of ouabain-induced concurrent apoptosis and necrosis in individual cultured cortical neurons. J. Neurosci. 22(4): 1350–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, S. P., 2003. Na(+), K(+)-ATPase: the new face of an old player in pathogenesis and apoptotic/hybrid cell death. Biochem. Pharmacol. 66(8): 1601–1609. [DOI] [PubMed] [Google Scholar]
- Yu, S. P., and D. W. Choi, 2000. Ions, cell volume, and apoptosis. Proc. Natl. Acad. Sci. USA 97(17): 9360–9362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, S. P., C. H. Yeh, S. L. Sensi, B. J. Gwag, L. M. Canzoniero et al., 1997. Mediation of neuronal apoptosis by enhancement of outward potassium current. Science 278(5335): 114–117. [DOI] [PubMed] [Google Scholar]