Abstract
Double-strand DNA breaks can be repaired by any of several alternative mechanisms that differ greatly in the nature of the final repaired products. We used a reporter construct, designated “Repair reporter 3” (Rr3), to measure the relative usage of these pathways in Drosophila germ cells. The method works by creating a double-strand break at a specific location such that expression of the red fluorescent protein, DsRed, in the next generation can be used to infer the frequency at which each pathway was used. A key feature of this approach is that most data come from phenotypic scoring, thus allowing large sample sizes and considerable precision in measurements. Specifically, we measured the proportion of breaks repaired by (1) conversion repair, (2) nonhomologous end joining (NHEJ), or (3) single-strand annealing (SSA). For conversion repair, the frequency of mitotic crossing over in the germ line indicates the relative prevalence of repair by double Holliday junction (DHJ) formation vs. the synthesis-dependent strand annealing (SDSA) pathway. We used this method to show that breaks occurring early in germ-line development were much more frequently repaired via single-strand annealing and much less likely to be repaired by end joining compared with identical breaks occurring later in development. Conversion repair was relatively rare when breaks were made either very early or very late in development, but was much more frequent in between. Significantly, the changes in relative usage occurred in a compensatory fashion, such that an increase in one pathway was accompanied by decreases in others. This negative correlation is interpreted to mean that the pathways for double-strand break repair compete with each other to handle a given breakage event.
A double-strand DNA break (DSB) can occur for a variety of reasons, including replication fork collapse, endonuclease activity, and the action of chemical agents or radiation. The resulting break must be repaired before the cell continues its cycle. Work in our lab and others has identified at least four distinguishable pathways for DSB repair in Drosophila mitotic cells. These pathways can be identified genetically by the resulting repair products.
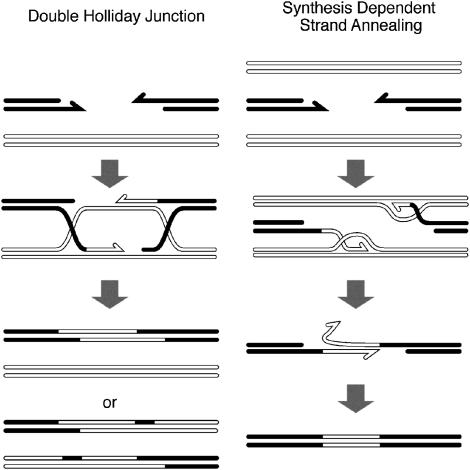
Two of these pathways (Figure 1) result in gene conversion, but have different genetic consequences. Only the double Holliday junction (DHJ) pathway can result in crossing over, whereas only synthesis-dependent strand annealing (SDSA) can yield three-partner or “bitemplate” repair. Yet, both can yield nonrecombinant gene conversion products. The SDSA mechanism appears to be more common than the DHJ in the mitotic germ cells of wild-type Drosophila (Nassif et al. 1994; Rong and Golic 2003). In meiotic cells, the choice between these two pathways is thought to determine whether a given DSB will result in gene conversion or crossover (Allers and Lichten 2001; Hunter and Kleckner 2001; Borner et al. 2004; Mazina et al. 2004).
Figure 1.
Comparison between double Holliday junction and SDSA models. Each strand of the broken duplex is shown as a solid line, and the template strands are open lines. Half arrowheads indicate 3′ ends. (Left) Double Holliday junction model showing both the crossover and noncrossover products (Szostak et al. 1983). (Right) The SDSA model. Each 3′ end is shown invading and synthesizing from a different homologous template. Other possibilities (not diagrammed) are that both could invade the same duplex or that only one end invades a template. The potential for bitemplate repair arises when different templates are used by each 3′ end. Eventually, the newly synthesized single-stranded DNA pairs with the other end of the break, which may also be newly synthesized or else resected. This pairing allows completion of the synthesis with each strand using the other as its template.
The nonhomologous end-joining pathway requires no extensive sequence homology. Broken ends are ligated together, often following limited resection to expose sites of microhomology, which are utilized in the ligation (Moore and Haber 1996). Extra bases of “filler” DNA are also commonly found inserted at breakpoints following nonhomologous end joining (NHEJ).
Repair via the single-strand annealing pathway occurs only for DSBs flanked by copies of a directly duplicated sequence (Fishman-Lobell et al. 1992). One strand is resected on each side of the break, exposing complementary sequences. These complementary sequences anneal and, after any required synthesis and trimming, both strands are ligated together. In Drosophila, this pathway appears to be the most common for breaks of this kind (Preston et al. 2002).
These four pathways, therefore, confer different levels of risk for causing mutations or chromosome instability. Gene conversion is typically the most faithful and can occur without producing a mutation, but it also provides a chance for local loss of heterozygosity. NHEJ usually leads to small insertion or deletion mutations. Single-strand annealing (SSA) always causes a deletion, and the Holliday junction pathway can cause crossing over between homologs (which leads to loss of heterozygosity) or between ectopic sites (leading to segmental aneuploidy). A cell can minimize the deleterious effects of break repair by using the most faithful pathway available. The large number of break repair mutations that cause elevated rates of cancer in humans confirms the importance of pathway choice (Miki et al. 1994; Ellis et al. 1995; Savitsky et al. 1995; Yu et al. 1996; Carney et al. 1998; Smith et al. 1998; Varon et al. 1998; Kitao et al. 1999; Riballo et al. 1999; Moshous et al. 2003; Wang and D'Andrea 2004; Sakiyama et al. 2005).
Our goal in this work is to develop a system in which the relative usage of several DSB repair pathways can be measured. A critical feature of the system is that more than one pathway is available to act on a single pool of DSB events. We exploit the different genetic consequences of these pathways to estimate what proportion of DSBs is repaired by each mechanism.
MATERIALS AND METHODS
Constructions:
The coding region of the I-SceI endonuclease was obtained from plasmid pgk3xnlsI-SceI (Maria Jasin). It was placed under the control of the Drosophila Ubiquitin (Ubi-p63E) promoter and fused to the second exon of Ubi-p63E, using pBUHA (J. Sekelsky, unpublished data) as an intermediate construct. The Ubiq∷I-SceI expression fusion was then cloned into P{CaSpeR4} to create P{Ubiq∷I-SceI}, or UIE, for germ-line transformation.
Stocks expressing the I-SceI endonuclease under the control of the β2-tubulin promoter (βIE) were obtained from Norbert Perrimon (Bellaiche et al. 1999).
The Repair reporter 3 (Rr3) was constructed from the fluorescent protein gene DsRed2 by PCR amplification from pDsRed2-C1 (BD Biosciences/ Clontech, Palo Alto, CA). The DsRed2 coding region was amplified in two fragments, which created a 147-bp repeat separated by a site for I-SceI endonuclease cleavage. The DsRed2 coding region with the duplication was cloned into the plasmid, pJM 839 (Reichhart and Ferrandon 1998), replacing the GFP coding region. This placed the interrupted DsRed2 gene under control of the Actin (Act5C) promoter and Drosomycin (Drs) 3′ control region, creating P{Rr3}. The conversion template used in cross 2, called P{Rr3EJ1}48C, was obtained as a novel end-joined product after I-SceI cleavage. It differs from P{Rr3}48C by a deletion of 4 bp and an insertion of 13 bp, such that the sequence surrounding the Rr3 cut site and the corresponding region in Rr3EJ1 are GTATATTACCCTGTTAT/CCCTAGCCGGG and GTATATTAtatctcccggccgTATCCCTAGCCGGG, respectively. The forward slash in the Rr3 sequence shows the position of the cut on the top strand. Lowercase in the Rr3EJ1 sequence shows the unique, inserted sequence.
Drosophila stocks and crosses:
Drosophila crosses were carried out with standard methods (Ashburner 1989). Genetic symbols can be found in Drysdale et al. (2005), except for Rr3, Rr3EJ1, UIE, and βIE, which are defined above. Genotypes of the G0 males in Table 1 for crosses 1 and 2 (respectively) were:
w ; P{Rr3}48C / + ; TM3 Sb P{UIE}72C
w ; P{Rr3EJ1}48C / al wgSp-1 P{Rr3}48C L sp ; TM3 Sb P{UIE}72C / TM6 Ubx.
The G0 genotypes in Table 2, starting with cross 1 in column 2, were:
w P{UIE}5B ; P{Rr3}48C / +
w P{UIE}5B ; P{Rr3EJ1}48C / al wgSp-1 P{Rr3}48C L sp
w ; P{Rr3}48C / + ; P{UIE}87F / TM6B
w ; P{Rr3EJ1}48C / al wgSp-1 P{Rr3}48C L sp ; P{UIE}87F / TM6B
w ; P{Rr3}48C / wgSp-1 P{UIE}2R
w ; P{Rr3}48C / CyO P{UIE}53D.
When endonuclease was supplied via maternal effect (Table 3) the G0 genotypes for crosses 1 and 2 were:
w ; P{Rr3}48C / + ; TM6B / +
w ; P{Rr3EJ1}48C / al wgSp-1 P{Rr3}48C L sp ; TM6B / +.
Finally, the G0 males in crosses 1 and 2 in which endonuclease was driven from a β2-tubulin promoter were:
w ; P{Rr3}48C / CyO ; βIE-3 / TM3 Sb
w ; P{Rr3EJ1}48C / al wgSp-1 P{Rr3}48C L sp ; βIE-3 / TM6B Ubx.
TABLE 1.
Relative usage of DSB repair pathways in the germline of males
| Pathway | Scoring | Counta | PCR proportion | Frequency (%) | SEb (%) |
|---|---|---|---|---|---|
| SSA (cross 1) | Proportion of red offspring among those lacking endonuclease | 1208/1767 | NA | 68.4 | ±1.7 |
| NHEJ (cross 1) | Proportion of nonred offspring among those with endonuclease | 341/1095 | NA | 31.1 | ±1.3 |
| SSA (cross 2) | Proportion of red offspring among those lacking endonuclease | 930/1408 | NA | 66.1 | ±1.6 |
| NHEJ (cross 2) | Proportion of nonred offspring among those with endonuclease | 348/1157 | 0.623c | 18.7 | ±1.7 |
| Conversion (cross 2) | (As above) | (As above) | 0.377d | 11.3 | ±1.5 |
Cross 1, as shown in Figure 4A, yielded the results in the top half, and cross 2 (Figure 4B) results are in the bottom half. The frequencies given are the proportion of all offspring in which the indicated type of repair event had occurred. Since the total was close to 100% for both crosses, we infer that essentially all of the progeny had undergone cleavage within the Rr3 element and repair by one of the pathways. The recombination frequency in cross 2 was only 0.42% (20/4752) with a standard error of 0.11%. All standard errors were computed from replicate information without bias from premeiotic clustering (see the appendix).
The progeny numbers shown come from 77 single-G0 male replicates of cross 1 and 56 of cross 2.
Standard errors were obtained using information from the single-male replicates as described in the appendix.
DNA was extracted from 122 nonred G1 offspring bearing endonuclease. PCR showed that 76 of these had a sequence different from Rr3EJ1, indicating NHEJ rather than conversion.
Of the 122 offspring tested by PCR above, the remaining 46 amplified with a primer specific for Rr3EJ1, indicating that conversion had occurred.
TABLE 2.
The effect on repair pathways by variation in the genomic position of I-SceI endonuclease
| Endonuclease source | % UIE-72Ca | % UIE-5Bb | % UIE-87Fc | % UIE-2Rd | % UIE-53De |
|---|---|---|---|---|---|
| SSA (1) | 68.4 ± 1.7 | 76.7 ± 1.3 | 62.5 ± 1.1 | 74.2 ± 1.0 | 73.0 ± 1.3 |
| NHEJ (1) | 31.1 ± 1.3 | 24.9 ± 1.7 | 34.6 ± 1.1 | 18.6 ± 0.6 | 25.2 ± 1.1 |
| Total (1) | 99.5 | 101.6 | 97.1 | 92.8 | 98.2 |
| SSA (2) | 66.1 ± 1.6 | 61.3 ± 1.6 | 74.5 ± 1.6 | ||
| NHEJ (2) | 18.7 ± 2.1 | 17.5 ± 1.0 | 16.2 ± 1.1 | ||
| Conversion | 11.3 ± 1.6 | 19.4 ± 1.2 | 7.7 ± 1.3 | ||
| Total (2) | 96.1 | 98.2 | 98.4 |
Estimates of repair pathway usage for cross 1 (top half) and cross 2 (bottom half) were obtained as in Table 1. Standard errors were computed from replicate information without bias from premeiotic clustering (see the appendix).
Endonuclease at cytological position 72C on the third chromosomal balancer TM3 Sb. Data are from Table 1.
Endonuclease source at cytological position 5B of the X chromosome. There were 60 single-G0 male replicates for cross 1, yielding 1257 fluorescent red G1 offspring among the 1639 lacking endonuclease for the SSA estimate. There were 550 nonred G1 offspring among 2211 with endonuclease for the NHEJ estimate. For cross 2, there were 115 single-G0 male replicates, and counts (as above) for SSA and NHEJ + conversion were 1156/1885 and 757/2053. We analyzed 617 of the nonred endonuclease-bearing G1 offspring by PCR and found 324 that had undergone conversion, with the remaining 293 classified as NHEJ. The crossover frequency was only 0.09% (7/7878) with a standard error of 0.04%.
Endonuclease source at cytological position 87F of chromosome 3. For cross 1 there were 65 replicates with scores for SSA and NHEJ being 1414/2263 and 893/2581. Cross 2 had 109 replicates and counts of 1369/1838 and 580/2421. PCR tests were performed on 441 offspring, with 142 conversions and 299 NHEJ. The crossover frequency of 0.02% (2/8852) had a standard error of 0.01%.
Endonuclease source on the right arm of chromosome 2. Since Rr3 was on the opposite homolog (cytological position 48C), none of the progeny received the paternal I-SceI endonuclease transgene. From 75 single-G0 male replicates of cross 1, the estimate for SSA was 4527/6102. To measure NHEJ, which requires endonuclease, we mated some of the parental males to endonuclease-bearing females. From 71 such crosses, we scored 490/2630 progeny for the NHEJ estimate. Cross 2 was not performed owing to the difficulty of assembling all components onto chromosome 2.
Endonuclease source is at cytological position 53D of the second-chromosome balancer CyO (Drysdale et al. 2005). As in the previous case, we performed separate sets of cross 1 replicates for SSA (60 replicates for counts of 3622/4959) and for NHEJ (74 replicates for counts of 747/2966). Cross 2 was not performed because of the second chromosomal endonuclease and the presence of a balancer.
TABLE 3.
The effect of developmental timing of double-strand breaks on choice of DSB repair pathways
| Endonuclease source | % UIE-72Ca (maternal) | % UIE-72Cb (zygotic onward) | % βIE-3c (meiotic prophase) |
|---|---|---|---|
| SSA (1) | 81.0 ± 2.4 | 68.4 ± 1.7 | 8.0 ± 0.5 |
| NHEJ (1) | 10.4 ± 2.7 | 31.1 ± 1.3 | 44.8 ± 1.5 |
| Total (1) | 91.4 | 99.5 | 52.8 |
| SSA (2) | 82.5 ± 2.1 | 66.1 ± 1.6 | 16.7 ± 2.7 |
| NHEJ (2) | 12.1 ± 1.9 | 18.7 ± 1.7 | 66.3 ± 2.3 |
| Conversion | 0.4 ± 1.7 | 11.3 ± 1.5 | 1.4 ± 1.7 |
| Total (2) | 95.1 | 96.1 | 84.4 |
Estimation of pathway usage was performed with the timing of breakage being regulated by the expression of I-SceI endonuclease. When endonuclease was expressed in the G0 male only through maternal effect (second column) DSB formation was restricted to early stages of development. In the third column (also in Table 1) endonuclease is expressed throughout development. The fourth column represents endonuclease expressed late in germ-line development of the G0 male by the use of a β2-tubulin promoter. As in the previous experiments, standard errors were computed from replicate information without bias from premeiotic clustering (see the appendix).
Crosses 1 and 2 were performed by a variation of the scheme in Figure 4. Parental G0 males do not carry the endonuclease transgene themselves, but obtain endonuclease through maternal effect. Some of these males were crossed to females lacking endonuclease to yield G1 progeny for SSA measurements, and others were crossed to endonuclease-bearing females. All progeny from the latter crosses can be scored for NHEJ in cross 1 or for NHEJ + conversion in cross 2 since endonuclease will be expressed through maternal effect. For cross 1, the number of replicates and the counts for SSA were 68 and 2782/3435, and for NHEJ they were 77 and 303/2923. For cross 2, the SSA frequency was estimated from 85 replicates and counts of 2840/3441, while NHEJ + conversion estimation used 144 replicates for counts of 788/6272. A total of 212 G1 offspring were tested by PCR, of which 205 were NHEJ and 7 were conversions. The crossover frequency of 0.3% (38/12,428) had a standard error of 0.1%.
See Table 1.
Endonuclease source, βIE-3 (Bellaiche et al. 1999) ensured DSB formation only near the end of germ-line development in the G0 males. As was the case for maternal-effect expression, it was necessary to perform separate crosses for SSA measurements vs. NHEJ or NHEJ + conversion, since the βIE-3 transgene did not produce the early endonuclease required for scoring mosaic G1 progeny. For cross 1, the SSA measurement came from 79 single-G0 male replicates yielding counts of 264/3305, and the NHEJ estimate came from 50 replicates and counts of 919/2051. In cross 2, we estimated SSA from 62 replicates and counts of 320/1912. The NHEJ + conversion estimates came from 35 replicates and counts of 810/1197. We tested 337 G1 offspring by PCR, with 330 of them proving to be NHEJ and 7 being conversions. (The crossover frequency was not measured for this group.)
PCR tests and flanking DNA sequencing:
DNA extraction for all PCR tests was done with individual flies as described (Gloor and Engels 1992). Primers included EJ3 (CCGGCTAGGGATACGGCCGGG), which is specific for the sequence of Rr3EJ1, and Red06 (GCCGTCCTCGAAGTTCATCA), which matches the sequence outside the 147-bp direct duplication of Rr3. These primers yield a band only if conversion from the homolog bearing Rr3EJ1 has occurred. All tests also included a pair of “DNA-control” primers that produce a defined fragment irrespective of repair events at Rr3. If the DNA-control fragment amplified but not the Rr3EJ1-specific fragment, that individual was classified as NHEJ. No classification was made if neither fragment amplified.
The genomic location of P insertions was determined by comparing a small segment of flanking DNA obtained with TAIL PCR (Liu and Whittier 1995) with the Drosophila genome sequence (Drysdale et al. 2005).
RESULTS
Repair reporter 3 structure and crossing schemes:
We designed a reporter construct, designated Rr3, in which double-strand breaks can be made at high frequencies by the endonuclease, I-SceI (Figure 2). This construct also carries a modified version of the gene for DsRed, a fluorescent protein. We observed that DsRed is easier to score in adult flies than GFP, a more commonly used reporter (Figure 3). The Rr3 construct carried on a P-element vector was recovered on chromosome 2 at position 48C by standard methods. The I-SceI endonuclease, which is driven by the ubiquitin (Ubi-p63E) gene promoter and hereafter designated UIE, was also inserted into the Drosophila genome by P-element transformation.
Figure 2.
Rr3 and two of its repair derivatives. (A) The reporter construct, Rr3, consists of a DsRed gene interrupted by the recognition sequence for the rare-cutting endonuclease, I-SceI. This cut site is flanked by a 147-bp direct duplication of part of the DsRed sequence, and the resulting modified DsRed gene is carried within a P element. The P element also carries a mini-white gene associated with the left P end. The intact Rr3 element does not express a functional DsRed gene product owing to the presence of the cut site and duplication. However, when a DSB is formed at the I-SceI cut site, repair via the SSA pathway results in a functional DsRed gene. The products of NHEJ repair also lack a working DsRed gene, and their I-SceI cut site is typically destroyed (“X”) by random alterations. (B) The two primary derivatives can be distinguished from each other and from the intact Rr3 element phenotypically as indicated. Mosaics are easily distinguishable from those not expressing DsRed, but they are difficult to differentiate from whole-bodied DsRed individuals. For that reason, flies lacking I-SceI endonuclease are used to score SSA frequencies.
Figure 3.
DsRed in adult flies. Two Drosophila females with a DsRed gene and two without as viewed in white light and with DsRed filters are shown. We find that DsRed is considerably easier to score in adults than GFP owing to the partial opacity of the Drosophila cuticle to the GFP excitation and emission frequencies. (The two DsRed females shown also have the markers y and f, but these are unrelated to the fluorescence.)
Figure 4 shows how Rr3 is employed to measure the relative usage of the four repair pathways in the germ line of males. Cross 1, shown in Figure 4A, provides measurements of NHEJ and SSA. The events of interest occur in the germ cells of the G0 male. His progeny (G1) that inherit the Rr3 chromosome fall into three categories: (1) Those in which SSA has occurred express the DsRed gene and display red fluorescence; (2) those in which NHEJ has occurred do not express DsRed; and (3) those in which neither type of repair has taken place exhibit red fluorescence only in those progeny that also inherit the I-SceI endonuclease gene. In that case, somatic breakage and repair via SSA results in mosaicism for a functional DsRed gene. We find that these G1 mosaics express enough DsRed somatically to make them nearly as fluorescent as whole-bodied DsRed individuals. They are distinguishable from wild type, but we did not attempt to distinguish them from whole-bodied DsRed flies. Thus, the frequency of SSA is measured as the proportion of red fluorescent offspring among those not receiving the endonuclease transgene, and the frequency of NHEJ was determined from the proportion of nonred offspring among those with endonuclease. Data presented below demonstrate the reliability of this classification scheme.
Figure 4.
Crosses to measure DSB repair pathway usage. Rr3 and the I-SceI endonuclease are shown on chromosomes 2 and 3, respectively, as an example, but other versions of these crosses can be used equivalently. Mosaicism for DsRed expression is indicated in the diagram for two of the genotypes. In practice, however, these mosaics are usually indistinguishable from full-bodied DsRed flies. Thus, mosaics and full-bodied DsRed offspring are classified together as “red” in our scoring. Dominant markers, not indicated in the diagram, enable classification of G1 offspring as to whether they received Rr3 and/or endonuclease. In some versions of these crosses, the endonuclease transgene resides on the X chromosome, allowing us to use the sex of the offspring for this purpose. The female G0 parents, not drawn, are endonuclease-free for the example shown. However, for situations where the I-SceI endonuclease is not transmitted from the G0 male or when it is not expressed somatically, it is necessary to use endonuclease-bearing G0 females for a portion of the crosses to score for NHEJ. (A) Cross 1 is used to measure SSA and NHEJ under conditions in which no template for conversion is available on the homolog. No PCR tests of the G1 offspring are needed. (B) Cross 2 measures conversion in addition to SSA and NHEJ. It is necessary to perform PCR tests of nonred (endonuclease-bearing) G1 offspring to distinguish between NHEJ and conversion. These PCR tests utilize a primer specific for Rr3EJ1. The loci designated “a” and “b” are arbitrary visible markers used to score for recombination. They can be located anywhere to the left and right (respectively) of Rr3. The G0 females carry the needed alleles to allow scoring of recombinants.
Note that conversion repair pathways are not measured in cross 1 because the sequence immediately surrounding the double-strand break does not match any sequence on the homolog. The sequences flanking P{Rr3} do match the homolog, but degradation of 7.5 kb on one side of the break and of 1.6 kb on the other would be required to “expose” this homology prior to conversion. Moreover, such events would result in loss of the w+ phenotype, which was observed only at trivial frequencies in our tests. Therefore, we used a second type of mating, cross 2, to measure the relative usage of conversion pathways.
Figure 4B shows how cross 2 allows us to monitor the relative usage of the two conversion pathways, SDSA and DHJ. It is similar to cross 1 except that Rr3 in the G0 male is now opposite to a modified derivative of Rr3 in which the I-SceI cut site has been replaced by an arbitrary sequence. This modified version, designated Rr3EJ1, was derived via NHEJ during cross 1 (see Figure 4 legend and materials and methods) and serves as the template for conversion repair. As shown in Figure 4B, the nonred, endonuclease-bearing G1 progeny from cross 2 include conversion events in which the Rr3EJ1 sequence has been copied in, as well as new NHEJ events. We use PCR to distinguish between these two possibilities. Amplification with a primer matching the novel sequence in Rr3EJ1 indicates conversion; however, if the DNA-control fragment amplified, but the Rr3EJ1-specific fragment did not, this implies NHEJ. Note that SSA was measured in the same way in cross 2 as in cross 1; however, NHEJ was determined with somewhat reduced sample sizes owing to the requirement for PCR.
The breakage and repair events in crosses 1 and 2 can occur in the premeiotic germ line and can yield clusters of nonindependent products. Therefore, we perform all crosses with individual G0 males, as opposed to mass matings. This technique ensures that each cross can be considered an independent experiment, thus allowing a rigorous statistical analysis (see appendix).
Confirmation of NHEJ classification:
The logic of cross 1 requires that all or nearly all of the nonred, endonuclease-bearing G1 offspring have NHEJ products. To test this assumption, we extracted DNA from a sample of such offspring and amplified the portion of Rr3 that contains the cut site. Among 345 tested, we found that 245 yielded bands that were slightly larger or smaller than the size of Rr3 on the basis of agarose gel electrophoresis, as would be expected from the imprecise nature of NHEJ. The remaining 100 had bands that were not easily distinguishable from the size of Rr3. To examine those cases more closely, we obtained the sequence of 94 of them in the vicinity of the cut site. We found that all but one had small changes indicative of NHEJ. The results (Figure 5) included a variety of typical NHEJ products. Most were simple deletions, including many with microhomology at the junctions. Others had inserted bases or more complex changes. The sole case that was identical to Rr3 might have had a lesion outside the sequenced portion, thus preventing DsRed expression. It is also possible that this case was a phenotypic mosaic whose DsRed expression was not detected. We conclude that the phenotypic classification of NHEJ is reliable, with the error rate being negligible at (100/345)(1/94) = 0.0031.
Figure 5.
Sequence data confirming NHEJ classification. The sequence near the cut site of Rr3 was determined for a sample of nonred endonuclease-bearing G1 offspring from cross 1. A total of 345 such offspring were examined by PCR, of which only 100 yielded amplicons of similar size to that of Rr3 on an agarose gel. Of those, we obtained sequence data from 94 individuals. (Top) The sequence in the vicinity of the cut site of Rr3 and some NHEJ derivatives. The four positions constituting the overhang of I-SceI cleavage are shown in red. Note that the depiction of the Rr3 sequence includes a 3-nucleotide gap introduced solely to assist alignment. Deletions are indicated by orange shading and insertions are indicated by blue shading. Microhomology, i.e., bases apparently originating from both sides of the break, is highlighted in green. Only sequences appearing more than once in the sample of 94 are shown. There were an additional 33 unique sequences, including the one case identical with Rr3. (Bottom) The size distribution of the 94 sequences relative to Rr3.
Relative frequencies of SSA, NHEJ, SDSA, and DHJ:
We obtained baseline measurements of the four pathways using crosses 1 and 2 with the endonuclease present on chromosome 3 (UIE-72C). In these crosses, the G0 males shown in Figure 4 obtained their Rr3 maternally and UIE paternally. This scheme ensures that no maternal-effect endonuclease is present, but ubiquitous expression will occur from the early embryo onward (zygotic).
The results (Table 1) show that approximately two-thirds of the progeny from each cross have undergone SSA repair. End joining is the next most frequent, being 31% in cross 1 and 20% in cross 2. Conversion using the homolog is possible only in cross 2, where it occurs ∼10% of the time. The frequency of crossing over in cross 2 was <0.5% (20/4732), suggesting that the DHJ pathway was rare and that most of the conversion events were from SDSA.
Not included in Table 1 was a set of controls similar to cross 2 except that there was no endonuclease. None of the 2681 progeny exhibited DsRed, confirming that an endonuclease-induced break is a prerequisite.
Remarkably, the frequencies sum to ∼100% for both crosses (99.5 and 96.2%, respectively), indicating that few progeny received the intact Rr3 construct. It should be emphasized that the estimates for SSA vs. NHEJ and conversion come from different pools of progeny: those without and with endonuclease, respectively. Therefore, the experimental design does not “force” the total to be near 100%.
It is also significant that the availability of conversion pathways in cross 2 corresponded with a decrease in nonconversion repair frequencies. This negative correlation between pathway usage frequencies is a recurring observation in all the experiments reported here and in related experiments (in preparation). We interpret this pattern as an indication of competition between the pathways. That is, a change in the usage of one pathway results in compensatory changes in one or more of the others. In fact, evidence of competition can be seen even in the variation of pathway usage among individual males in crosses 1 and 2. Figure 6A shows the negative correlation between SSA and the combined NHEJ + conversion phenotypic category among the 56 individual G0 males used for cross 2 in Table 1. Overall, six of the crosses in this report were suitable for this kind of analysis, and all six had negative correlations, three of which were significant at the 0.05 level. We conclude that the negative correlation between pathways occurs in the random developmental variation between individuals as well as in the systematic effects such as the difference between crosses 1 and 2.
Figure 6.
Negative correlation between pathway usage. (A) Each point represents the progeny counts from a single G0 male. The negative correlation between SSA and NHEJ + conversion (−0.45) was significant by the permutation test at P < 0.001. (B) Each point represents a different endonuclease source. The two points near the top left corner come from the maternal-effect expression of UIE-72C in Table 3, and the other eight points come from Table 2. Calculation of the significance of the negative correlation is described in the appendix. A similar calculation excluding the two maternal-effect cases also indicates a highly significant (P = 0.0014) negative correlation. (C) Frequency distribution of the sum of SSA and NHEJ + conversion frequencies for the offspring counts of individual G0 males. The totals are clustered around 1.0, but the degree of scatter suggests there is no constraint for the sum to be constant within each G0 germ line.
Effects of genomic position of the endonuclease:
We repeated the previous experiments with four other insertions of UIE to determine whether the genomic position of the endonuclease affected repair pathway usage. The results (Table 2) do not include cross 2 for those cases where UIE is located on the same chromosome as Rr3. The overall conclusions reinforce the previous results: SSA remains the most common pathway used; DHJ is rarely used, with crossing over being <0.5% from each set of cross 2 results. However, there was significant variation in the specific frequencies. For example, both UIE-5B and UIE-2R had relatively high SSA frequencies and low NHEJ frequencies from cross 1, whereas UIE-87F had a particularly low frequency of conversion from cross 2. These differences were all significant below the 0.05 level. One technical implication from this result is that any experiment using Rr3 to look for subtle effects must be designed so that the controls and experimentals use the same genomic location for their endonuclease source.
The effect of endonuclease location provided another opportunity to examine the negative correlation between pathways. Figure 6B shows the SSA frequencies plotted against NHEJ + conversion estimates for the data in Table 2. The strong negative correlation is apparent. As above, we interpret this negative correlation as an indication of competition between the double-strand break repair pathways.
What is the underlying cause of the observed variation in pathway usage with endonuclease location? The relationship between DSB repair and cell cycle stage has been well examined (Takata et al. 1998; Adachi et al. 2001; Karathanasis and Wilson 2002; Rothkamm et al. 2003; Aylon et al. 2004; Ferreira and Cooper 2004; Saleh-Gohari and Helleday 2004; Aylon and Kupiec 2005; Weinert et al. 2005), and evidence is amassing that it can be a critical factor. One possibility is that the level of endonuclease may affect the proportion of breaks that are suffered in G1 vs. late-S/G2. For example, if a low level of endonuclease were present throughout the cell cycle, breaks may occur only when the site is most accessible, perhaps in S-phase. Much higher levels of endonuclease, also present throughout the cell cycle, may be able to produce breaks at times of lower accessibility, e.g., at G1 and G2.
Another possibility is that the choice of double-strand break repair pathway depends, in part, upon the developmental stage of the organism. It is likely that local enhancers have minor effects on the developmental timing of endonuclease expression. If the I-SceI endonuclease is being expressed with different profiles, and if repair pathways change with developmental timing, it would account for the observed variation. The following experiment was designed to test whether the choice of repair pathways is sensitive to developmental timing of DNA breakage.
The effect of developmental stage:
In the previous experiments, UIE-72C is part of the G0 male's genotype and is inherited from the father. Therefore ubiquitous expression of endonuclease is expected to begin at cell cycle 14 when zygotic genes are initially expressed (Foe et al. 1993) and continue throughout development. We used two methods to vary the developmental stage of endonuclease production in the parental males of crosses 1 and 2. The first method utilizes the same UIE-72C insert, but expressed only as maternal effect in the G0 males and thus only early in development. This change was accomplished by (1) employing the reciprocal cross to produce the G0 males of crosses 1 and 2 such that Rr3 comes from the father, and the mother carries I-SceI endonuclease, and (2) selecting G0 sons from that cross that did not receive the UIE-72C construct. These G0 males were then used in crosses 1 and 2 in the same way as above except that a subset of them were mated to UIE-72C females to yield G1 offspring in which endonuclease is expressed, thus allowing us to score for NHEJ and conversion.
The second method employs a different I-SceI endonuclease construct, designated βIE-3, in which the endonuclease gene is driven by the β2-tubulin promoter (Bellaiche et al. 1999). With this construct, expression of endonuclease occurs only late in development of the germ line, after formation of primary spermatocytes for a period of 90 hr during meiotic prophase (Fuller 1993).
The results (Table 3) allow a comparison of the repair pathway utilization at three rough stages of development: early (maternal), middle (zygotic onward), and late (βIE-3). In comparing the maternal and zygotic cases, we see that end joining is greatly reduced early in development and conversion is almost nonexistent. SSA increases in partial compensation for the reduction in the other pathways. These differences must be attributed to developmental stage, since the endonuclease construct and other aspects of the scheme are otherwise the same. Note that the magnitude of the differences is greater than any differences seen from merely changing the genomic position of the endonuclease gene (Table 2).
Similarly, comparison of the pathway usage late in development (last column of Table 3) with the zygotically expressed UIE-72C group also indicates clear sensitivity to developmental stage. We see that SSA is further reduced late in development, continuing the trend. However, conversion events were also rare in the βIE-3 group, indicating that conversion pathways are used extensively only in the middle portion of germ-line development. The NHEJ pathway becomes the primary mechanism for double-strand break repair in spermatocytes.
Our interpretation of the comparison between the βIE-3 group and those using the UEI-3R endonuclease construct is complicated by the difference in genomic location of the endonuclease construct. However, we see that the differences in pathway usage owing to variation in genomic location are much less pronounced (Table 2) than the effect we see with βIE-3. For that reason, it seems clear that most or all of the dramatic effect of expressing the endonuclease gene from a β2-tubulin promoter can be attributed to the promoter itself rather than to the genomic location of the βIE-3 element.
Unlike most of the previous experiments, the sum over pathways is significantly <100% when maternal effect or βIE-3 are the endonuclease sources. This difference can be attributed to the relatively short window of endonuclease expression in these groups, leaving a portion of the Rr3 chromosomes uncut.
The combined results in Tables 2 and 3 support the suggestion that the choice of repair pathway is highly sensitive to the stage of development at which the break occurs. Under this interpretation, the variation seen in Table 2 represents the relatively subtle differences in expression patterns coinciding with variation in genomic location of the endonuclease construct. Table 3 shows a much more precipitous effect, presumably resulting from the more pronounced differences in the timing of double-strand breaks, depending on whether the I-SceI endonuclease is expressed early, in the middle, or late in germ-line development.
DISCUSSION
Rr3 for measuring relative usage of DSB repair pathways:
Part of our goal in this work was to test the utility of Rr3 for determining the frequency at which each DSB repair pathway is used. The method is not suitable for measuring absolute frequencies, but rather for detecting how the relative usage of each pathway changes with an outside variable, such as developmental stage. Specific characteristics of the system, such as the length of the direct duplication within Rr3 (147 bp), are likely to influence the choice of DSB repair pathway (Sugawara et al. 2000; Preston et al. 2002). However, by holding these characteristics constant while changing another variable of interest, we can determine how the variable influences the pathway preferences.
A key benefit of the Rr3 method is that most of the data come from phenotypic scoring rather than from molecular tests. This feature allows for larger sample sizes owing to the greater efficiency of scoring for phenotypes. For cross 1, we found that phenotypic scoring alone was sufficient to classify progeny as SSA or NHEJ with an error rate of only 0.003. For cross 2, PCR tests are needed to distinguish NHEJ from conversion, but these tests need be performed only on the nonred, endonuclease-bearing category, which composes <20% of the G1 offspring when UIE was used for endonuclease (Tables 1–3). The resulting large sample sizes, which are made up of many statistically independent single-G0 male crosses, allowed us to measure the relative pathway usage frequencies with enough precision to detect even subtle differences between groups.
The 85 replicates from cross 2 with maternal-effect endonuclease (Table 3, first column) provide an indication of the reliability of the method. Those data were actually pooled from two experiments that were performed ∼2 years apart, but that gave entirely consistent results. The first included 36 replicates and yielded an estimate of 86.0% for SSA (±2.7%), while the second had 49 replicates with an SSA frequency of 81.7% (±2.7%). The difference was not significant (P = 0.58). Estimates of NHEJ + conversion were also consistent between the two experiments.
The technique used here to examine the effect of developmental stage can also be applied to a variety of other questions. In one particularly important class of questions, Rr3 can be used to determine which DSB repair pathway(s) are affected by various DNA repair mutations. For example, from our experiments we found that the double Holliday junction pathway was rarely used in wild-type individuals, but the same is not true in some mutant backgrounds (D. Johnson-Schlitz, personal communication). Another class of questions can be answered by making use of the fact that Rr3 is itself a mobile element and can be positioned at various places in the genome. Our procedure made use of only one position (48C), leaving open the question of whether DSB repair is dependent on the location of the break. Other environmental and biological factors can also be varied in the presence of Rr3 to determine their effect on DSB repair. The system, as presented here, is limited to the germ line of males, but variations can be designed for other situations. We conclude that Rr3 provides a powerful and versatile method for measuring the relative usage of DSB repair pathways in a broad range of situations.
Comparison with the repair of P-element-induced breaks:
Our observations of the repair of Rr3 breaks are in general agreement with previous data in Drosophila, much of which employed the unusual breaks created by P elements. Whereas I-SceI cleavage produces ends with complementary 4-base, 3′ overhangs, the ends formed by P transposition contain 17-base single-stranded, 3′ overhangs that are not complementary (Beall and Rio 1997). Yet the overall conclusions are similar for both types of ends: (1) SDSA is used much more frequently than DHJ as evidenced by low crossover rates (Engels et al. 1990; Johnson-Schlitz and Engels 1993; Nassif and Engels 1993); (2) SSA can be very efficient (Preston et al. 2002; Rong and Golic 2003); and (3) the majority of NHEJ products have simple deletions, insertions, or a combination of deletion and insertion (Rong and Golic 2003; McVey et al. 2004b; Weinert et al. 2005). With both P- and I-SceI-induced breaks, many of the simple deletion products appear to exploit microhomology to align the ends (Staveley et al. 1995; Romeijn et al. 2005).
Like much, although not all (Gloor et al. 2000; Weinert et al. 2005), of the earlier P-element data, the present Rr3 data focus on the premeiotic germ line. All the experiments presented here examine events in the male germ line while some earlier experiments have looked at the female germ line (Johnson-Schlitz and Engels 1993). Previous work has investigated other aspects of DSB repair that we have not tested with Rr3 such as the length of conversion tracts (Gloor et al. 1991), the effects of polymorphisms (Nassif and Engels 1993; Dray and Gloor 1997; Coveny et al. 2002), the repair efficiency of large gaps (Nassif et al. 1994; McVey et al. 2004a), and the preference hierarchy of template location (i.e., sister chromatid vs. allelic vs. ectopic sites in cis or in trans) (Johnson-Schlitz and Engels 1993; Engels et al. 1994; Keeler et al. 1996; Lankenau et al. 1996; Lankenau and Gloor 1998).
Unmeasured DSB repair pathways:
It is likely that double-strand breaks can be repaired by mechanisms other than those directly measured here. One such pathway is breakage-induced replication, a conversion pathway in which DNA synthesis can continue to the end of the chromosome (Kraus et al. 2001). Such events would be picked up by the Rr3 assay, but would be indistinguishable from DHJ repair. In our experiments, Rr3 is located near the middle of a chromosome arm and thus would require an extremely long DNA synthesis tract if breakage-induced replication were to occur.
Any conversion event in which the sister chromatid serves as the template would have no genetic consequences and would appear as unchanged in our assay. Previous data using P elements suggest that this may occur frequently (Preston et al. 2002). However, the product would remain available to recutting by I-SceI endonuclease subsequently during germ-line development. In fact, the cycle of breakage and copying from the sister could be repeated any number of times until the process is finally terminated by SSA, NHEJ, aberrant conversion from the sister, or conversion from the homolog. The possibility of such a cycle does not affect our interpretation that the measurements reported here represent the relative frequencies of SSA, NHEJ, and conversion. These measurements should not, however, be taken as absolute frequencies of those repair events, since the sister chromatid conversions remain unmeasured.
Possible basis of the developmental stage effect:
Our results indicate that SSA is the dominant pathway used early in development of the germ line, but it is used somewhat less during intermediate stages and is relatively infrequent just before meiosis. NHEJ, however, follows the opposite pattern, becoming progressively more prevalent as development proceeds. Conversion repair occurs only rarely in the early and late stages of germ-line development, but accounts for a substantial portion of the events during intermediate stages.
Some speculation on the causes of these differences is possible. During the syncytial phase of embryonic development, which includes cycles 1–13, no zygotic transcription occurs and the cell cycle duration averages only 10 min (Foe et al. 1993). The extremely short cell cycle could be a limiting factor throughout this period, and repair pathways that can be completed rapidly are likely to be favored. Moreover, the absence of G1 and G2 phases during the syncytial cycles could alter repair pathway preference.
Although at least some DNA damage checkpoints operate in the early embryo (Fogarty et al. 1997; Sibon et al. 1999; Masrouha et al. 2003; Xu and Du 2003; Brodsky et al. 2004), it is not clear if they differ from what occurs in later development. Differences in the prevalence of SSA vs. NHEJ between mouse oocytes and preimplantation embryos have been reported (Fiorenza et al. 2001).
At the other extreme of germ-line development, we find that NHEJ becomes the predominant pathway at the expense of SSA and conversion just prior to meiosis. Note that NHEJ is the only pathway that does not involve extensive pairing of homologous sequences. Since male meiosis in Drosophila does not include crossing over, it is possible that components necessary for SSA and conversion are less readily available, leaving NHEJ as the preferred pathway.
The prevalence of different break repair pathways has been tested in brewer's and fission yeast, as well as in Arabidopsis, chicken, and mammalian cells (Lee et al. 1999; Wang et al. 2001; Wilson 2002; Orel et al. 2003; Prudden et al. 2003; Stark et al. 2004; Elliott et al. 2005; Nakanishi et al. 2005). In these experiments as in ours, whenever SSA was available it was relatively efficient. The relative use of NHEJ and conversion varied dramatically from organism to organism and sometimes within an organism. It seems that cell-specific factors strongly influence pathway choice. Using a somewhat similar system in Drosophila, Rong and Golic compared results of I-SceI endonuclease expressed by heat shock to results of leaky expression without induction (Rong and Golic 2003). In this case, not only the level of endonuclease was varied but also it is likely that the developmental timing differed as well. Overall they saw much higher levels of conversion, using a template on the homolog and lower NHEJ frequencies than we observed. As we show here, differences in endonuclease expression can produce significant effects on the relative pathway frequencies. It is also likely that differences between Rr3 and their assay construct contributed to the conflicting results. Their construct had much longer direct repeats and a much greater separation between repeats than Rr3. Furthermore, there may well be an effect of the heat-shock process itself, which could skew the results.
Competition between pathways:
One common theme in the experiments reported here is the tendency for pathway usage frequencies to change in a compensatory fashion relative to each other. Increased usage of one pathway coincides with a decrease in one or more of the others. This negative correlation was seen regardless of the underlying cause of the variation. It was evident in the random variation between germ cells of otherwise-identical males (Figure 6A) as well as in the more systematic changes resulting from variation in endonuclease expression (Figure 6B and Table 3). We interpret these compensatory changes as an indication of competition between pathways for double-strand break repair. Our data are inconsistent with the trivial explanation in which a negative correlation comes about simply because our experimental design forces the total of the pathway usage frequencies to be ∼100%. This might be the case if there was a substantial amount of selection in the germ line due to cell death from unrepaired breaks. Note from Figure 6C that the totals for the individual males vary widely, with some being ≫100% and others being much less. Yet, the negative correlation remains clear and highly significant (Figure 6A).
Thus our data support the view that DNA repair pathways compete as opposed to the alternative view that each DSB becomes committed to a particular pathway soon after its formation. Although the idea of competition between pathways is increasingly popular, there are at least two cases for early commitment: During meiosis in Saccharomyces cerevisiae, there is strong evidence that the decision to repair a particular break by either a crossover or a noncrossover pathway occurs quite early (Allers and Lichten 2001; Borner et al. 2004). There is also increasing evidence that pathway choice in eukaryotes is dependant on the stage of the cell cycle (Aylon et al. 2004; Ferreira and Cooper 2004; Saleh-Gohari and Helleday 2004). This dependence implies that a pathway bias is established even before the break is made. Note that neither of these circumstances completely excludes the possibility of competition. In fact, cell cycle bias and pathway competition have been observed concurrently (Ira et al. 2004).
The existence of compensatory changes can affect the interpretation of previous work. For example, any observation in which usage of a particular pathway is increased in a mutant background might reflect only a compensatory change rather than a direct involvement by the gene in the observed pathway. We suggest that a more informative approach is to monitor multiple pathways simultaneously with Rr3 or a similar system.
Acknowledgments
We thank Dena Johnson-Schlitz for major help and ideas. Dirk Lankenau provided valuable comments on the manuscript, as did two anonymous reviewers. This work was supported by NIH public service grant GM30948.
APPENDIX
The progeny from each G0 male used in crosses 1 and 2 (Figure 4) can be considered as an independent trial. However, within the progeny of a single G0 male, the individual offspring cannot be assumed independent, owing to the premeiotic nature of the breakage and repair events. Specifically, more than one offspring from a given male can carry the product of a single event. To deal with this situation, we performed all experiments by mating G0 males individually to several females and treating the progeny of each such cross as an independent replicate. This independence was then used as follows for performing hypothesis tests and computing standard errors for estimates.
The method of permutation tests (Fisher 1935) was employed for all hypothesis testing. This approach makes use of the independence between G0 males without being affected by the nonindependence within each male. While computationally intensive, permutation tests are preferable to standard parametric methods for these data since the premeiotic events result in substantial nonnormality. Nonparametric tests, such as those based on ranks (Lehmann 1975), would also be suitable, but those methods have less power than the permutation tests used here, yet they are no more robust.
Two types of permutation tests were employed in this study: one to compare means and one to test for correlations. As an example of the former, consider the SSA frequencies from cross 1 in Table 2. To test whether the measured SSA frequency with endonuclease from UIE-5B (76.7%) was significantly different from that from UIE-72C (68.4%), we take as our null distribution all possible ways in which the 137 total G0 crosses can be broken into subsets of 60 and 77. The desired P-value is the proportion of these cases in which the difference between the two SSA frequencies is equal to or greater than the observed difference. The number of such subsets, 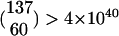 , is too great to examine exhaustively. Therefore, we selected 100 million random subsets, finding only 4924 whose difference equaled or exceeded that of the observed sample in the same direction and 5862 in the opposite direction. Therefore, our P-value is 1.08 × 10−4. As an example of a correlation test, consider the data in Figure 6B. There are 10 data points, each consisting of an SSA frequency and the corresponding NHEJ + conversion frequency. The null distribution consists of the 10! = 3,628,800 ways in which the SSA frequencies can be matched with the NHEJ + conversion frequencies. In this case, it was feasible to perform an exhaustive calculation by computing the product-moment correlation for each permutation. There were 138 cases in which the correlation was at least as great in the negative direction as the observed value of −0.948. Therefore, the P-value for this test is 138/3,628,800 = 3.8 × 10−5. In general, we carried out the exhaustive calculations wherever the number of cases was <10 million and used random sampling of at least 10 million cases otherwise.
, is too great to examine exhaustively. Therefore, we selected 100 million random subsets, finding only 4924 whose difference equaled or exceeded that of the observed sample in the same direction and 5862 in the opposite direction. Therefore, our P-value is 1.08 × 10−4. As an example of a correlation test, consider the data in Figure 6B. There are 10 data points, each consisting of an SSA frequency and the corresponding NHEJ + conversion frequency. The null distribution consists of the 10! = 3,628,800 ways in which the SSA frequencies can be matched with the NHEJ + conversion frequencies. In this case, it was feasible to perform an exhaustive calculation by computing the product-moment correlation for each permutation. There were 138 cases in which the correlation was at least as great in the negative direction as the observed value of −0.948. Therefore, the P-value for this test is 138/3,628,800 = 3.8 × 10−5. In general, we carried out the exhaustive calculations wherever the number of cases was <10 million and used random sampling of at least 10 million cases otherwise.
We also made use of the independence between G0 males to compute standard errors for our estimates of repair pathway frequencies. Let mi be the number of progeny in cross i in which a particular event has occurred and ni be the total number of progeny from the cross that can be scored for that event. Define 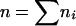 ,
,  , and let pw = m/n be our estimator of the event's frequency. As was previously shown (Engels 1979), two unbiased estimators of the variance of pw are
, and let pw = m/n be our estimator of the event's frequency. As was previously shown (Engels 1979), two unbiased estimators of the variance of pw are
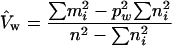 |
and
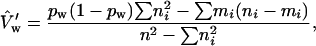 |
the former being preferable when pw ≪ 1 and the latter being preferable otherwise. The standard errors we used for SSA and NHEJ + conversion frequencies in Tables 1–3 were 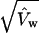 whenever pw < 10% and
whenever pw < 10% and  otherwise. For cross 2 we also report estimates in the form pwr, where r is the ratio determined by PCR. In this case, we must also take into account the uncertainty in r by the approximation
otherwise. For cross 2 we also report estimates in the form pwr, where r is the ratio determined by PCR. In this case, we must also take into account the uncertainty in r by the approximation
 |
which is valid if r and pw are uncorrelated. Some of our data allowed us to test this assumption, since both r and pw were measured for individual G0 males. We analyzed one such data set consisting of 278 individual G0 crosses and found no evidence of correlation (P > 0.05). Therefore, we used the above approximation and reported 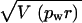 as the standard error for those frequencies in Tables 1–3 in which a PCR ratio was involved.
as the standard error for those frequencies in Tables 1–3 in which a PCR ratio was involved.
References
- Adachi, N., T. Ishino, Y. Ishii, S. Takeda and H. Koyama, 2001. DNA ligase IV-deficient cells are more resistant to ionizing radiation in the absence of Ku70: implications for DNA double-strand break repair. Proc. Natl. Acad. Sci. USA 98: 12109–12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allers, T., and M. Lichten, 2001. Differential timing and control of noncrossover and crossover recombination during meiosis. Cell 106: 47–57. [DOI] [PubMed] [Google Scholar]
- Ashburner, M., 1989. Drosophila: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Aylon, Y., and M. Kupiec, 2005. Cell cycle-dependent regulation of double-strand break repair: a role for the CDK. Cell Cycle 4: 259–261. [PubMed] [Google Scholar]
- Aylon, Y., B. Liefshitz and M. Kupiec, 2004. The CDK regulates repair of double-strand breaks by homologous recombination during the cell cycle. EMBO J. 23: 4868–4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall, E. L., and D. C. Rio, 1997. Drosophila P-element transposase is a novel site-specific endonuclease. Genes Dev. 11: 2137–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellaiche, Y., V. Mogila and N. Perrimon, 1999. I-SceI endonuclease, a new tool for studying DNA double-strand break repair mechanisms in Drosophila. Genetics 152: 1037–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borner, G. V., N. Kleckner and N. Hunter, 2004. Crossover/noncrossover differentiation, synaptonemal complex formation, and regulatory surveillance at the leptotene/zygotene transition of meiosis. Cell 117: 29–45. [DOI] [PubMed] [Google Scholar]
- Brodsky, M. H., B. T. Weinert, G. Tsang, Y. S. Rong, N. M. McGinnis et al., 2004. Drosophila melanogaster MNK/Chk2 and p53 regulate multiple DNA repair and apoptotic pathways following DNA damage. Mol. Cell. Biol. 24: 1219–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney, J. P., R. S. Maser, H. Olivares, E. M. Davis, M. Le Beau et al., 1998. The hMre11/hRad50 protein complex and Nijmegen breakage syndrome: linkage of double-strand break repair to the cellular DNA damage response. Cell 93: 477–486. [DOI] [PubMed] [Google Scholar]
- Coveny, A. M., T. Dray and G. B. Gloor, 2002. The effect of heterologous insertions on gene conversion in mitotically dividing cells in Drosophila melanogaster. Genetics 161: 249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dray, T., and G. B. Gloor, 1997. Homology requirements for targeting heterologous sequences during P-induced gap repair in Drosophila melanogaster. Genetics 147: 689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drysdale, R. A., M. A. Crosby, W. Gelbart, K. Campbell, D. Emmert et al., 2005. FlyBase: genes and gene models. Nucleic Acids Res. 33: D390–D395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott, B., C. Richardson and M. Jasin, 2005. Chromosomal translocation mechanisms at intronic alu elements in mammalian cells. Mol. Cell 17: 885–894. [DOI] [PubMed] [Google Scholar]
- Ellis, N. A., J. Groden, T. Z. Ye, J. Straughen, D. J. Lennon et al., 1995. The Bloom's syndrome gene product is homologous to RecQ helicases. Cell 83: 655–666. [DOI] [PubMed] [Google Scholar]
- Engels, W. R., 1979. The estimation of mutation rates when premeiotic events are involved. Environ. Mutagen. 1: 37–43. [DOI] [PubMed] [Google Scholar]
- Engels, W. R., D. M. Johnson-Schlitz, W. B. Eggleston and J. Sved, 1990. High-frequency P element loss in Drosophila is homolog dependent. Cell 62: 515–525. [DOI] [PubMed] [Google Scholar]
- Engels, W. R., C. R. Preston and D. M. Johnson-Schlitz, 1994. Long-range cis preference in DNA homology search over the length of a Drosophila chromosome. Science 263: 1623–1625. [DOI] [PubMed] [Google Scholar]
- Ferreira, M. G., and J. P. Cooper, 2004. Two modes of DNA double-strand break repair are reciprocally regulated through the fission yeast cell cycle. Genes Dev. 18: 2249–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorenza, M. T., A. Bevilacqua, S. Bevilacqua and F. Mangia, 2001. Growing dictyate oocytes, but not early preimplantation embryos, of the mouse display high levels of DNA homologous recombination by single-strand annealing and lack DNA nonhomologous end joining. Dev. Biol. 233: 214–224. [DOI] [PubMed] [Google Scholar]
- Fisher, R. A., 1935. Design of Experiments. Oliver & Boyd, Edinburgh.
- Fishman-Lobell, J., N. Rudin and J. E. Haber, 1992. Two alternative pathways of double-strand break repair that are kinetically separable and independently modulated. Mol. Cell. Biol. 12: 1292–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foe, V. E., G. M. Odell and B. A. Edgar, 1993. Mitosis and morphogenesis in the Drosophila embryo: point and counterpoint, pp. 149–300 in The Development of Drosophila melanogaster, edited by M. Bate and A. Martinez Arias. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Fogarty, P., S. D. Campbell, R. Abu-Shumays, B. S. Phalle, K. R. Yu et al., 1997. The Drosophila grapes gene is related to checkpoint gene chk1/rad27 and is required for late syncytial division fidelity. Curr. Biol. 7: 418–426. [DOI] [PubMed] [Google Scholar]
- Fuller, M., 1993. Spermatogenesis, pp. 71–147 in The Development of Drosophila melanogaster, edited by M. Bate and A. Martinez Arias. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Gloor, G., and W. Engels, 1992. Single-fly DNA preps for PCR. Dros. Inf. Serv. 71: 148–149. [Google Scholar]
- Gloor, G. B., N. A. Nassif, D. M. Johnson-Schlitz, C. R. Preston and W. R. Engels, 1991. Targeted gene replacement in Drosophila via P element-induced gap repair. Science 253: 1110–1117. [DOI] [PubMed] [Google Scholar]
- Gloor, G. B., J. Moretti, J. Mouyal and K. J. Keeler, 2000. Distinct P-element excision products in somatic and germline cells of Drosophila melanogaster. Genetics 155: 1821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter, N., and N. Kleckner, 2001. The single-end invasion: an asymmetric intermediate at the double-strand break to double-holliday junction transition of meiotic recombination. Cell 106: 59–70. [DOI] [PubMed] [Google Scholar]
- Ira, G., A. Pellicioli, A. Balijja, X. Wang, S. Fiorani et al., 2004. DNA end resection, homologous recombination and DNA damage checkpoint activation require CDK1. Nature 431: 1011–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Schlitz, D. M., and W. R. Engels, 1993. P-element-induced interallelic gene conversion of insertions and deletions in Drosophila melanogaster. Mol. Cell. Biol. 13: 7006–7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karathanasis, E., and T. E. Wilson, 2002. Enhancement of Saccharomyces cerevisiae end-joining efficiency by cell growth stage but not by impairment of recombination. Genetics 161: 1015–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeler, K. J., T. Dray, J. E. Penney and G. B. Gloor, 1996. Gene targeting of a plasmid-borne sequence to a double-strand DNA break in Drosophila melanogaster. Mol. Cell. Biol. 16: 522–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitao, S., A. Shimamoto, M. Goto, R. W. Miller, W. A. Smithson et al., 1999. Mutations in RECQL4 cause a subset of cases of Rothmund-Thomson syndrome. Nat. Genet. 22: 82–84. [DOI] [PubMed] [Google Scholar]
- Kraus, E., W. Y. Leung and J. E. Haber, 2001. Break-induced replication: a review and an example in budding yeast. Proc. Natl. Acad. Sci. USA 98: 8255–8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankenau, D. H., and G. B. Gloor, 1998. In vivo gap repair in Drosophila: a one-way street with many destinations. BioEssays 20: 317–327. [DOI] [PubMed] [Google Scholar]
- Lankenau, D. H., V. G. Corces and W. R. Engels, 1996. Comparison of targeted-gene replacement frequencies in Drosophila melanogaster at the forked and white loci. Mol. Cell. Biol. 16: 3535–3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S. E., F. Paques, J. Sylvan and J. E. Haber, 1999. Role of yeast SIR genes and mating type in directing DNA double-strand breaks to homologous and non-homologous repair paths. Curr. Biol. 9: 767–770. [DOI] [PubMed] [Google Scholar]
- Lehmann, E. L., 1975. Nonparametrics. McGraw-Hill, New York.
- Liu, Y. G., and R. F. Whittier, 1995. Thermal asymmetric interlaced PCR: automatable amplification and sequencing of insert end fragments from P1 and YAC clones for chromosome walking. Genomics 25: 674–681. [DOI] [PubMed] [Google Scholar]
- Masrouha, N., L. Yang, S. Hijal, S. Larochelle and B. Suter, 2003. The Drosophila chk2 gene loki is essential for embryonic DNA double-strand-break checkpoints induced in S phase or G2. Genetics 163: 973–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazina, O. M., A. V. Mazin, T. Nakagawa, R. D. Kolodner and S. C. Kowalczykowski, 2004. Saccharomyces cerevisiae Mer3 helicase stimulates 3′-5′ heteroduplex extension by Rad51; implications for crossover control in meiotic recombination. Cell 117: 47–56. [DOI] [PubMed] [Google Scholar]
- McVey, M., M. Adams, E. Staeva-Vieira and J. J. Sekelsky, 2004. a Evidence for multiple cycles of strand invasion during repair of double-strand gaps in Drosophila. Genetics 167: 699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVey, M., D. Radut and J. J. Sekelsky, 2004. b End-joining repair of double-strand breaks in Drosophila melanogaster is largely DNA ligase IV independent. Genetics 168: 2067–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki, Y., J. Swensen, D. Shattuck-Eidens, P. A. Futreal, K. Harshman et al., 1994. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 266: 66–71. [DOI] [PubMed] [Google Scholar]
- Moore, J. K., and J. E. Haber, 1996. Cell cycle and genetic requirements of two pathways of nonhomologous end-joining repair of double-strand breaks in Saccharomyces cerevisiae. Mol. Cell. Biol. 16: 2164–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshous, D., C. Pannetier, Rd. R. Chasseval, Fl. F. Deist, M. Cavazzana-Calvo et al., 2003. Partial T and B lymphocyte immunodeficiency and predisposition to lymphoma in patients with hypomorphic mutations in Artemis. J. Clin. Invest. 111: 381–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi, K., Y. G. Yang, A. J. Pierce, T. Taniguchi, M. Digweed et al., 2005. Human Fanconi anemia monoubiquitination pathway promotes homologous DNA repair. Proc. Natl. Acad. Sci. USA 102: 1110–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassif, N., and W. Engels, 1993. DNA homology requirements for mitotic gap repair in Drosophila. Proc. Natl. Acad. Sci. USA 90: 1262–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassif, N. A., J. Penney, S. Pal, W. R. Engels and G. B. Gloor, 1994. Efficient copying of nonhomologous sequences from ectopic sites via P element-induced gap repair. Mol. Cell. Biol. 14: 1613–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orel, N., A. Kyryk and H. Puchta, 2003. Different pathways of homologous recombination are used for the repair of double-strand breaks within tandemly arranged sequences in the plant genome. Plant J. 35: 604–612. [DOI] [PubMed] [Google Scholar]
- Preston, C. R., W. Engels and C. Flores, 2002. Efficient repair of DNA breaks in Drosophila: evidence for single-strand annealing and competition with other repair pathways. Genetics 161: 711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudden, J., J. S. Evans, S. P. Hussey, B. Deans, P. O'Neill et al., 2003. Pathway utilization in response to a site-specific DNA double-strand break in fission yeast. EMBO J. 22: 1419–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichhart, J. M., and D. Ferrandon, 1998. Green balancers. Dros. Inf. Serv. 81: 201–202. [Google Scholar]
- Riballo, E., S. E. Critchlow, S. H. Teo, A. J. Doherty, A. Priestley et al., 1999. Identification of a defect in DNA ligase IV in a radiosensitive leukaemia patient. Curr. Biol. 9: 699–702. [DOI] [PubMed] [Google Scholar]
- Romeijn, R. J., M. M. Gorski, M. A. van Schie, J. N. Noordermeer, L. H. Mullenders et al., 2005. Lig4 and rad54 are required for repair of DNA double-strand breaks induced by P-element excision in Drosophila. Genetics 169: 795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong, Y. S., and K. G. Golic, 2003. The homologous chromosome is an effective template for the repair of mitotic DNA double-strand breaks in Drosophila. Genetics 165: 1831–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothkamm, K., I. Kruger, L. H. Thompson and M. Lobrich, 2003. Pathways of DNA double-strand break repair during the mammalian cell cycle. Mol. Cell. Biol. 23: 5706–5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakiyama, T., T. Kohno, S. Mimaki, T. Ohta, N. Yanagitani et al., 2005. Association of amino acid substitution polymorphisms in DNA repair genes TP53, POLI, REV1 and LIG4 with lung cancer risk. Int. J. Cancer 114: 730–737. [DOI] [PubMed] [Google Scholar]
- Saleh-Gohari, N., and T. Helleday, 2004. Conservative homologous recombination preferentially repairs DNA double-strand breaks in the S phase of the cell cycle in human cells. Nucleic Acids Res. 32: 3683–3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitsky, K., A. Bar-Shira, S. Gilad, G. Rotman, Y. Ziv et al., 1995. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science 268: 1749–1753. [DOI] [PubMed] [Google Scholar]
- Sibon, O. C., A. Laurencon, R. Hawley and W. E. Theurkauf, 1999. The Drosophila ATM homologue Mei-41 has an essential checkpoint function at the midblastula transition. Curr. Biol. 9: 302–312. [DOI] [PubMed] [Google Scholar]
- Smith, L., S. J. Liu, L. Goodrich, D. Jacobson, C. Degnin et al., 1998. Duplication of ATR inhibits MyoD, induces aneuploidy and eliminates radiation-induced G1 arrest. Nat. Genet. 19: 39–46. [DOI] [PubMed] [Google Scholar]
- Stark, J. M., A. J. Pierce, J. Oh, A. Pastink and M. Jasin, 2004. Genetic steps of mammalian homologous repair with distinct mutagenic consequences. Mol. Cell. Biol. 24: 9305–9316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staveley, B. E., T. R. Heslip, R. B. Hodgetts and J. B. Bell, 1995. Protected P-element termini suggest a role for inverted-repeat-binding protein in transposase-induced gap repair in Drosophila melanogaster. Genetics 139: 1321–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara, N., G. Ira and J. E. Haber, 2000. DNA length dependence of the single-strand annealing pathway and the role of Saccharomyces cerevisiae RAD59 in double-strand break repair. Mol. Cell. Biol. 20: 5300–5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szostak, J. W., T. L. Orr-Weaver, R. J. Rothstein and F. W. Stahl, 1983. The double-strand-break repair model for recombination. Cell 33: 25–35. [DOI] [PubMed] [Google Scholar]
- Takata, M., M. S. Sasaki, E. Sonoda, C. Morrison, M. Hashimoto et al., 1998. Homologous recombination and non-homologous end-joining pathways of DNA double-strand break repair have overlapping roles in the maintenance of chromosomal integrity in vertebrate cells. EMBO J. 17: 5497–5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varon, R., C. Vissinga, M. Platzer, K. M. Cerosaletti, K. H. Chrzanowska et al., 1998. Nibrin, a novel DNA double-strand break repair protein, is mutated in Nijmegen breakage syndrome. Cell 93: 467–476. [DOI] [PubMed] [Google Scholar]
- Wang, H., Z. C. Zeng, T. A. Bui, E. Sonoda, M. Takata et al., 2001. Efficient rejoining of radiation-induced DNA double-strand breaks in vertebrate cells deficient in genes of the RAD52 epistasis group. Oncogene 20: 2212–2224. [DOI] [PubMed] [Google Scholar]
- Wang, X., and A. D. D'Andrea, 2004. The interplay of Fanconi anemia proteins in the DNA damage response. DNA Repair 3: 1063–1069. [DOI] [PubMed] [Google Scholar]
- Weinert, B. T., B. Min and D. C. Rio, 2005. P element excision and repair by non-homologous end joining occurs in both G1 and G2 of the cell cycle. DNA Repair 4: 171–181. [DOI] [PubMed] [Google Scholar]
- Wilson, T. E., 2002. A genomics-based screen for yeast mutants with an altered recombination/end-joining repair ratio. Genetics 162: 677–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, J., and W. Du, 2003. Drosophila chk2 plays an important role in a mitotic checkpoint in syncytial embryos. FEBS Lett. 545: 209–212. [DOI] [PubMed] [Google Scholar]
- Yu, C. E., J. Oshima, Y. H. Fu, E. M. Wijsman, F. Hisama et al., 1996. Positional cloning of the Werner's syndrome gene. Science 272: 258–262. [DOI] [PubMed] [Google Scholar]