Abstract
Myxococcus xanthus has two different mechanisms of motility, adventurous (A) motility, which permits individual cells to glide over solid surfaces, and social (S) motility, which permits groups of cells to glide. To identify the genes involved in S-gliding motility, we mutagenized a ΔaglU (A−) strain with the defective transposon, magellan-4, and screened for S− mutants that form nonmotile colonies. Sequence analysis of the sites of the magellan-4 insertions in these mutants and the alignment of these sites with the M. xanthus genome sequence show that two-thirds of these insertions lie within 27 of the 37 nonessential genes known to be required for social motility, including those necessary for the biogenesis of type IV pili, exopolysaccharide, and lipopolysaccharide. The remaining insertions also identify 31 new, nonessential genes predicted to encode both structural and regulatory determinants of S motility. These include three tetratricopeptide repeat proteins, several regulators of transcription that may control the expression of genes involved in pilus extension and retraction, and additional enzymes involved in polysaccharide metabolism. Three insertions that abolish S motility lie within genes predicted to encode glycolytic enzymes, suggesting that the signal for pilus retraction may be a simple product of exopolysaccharide catabolism.
MYXOCOCCUS xanthus is a soil bacterium that can glide over solid surfaces without the use of flagella. The genetic analysis of its ability to glide shows that M. xanthus has two different sets of genes involved in two very different mechanisms of motility. Most single mutations affect the ability of M. xanthus to glide either as single cells or as groups of cells, but not as both. General searches for mutants of M. xanthus with motility defects have shown that single-cell (adventurous, A) motility and group-dependent (social, S) motility are genetically separable and that each type of motility requires the functions of large, nonoverlapping sets of genes (Hodgkin and Kaiser 1979a,b; Macneil et al. 1994a,b; Youderian et al. 2003). Mutations in only three genes, mglA, which encodes a small GTPase (Stephens and Kaiser 1987; Stephens et al. 1989; Hartzell and Kaiser 1991a,b; Hartzell 1997), agmA, which is predicted to encode a critical amidase involved in cell wall biogenesis (Youderian et al. 2003), and epsI/nla24, predicted to encode an activator of transcription (Caberoy et al. 2003; Lancero et al. 2004; Lu et al. 2005), have been shown to abolish both mechanisms of motility simultaneously.
Mutants defective in A motility form colonies with groups of motile cells, but without individual motile cells, at their edges. Adventurous motility in M. xanthus requires the functions of at least 30 different genes, many of which have no known homologs in nongliding bacteria (Youderian et al. 2003). Loss-of-function mutations in each of these genes do not affect social motility. Although it has been proposed that nozzles that cluster at the cell poles may generate force by directional secretion of polymer (Wolgemuth et al. 2002), genetic and biochemical support for this model is forthcoming. In contrast, social motility in M. xanthus involves the interaction between two organelles, type IV pili and exopolysaccharide/fibril material, and is much better understood.
Mutants defective in S motility form colonies with individual motile cells, but without groups of motile cells, at their edges. Social motility results from a cycle in which the cell extends a type IV pilus, the pilus tip attaches to a receptor, and the pilus is retracted (Wu and Kaiser 1995; Merz et al. 2000; Sun et al. 2000; Skerker and Berg 2001; Li et al. 2003). This mechanism appears to be conserved among diverse Gram-negative bacteria, including M. xanthus, Pseudomonas aeruginosa, and Haemophilus influenzae (see Mattick 2002, for review). For M. xanthus, the substrate for pilus attachment is presented on the surface of a partner (receptor) cell within about one cell length of a pilus-producing cell (Hodgkin and Kaiser 1977; Wu and Kaiser 1995). Previous biochemical studies have shown that peritrichous fibrils constitute the receptor organelle required for S motility; these are composed of both protein and exopolysaccharide (EPS) components (Behmlander and Dworkin 1991; Behmlander and Dworkin 1994). The EPS component, but not the protein component, of fibrils appears to be essential for social motility and cell–cell adhesion. Mutants defective in EPS production are defective in S motility (Lu et al. 2005), and EPS stimulates pilus retraction (Li et al. 2003).
Our current understanding of S motility is missing several key elements. Mutants defective in lipopolysaccharide (LPS) production also are defective in S motility (Bowden and Kaplan 1998; Yang et al. 2000a); however, as yet, we do not know what role LPS plays in the mechanism of social motility. Also, we do not understand the molecular mechanism of pilus extension and retraction, nor have we identified the full set of genes encoding the cytoplasmic and membrane components of this molecular motor. Other important details missing from our picture of the mechanism of S motility in M. xanthus include an understanding of how the signals that trigger pilus retraction are generated and transduced upon the contact of a pilus-bearing cell with a receptor-bearing cell, how the next cycle of pilus extension and retraction is reinitiated after cell–cell contact, and what the signals are precisely for pilus extension and retraction. It is not known whether the cycle of pilus extension and retraction involves the polymerization and depolymerization of the pilus. To begin to define the genes encoding products involved in these processes, we have conducted an extensive screen for mutants with transposon insertions in the nonessential genes required for S motility.
MATERIALS AND METHODS
Bacterial strains and growth conditions:
The M. xanthus strains we have generated in this study are derivatives of the wild-type strain DK1622, and its A− mutant derivative MxH1777 (ΔaglU) (White and Hartzell, 2000), and are listed in Table 1. M. xanthus was grown at 32° in CTPM liquid medium (1% casitone, 10 mm Tris pH 7.6, 1 mm potassium phosphate pH 7.5, 5 mm MgSO4) and CTPM agar (1.5%) plates; CTPM was supplemented with kanamycin (Km; 40 μg/ml). Plasmids were introduced into M. xanthus by electroporation (Kashefi and Hartzell 1995; Youderian et al. 2003). Escherichia coli strain DH5α (λ pir) was used for the construction of plasmids and the preparation of plasmid DNA. Plasmids were introduced into this strain by electroporation, and derivatives with plasmids were grown in LB medium supplemented with Km (40 μg/ml). Plasmid pMycoMar, donor of the mini-mariner element magellan-4, has been described (Rubin et al. 1999). Restriction endonucleases and DNA modifying enzymes were from New England Biolabs (Beverly, MA) and were used under recommended conditions for plasmid constructions.
TABLE 1.
Insertions of magellan-4 that impair social gliding motility
| Coordinates | Locus | Insertion | Strain | Gene |
|---|---|---|---|---|
| 0421763–0421764 | MXAN0358 | mis-23 | MxH1521 | sgmA; ileS |
| 0500448–0500449 | MXAN0440 | mis-66 | MxH1561 | sgmB |
| 1287846–1287847 | MXAN1106 | mis-15 | MxH1513 | sgmC |
| 1288161–1288162 | MXAN1106 | mis-77 | MxH1572 | sgmC |
| 1946011–1946012 | MXAN1641 | mis-146 | MxH1629 | sgmD |
| 2122234–2122235 | MXAN1795 | mis-17 | MxH1515 | sgmE |
| 2259176–2259177 | MXAN1926 | mis-61 | MxH1557 | mglB |
| 2462808–2462809 | MXAN2128 | mis-114 | MxH1604 | sgmF |
| 2542123–2542124 | MXAN2203 | mis-7 | MxH1505 | sgmG |
| 2939846–2939847 | MXAN2526 | mis-10 | MxH1508 | sgmH |
| 2981508–2981509 | MXAN2561 | mis-96 | MxH1589 | sgmI |
| 3422255–3422256 | MXAN2921 | mis-112 | MxH1603 | sgmJ |
| 3422987–3422988 | MXAN2922 | mis-119 | MxH1609 | sgmK |
| 3422987–3422988 | MXAN2922 | mis-129 | MxH1616 | sgmK |
| 3589491–3589492 | MXAN3060 | mis-141 | MxH1626 | cglB |
| 3589491–3589492 | MXAN3060 | mis-147 | MxH1630 | cglB |
| 3611423–3611424 | MXAN3084 | mis-2 | MxH1501 | tgl |
| 3611426–3611427 | MXAN3084 | mis-85 | MxH1580 | tgl |
| 3611426–3611427 | MXAN3084 | mis-88 | MxH1583 | tgl |
| 4055186–4055187 | MXAN3474 | mor-105 | MxH1598 | stk |
| 4084951–4084952 | MXAN3506 | mis-19 | MxH1517 | sgmL |
| 4084987–4084988 | MXAN3506 | mis-131 | MxH1618 | sgmL |
| 4085119–4085120 | MXAN3506 | mis-26 | MxH1524 | sgmL |
| 4085119–4085120 | MXAN3506 | mis-127 | MxH1614 | sgmL |
| 4477791–4477792 | MXAN3759 | mis-142 | MxH1627 | sgmM |
| 4563759–4563760 | MXAN3797 | mis-51 | MxH1548 | sgmN |
| 4619415–4619416 | MXAN3840 | mis-121 | MxH1611 | scpB |
| 4672427–4672428 | MXAN3886 | mis-116 | MxH1606 | agmA |
| 5036166–5036167 | MXAN4089 | mor-115 | MxH1605 | carR |
| 5096652–5096653 | MXAN4149 | mis-75 | MxH1570 | frzS |
| 5098300–5098301 | MXAN4150 | mis-68 | MxH1563 | sgmO |
| 5788164–5788165 | MXAN4613 | mis-135 | MxH1622 | sgmP |
| 5791219–5791220 | MXAN4616 | mis-130 | MxH1617 | sgmQ |
| 5792644–5792645 | MXAN4616 | mis-101 | MxH1594 | sgmQ |
| 5792644–5792645 | MXAN4616 | mis-120 | MxH1610 | sgmQ |
| 5800139–5800140 | MXAN4620 | mis-70 | MxH1565 | sgmR |
| 5801403–5801404 | MXAN4621 | mis-43 | MxH1540 | rfbC |
| 5804537–5804538 | MXAN4621 | mis-102 | MxH1595 | rfbC |
| 5805964–5805965 | MXAN4622 | mis-41 | MxH1538 | rfbB |
| 5807214–5807215 | MXAN4623 | mis-21 | MxH1519 | rfbA |
| 5819677–5819678 | MXAN4639 | mis-28 | MxH1526 | sgmS |
| 5822620–5822621 | MXAN4640 | mis-9 | MxH1507 | sgmT |
| 5901872–5901873 | MXAN4707 | mis-55 | MxH1551 | sgmU |
| 6413442–6413443 | MXAN5134 | mis-122 | MxH1612 | mutS |
| 6640018–6640019 | MXAN5333 | mis-24 | MxH1522 | sgmV |
| 6947398–6947399 | MXAN5592 | mis-42 | MxH1539 | sgmW |
| 7140653–7140654 | MXAN5766 | mis-100 | MxH1593 | sgmX |
| 7142162–7142163 | MXAN5766 | mis-13 | MxH1511 | sgmX |
| 7145304–7145305 | MXAN5770 | mis-73 | MxH1568 | sgmY |
| 7146533–7146534 | MXAN5772 | mis-97 | MxH1590 | pilQ |
| 7147173–7147174 | MXAN5772 | mis-63 | MxH1559 | pilQ |
| 7148427–7148428 | MXAN5772 | mis-50 | MxH1547 | pilQ |
| 7148895–7148896 | MXAN5772 | mis-29 | MxH1527 | pilQ |
| 7148895–7148896 | MXAN5772 | mis-30 | MxH1528 | pilQ |
| 7149164–7149165 | MXAN5773 | mis-103 | MxH1596 | pilP |
| 7149521–7149522 | MXAN5772 | mis-31 | MxH1529 | pilP |
| 7149933–7149934 | MXAN5774 | mis-78 | MxH1573 | pilO |
| 7149933–7149934 | MXAN5774 | mis-79 | MxH1574 | pilO |
| 7151318–7151319 | MXAN5776 | mis-39 | MxH1536 | pilM |
| 7151453–7151454 | MXAN5776 | mis-44 | MxH1541 | pilM |
| 7152059–7152060 | MXAN5776 | mis-118 | MxH1608 | pilM |
| 7152059–7152060 | MXAN5776 | mis-128 | MxH1615 | pilM |
| 7152136–7152137 | MXAN5776 | mis-94 | MxH1588 | pilM |
| 7152564–7152565 | MXAN5777 | mis-108 | MxH1601 | nla23 |
| 7152889–7152890 | MXAN5777 | mis-58 | MxH1554 | nla23 |
| 7152889–7152890 | MXAN5777 | mis-86 | MxH1581 | nla23 |
| 7152889–7152890 | MXAN5777 | mis-89 | MxH1584 | nla23 |
| 7156382–7156383 | MXAN5780 | mis-64 | MxH1560 | pilI |
| 7156382–7156383 | MXAN5780 | mis-133 | MxH1620 | pilI |
| 7156717–7156718 | MXAN5780 | mis-69 | MxH1564 | pilI |
| 7156717–7156718 | MXAN5780 | mis-104 | MxH1597 | pilI |
| 7157002–7157003 | MXAN5781 | mis-74 | MxH1569 | pilH |
| 7157880–7157881 | MXAN5782 | mis-144 | MxH1628 | pilG |
| 7157964–7157965 | MXAN5782 | mis-99 | MxH1592 | pilG |
| 7158021–7158022 | MXAN5782 | mis-76 | MxH1571 | pilG |
| 7158154–7158155 | MXAN5782 | mis-80 | MxH1575 | pilG |
| 7158204–7158205 | MXAN5782 | mis-33 | MxH1531 | pilG |
| 7158273–7158274 | MXAN5782 | mis-83 | MxH1578 | pilG |
| 7158288–7158289 | MXAN5782 | mis-4 | MxH1502 | pilG |
| 7158344–7158345 | MXAN5782 | mis-1 | MxH1500 | pilG |
| 7158429–7158430 | MXAN5782 | mis-106 | MxH1599 | pilG |
| 7158429–7158430 | MXAN5782 | mis-107 | MxH1600 | pilG |
| 7159294–7159295 | MXAN5783 | mis-91 | MxH1585 | pilA |
| 7159294–7159295 | MXAN5783 | mis-92 | MxH1586 | pilA |
| 7159632–7159633 | MXAN5783 | mis-84 | MxH1579 | pilAa |
| 7160010–7160011 | MXAN5784 | mis-132 | MxH1619 | pilR |
| 7162816–7162817 | MXAN5786 | mis-14 | MxH1512 | pilC |
| 7162902–7162903 | MXAN5786 | mis-32 | MxH1530 | pilC |
| 7163164–7163165 | MXAN5786 | mis-134 | MxH1621 | pilC |
| 7163494–7163495 | MXAN5786 | mis-11 | MxH1509 | pilC |
| 7163494–7163495 | MXAN5786 | mis-60 | MxH1556 | pilC |
| 7163494–7163495 | MXAN5786 | mis-123 | MxH1613 | pilC |
| 7164417–7164418 | MXAN5787 | mis-34 | MxH1532 | pilT |
| 7164529–7164530 | MXAN5787 | mis-82 | MxH1577 | pilT |
| 7164529–7164530 | MXAN5787 | mis-87 | MxH1582 | pilT |
| 7166434–7166435 | MXAN5788 | mis-36 | MxH1534 | pilB |
| 7231003–7231004 | MXAN5831 | mis-148 | MxH1631 | sgmZ; glgP |
| 7572031–7572032 | MXAN6125 | mis-109 | MxH1602 | sgnA |
| 8039216–8039217 | MXAN6518 | mis-40 | MxH1537 | sgnB |
| 8154610–8154611 | MXAN6627 | mis-52 | MxH1549 | sgnC |
| 8200210–8200211 | MXAN6671 | mis-38 | MxH1535 | sglK |
| 8200360–8200361 | MXAN6671 | mis-45 | MxH1542 | sglK |
| 8200465–8200466 | MXAN6671 | mis-48 | MxH1545 | sglK |
| 8200465–8200466 | MXAN6671 | mis-49 | MxH1546 | sglK |
| 8461333–8461334 | MXAN6908 | mis-35 | MxH1533 | sgnD; pgi |
| 8461447–8461448 | MXAN6908 | mis-71 | MxH1566 | sgnD; pgi |
| 8461447–8461448 | MXAN6908 | mis-93 | MxH1587 | sgnD; pgi |
| 8982735–8982736 | MXAN7360 | mis-59 | MxH1555 | sgnEa |
| 9039673–9039674 | MXAN7415 | mis-18 | MxH1516 | epsZ |
| 9039673–9039674 | MXAN7415 | mis-20 | MxH1518 | epsZ |
| 9039673–9039674 | MXAN7415 | mis-47 | MxH1544 | epsZ |
| 9040255–9040256 | MXAN7415 | mis-57 | MxH1553 | epsZ |
| 9040369–9040370 | MXAN7415 | mis-72 | MxH1567 | epsZ |
| 9042228–9042229 | MXAN7417 | mis-117 | MxH1607 | epsY |
| 9042228–9042229 | MXAN7417 | mis-136 | MxH1623 | epsY |
| 9042228–9042229 | MXAN7417 | mis-138 | MxH1624 | epsY |
| 9045962–9045963 | MXAN7421 | mis-62 | MxH1558 | epsV |
| 9046689–9046690 | MXAN7422 | mis-22 | MxH1520 | epsU |
| 9047313–9047314 | MXAN7422 | mis-46 | MxH1543 | epsU |
| 9063422–9063423 | MXAN7441 | mis-81 | MxH1576 | epsH |
| 9065683–9065684 | MXAN7442 | mis-6 | MxH1504 | sgnF |
| 9065986–9065987 | MXAN7442 | mis-27 | MxH1525 | sgnF |
| 9066004–9066005 | MXAN7442 | mis-67 | MxH1562 | sgnF |
| 9070550–9070551 | MXAN7445 | mis-25 | MxH1523 | epsE |
| 9071704–9071705 | MXAN7448 | mis-56 | MxH1552 | epsD |
| 9072040–9072041 | MXAN7448 | mis-53 | MxH1550 | epsD |
| 9072433–9072434 | MXAN7448 | mis-8 | MxH1506 | epsD |
| 9072508–9072509 | MXAN7448 | mis-16 | MxH1514 | epsD |
| 9072734–9072735 | MXAN7449 | mis-5 | MxH1503 | epsC |
| 9072805–9072806 | MXAN7449 | mis-12 | MxH1510 | epsC |
| 9072805–9072806 | MXAN7449 | mis-139 | MxH1625 | epsC |
| 9076128–9076129 | MXAN7451 | mis-98 | MxH1591 | epsA |
The 132 insertions of magellan-4 in the M. xanthus genome described in this report are listed in order of their sites within the genome sequence, which can be found at http://cmr.tigr.org/tigr-scripts/CMR/GenomePage.cgi?org=gmx. Given are the gene numbers in which the insertions are situated, the allele numbers of the insertions, the strain numbers of the derivatives of MxH1777 with each insertion, and the gene names.
An insertion lies upstream of the coding sequence indicated.
Isolation and phenotypic screening of potential social motility mutants:
Electroporation of MxH1777 cells with plasmid pMycoMar was performed as described (Youderian et al. 2003). Electroporation mixes were plated on CTPM Km agar and incubated for 5 days at 32°. After incubation, plates were screened visually to identify small colonies with smooth edges, the phenotype of A−S− double mutants. Mutants were purified twice, and the phenotypes of single colonies formed by each mutant were compared after each purification step. Colonies displaying reduced motility after the initial plating were chosen conservatively for purification, and, of these, 149/154 colonies displayed a stable A−S− phenotype. We found that one of the 149 mutants, MxH1777 mor-105, formed colonies with an unusual morphology; these colonies were smaller in diameter and more than twice the height of the colonies formed by the parent or other mutant strains and have a wrinkled appearance. Only 5/154 colonies chosen after the initial plating were found to form colonies with the same size as the parental (A−S+) strain after purification. Four of these were not considered in our subsequent analyses, but the fifth mutant was passed through our sequence analysis, because it had a colony morphology strikingly different from that of the parent and formed bright orange colonies. This mutant was designated MxH1777 mor-115 (for “morphology”). The alleles of magellan-4 insertions in all other mutants were designated mis (“mutation in S”) followed by their isolation number. Five of the 149 mutants, with alleles mis-121, mis-122, mis-141, mis-146, and mis-147, were found to form colonies with sizes intermediate between those formed by the parental strain and mutants with more severe motility defects (see results).
Cloning of M. xanthus genomic DNA flanking magellan-4 insertions in S genes:
To subclone magellan-4 insertions in S genes, M. xanthus genomic DNA was isolated from vegetative cultures of MxH1777 S∷magellan-4 strains, using the method for the rapid isolation of myxophage Mx8 DNA (Magrini et al. 1997). Each strain was grown to a density of 5 × 108/ml in CTPM Km medium, and 1 ml of cells were harvested by low speed centrifugation and resuspended in 0.4 ml distilled water. Buffer [50 μl of 2.0 m Tris–HCl (pH 8.0) and 0.1 m ethylenediamine tetraacetate (EDTA)], 2 μl diethylpyrocarbonate, and 10 μl 10% sodium dodecyl sulfate (SDS) were added. Mixtures were incubated at 75° for 5 min, and 50 μl of 5 m potassium acetate was added to precipitate SDS-protein complexes. After incubation at 4° for 2 hr, mixtures were microcentrifuged at 10,000 × g for 15 min. Supernatants were precipitated with 2.5 volume ethanol, and pellets were rinsed in 70% ethanol, 10 mm Tris–HCl (pH 8.0), 1 mm EDTA, and 10 mm MgCl2 (DNA wash solution) for 5 min. DNA pellets were air dried, resuspended in 200 μl 10 mm Tris–HCl (pH 8.0) and 0.1 mm EDTA, and stored routinely at −20°. Genomic DNA (2 μl) was cleaved with BssHI or NotI for 8–12 hr in a total volume of 12 μl, enzymes were inactivated by incubation at 75° for 15 min, and the mixtures were placed on a 0.025-μm filter (Millipore, Bedford, MA) and dialyzed against 1000 volume distilled water for 30 min. Cleaved DNA (8 μl) was treated with T4 DNA ligase in a total volume of 10 μl at 25° for 4–12 hr, incubated at 65° for 20 min, and drop dialyzed prior to electroporation into E. coli DH5α (λ pir). Approximately 1–100 KmR electroporants were obtained after electroporation of 1 μl of each ligated DNA and were recovered on LB Km plates after incubation at 37° for 24 hr. We were successful in cloning and sequencing the magellan-4 insertions present in 134/154 mutants. The MxH (M. xanthus Hartzell) strain names for mutants with mis or mor insertions are listed in Table 1.
Sequence analysis:
Plasmid DNAs with subcloned BssHII fragments were sequenced initially with primers Mar1 and Mar2 (Biosource, Camarillo, TX), complementary to the ends of magellan-4 (Youderian et al. 2003). In several cases, we extended these sequences with additional primers or by sequencing plasmids with NotI subclones from the same mutant. Sequences were assembled into contigs and then used in BLASTn searches (Altschul et al. 1990) against the DK1622 genome sequence to identify the TA target site for each magellan-4 insertion, given as the coordinates of the M. xanthus sequence (http://cmr.tigr.org/tigr-scripts/CMR/GenomePage.cgi?org=gmx) (Table 1). In all cases, these searches yielded a unique dinucleotide target site of insertion without accompanying deletion or rearrangement. Comparison of our sequences with the M. xanthus genome confirmed the identity of >50 kb of the assembled sequence (our unpublished results).
RESULTS
Genetic screen for magellan-4 insertions in S motility genes:
Transient cis-complementation of defective transposons (DNA elements flanked by transposon ends that are lacking an active transposase gene) by transposase has proven to be a powerful method for performing insertion mutagenesis with transposons. This is because stable insertions that do not undergo additional rounds of transposition are generated in the absence of the transposase gene (Hughes and Roth 1988).
Various strategies have been developed for the transient cis-complementation of defective transposons carried on either linear or circular DNA donor molecules, using transduction, conjugation, or transformation as mechanisms for their delivery. One such strategy involves the transformation of a recipient cell with a plasmid that cannot replicate in recipient cells and that expresses transposase in cis to a defective transposon carrying a selectable antibiotic-resistance determinant. Selection for antibiotic-resistant transformants enriches for recombinants in which the defective transposon has inserted into the target genome; their subsequent outgrowth results in the segregative loss of the donor plasmid molecule, giving rise to mutants with stable insertions.
Plasmid pMycoMar (Rubin et al. 1999) is one such plasmid donor for the transposition of the defective magellan-4 element. This minitransposon has the ends of a Himar element, a transposon in the mariner superfamily (Hartl et al. 1997) that moves from donor to recipient DNA molecules by a “cut and paste” mechanism, dependent only upon transposase (Lampe et al. 1996). These ends flank the npt gene from bacterial transposon Tn5, which confers resistance to kanamycin (KmR), as well as the R6Kγ origin of replication, which can function only when the Pir initiation protein is supplied in trans. Adjacent to the defective magellan-4 element, pMycoMar can express mariner transposase from the mycobacterial T6 promoter (Barsom and Hatfull 1996). The G + C-rich T6 promoter is active in Mycobacterium tuberculosis but inactive in E. coli (Rubin et al. 1999). We have shown that this promoter also functions well in M. xanthus (Youderian et al. 2003). In an E. coli host that expresses the Pir protein, pMycoMar replicates autonomously, but does not express transposase. In M. tuberculosis and M. xanthus, pMycoMar cannot replicate autonomously and expresses transposase. After electroporation of M. xanthus with pMycoMar, transient cis-complementation results in the transposition of magellan-4 into the target genome and the generation of KmR insertion mutants; the donor plasmid is lost upon cell division (Youderian et al. 2003).
Because magellan-4 has the R6Kγ origin of replication, it permits the rapid sequence analysis of insertion mutations. When the chromosomal DNA of a mutant with a magellan-4 insertion is restricted with an endonuclease that does not cleave within the element, ligated, and used to transform an E. coli pir+ recipient strain, KmR recombinants are recovered. Such recombinants carry plasmids with the magellan-4 element and regions of the target genome flanking the site of magellan-4 insertion. Sequences of the sites of each insertion in each plasmid can be determined by using primers internal to, and pointing outward from, the ends of transposed magellan-4 elements.
In another article, we described the use of pMycoMar to generate insertions in adventurous (A) motility genes in M. xanthus. We electroporated a strain of M. xanthus deficient in S motility with pMycoMar, screened for KmR recombinants that have a nonmotile colony morphology (resulting from an S–A∷magellan-4 genotype), and found such mutants with a frequency of ∼1% (115/12,000). We concluded from this result that magellan-4 has a much broader spectrum of targets in M. xanthus than transposon Tn5, because similar approaches with Tn5 yield only ∼0.2% mutants that form nonmotile colonies. This interpretation is supported by the results of sequence analysis, which enabled us to identify 34 different genes disrupted by 115 independent insertions of magellan-4. These 34 genes included the four genes previously known to be involved in A motility. Because 15 of the 30 new genes we identified in this search were represented only by single insertions of magellan-4, we concluded that we have yet to saturate the genes required for A motility using this transposon (Youderian et al. 2003).
In this report, we describe the results of the reciprocal experiment. We started with a mutant defective in A, but not in S, motility. We chose the ΔaglU-1777 mutant as our starting strain, because we have demonstrated that this allele, when paired with several different S mutant alleles, results in a nonmotile colony phenotype (White and Hartzell 2000). We electroporated this mutant with plasmid pMycoMar and screened for KmR recombinants that have a nonmotile colony morphology (resulting from a ΔaglU S∷magellan-4 genotype). Again, we find such mutants with a frequency of ∼1% (149/15,000), a frequency about fivefold higher than that obtained with Tn5 in similar mutagenesis experiments (Macneil et al. 1994a). These mutants were purified twice, to confirm that their defects in motility breed true, and grown in liquid CTPM medium (or on plates) to prepare genomic DNA for sequence analysis. Chromosomal DNA from nonmotile, ΔaglU S∷magellan-4 double mutants was purified, cleaved, and ligated and used to transform an E. coli recipient strain that produces the Pir protein; plasmids were prepared from KmR electroporants of E. coli and sequenced. As shown in Table 1, we determined the sequences of the sites of magellan-4 insertions in each of the plasmids derived from 132 of the 149 independent mutants. All of the insertions were found within the preferred TA dinucleotide target sequence duplicated by magellan-4 upon insertion. For the majority of these insertions, we determined that the TA target site had been duplicated by insertion, by sequencing both insertion junctions.
Insertions of magellan-4 in genes required for type IV pilin biogenesis:
Among the sequenced insertions of magellan-4 that affect S motility, 50 were found in one cluster of genes required for type IV pilin biosynthesis, located in a 27-kb region of the M. xanthus genome (Figure 1). These insertions are distributed among 40 different target sites, and insertions that occur at the same site are often found in opposite orientations. The most frequent target for insertion in this region was the pilG gene, in which 10 insertions were recovered at 9 different sites (with the pair of transposon insertions at the same site found in opposite orientations). These results confirm that the spectrum of insertions made by magellan-4 in M. xanthus is extremely broad and shows little preference in choosing AT target sites within the same gene.
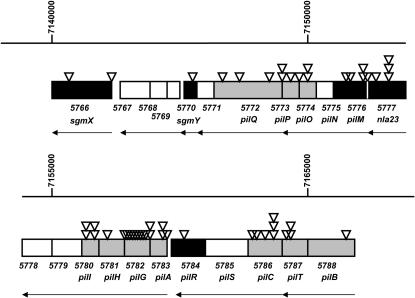
Figure 1.
magellan-4 insertions identify 15 different genes required for S motility in the M. xanthus pil gene cluster. The 27-kb region of the M. xanthus genome with the pilA (pilin) and adjacent genes (boxes) is shown; the directions of transcription are indicated by arrows. Coordinates and gene numbers are those of the M. xanthus genome sequence ( http://cmr.tigr.org/tigr-scripts/CMR/GenomePage.cgi?org=gmx). Positions of magellan-4 insertions are indicated by triangles. Shaded boxes indicate genes shown previously to be required for S motility; solid boxes indicate the new genes identified in this study. The top and bottom represent contiguous sequence.
As shown in Figure 1, The 23 predicted coding sequences in this region of the genome are transcribed in the same direction, most likely as at least five separate operons. These include the pilA gene (MXAN5783), which encodes pilin (Wu and Kaiser 1996); the pilG (MXAN5782), pilH (MXAN5781), and pilI (MXAN5780) genes (Wu et al. 1998); and the pilB (MXAN5788), pilT (MXAN5787), pilC (MXAN5786), and pilQ (MXAN5772) genes, each of which has been shown to be essential for S motility (Wu et al. 1997; Wall et al. 1999). Insertions of magellan-4 were obtained in each of these genes, as well as in pilP (MXAN5773), pilO (MXAN5774), pilM (MXAN5776), pilR (MXAN5784), and nla23(MXAN5777), predicted to encode a response regulator homologous to PilR (Wall et al. 1999; Caberoy et al. 2003). No insertions were found in the large pilS (MXAN5785) gene, consistent with the finding that it is not essential for S motility (Wall et al. 1999).
In addition to genes in this cluster known or suspected to be required for S motility, insertions were recovered in gene MXAN5770, immediately downstream from MXAN5771, which is not required for S motility (Wu and Kaiser 1996). MXAN5770 is predicted to encode a conserved hypothetical protein. Two different insertions were recovered in nearby gene MXAN5766, predicted to encode a product with tetratricopeptide (TPR) repeats (see below).
Insertions of magellan-4 in genes required for exopolysaccharide biosynthesis:
Among the independent magellan-4 insertions that disrupt S motility, 24 disrupt genes known to be required for exopolysaccharide (EPS) biosynthesis. Recently, Lu et al. (2005) identified a large group of genes involved in EPS biosynthesis, which also is required for S motility. Their strategy to identify these genes was to mutagenize M. xanthus by electroporation with a plasmid library made with a KmR vector that carries random, ∼500-bp inserts of M. xanthus DNA prepared by physical shearing. Because their plasmid vector cannot replicate in M. xanthus, KmR recombinants must arise due to the integration of the plasmid, which occurs most frequently by homologous recombination in this host, as in the yeast Saccharomyces cerevisiae. Thus, this strategy is equivalent to that of inactivating a yeast gene by the integration of a plasmid that carries an insert missing the 5′ and 3′ ends of a gene; integration of a plasmid results in a merodiploid in which the integrated vector is sandwiched between two incomplete copies of the gene, one missing its 5′ end and one missing its 3′ end, required for function (Neff et al. 1983).
Using this strategy, they screened among 5000 “integrative disruption” mutants and found 68 with reduced motility. Four of these 68 mutants are unable to form fruiting bodies, a phenotype of many S motility mutants. Also, they do not bind calcofluor white, which is an indicator dye for EPS production (Ramaswamy et al. 1997). The integrated plasmids in three of these four integrative disruption mutants were found to flank a 37-kb region of the M. xanthus genome, which they designated the “eps” region.
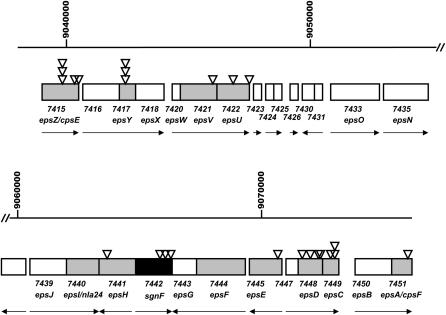
Sequence analysis shows that this region includes genes predicted to encode products homologous to those of the genes known to be involved in EPS biosynthesis in other bacteria. In-frame deletion and insertion mutations in 11 genes within this region impair social motility (Figure 2). These are: epsA (MXAN7451), epsC (MXAN7449), epsD (MXAN7448), epsE (MXAN7445), epsF (MXAN7444), epsH (MXAN7441), epsI/nla24 (MXAN7440), epsU (MXAN7422), epsV (MXAN7421), epsY (MXAN7417), and epsZ (MXAN7415) (Lu et al. 2005). As shown in Table 1, 24/131 of the independent magellan-4 insertions that disrupt S motility fall within 9 of these 11 genes. Three insertions interrupt the coding sequence MXAN7442, which lies between epsG and epsH and is predicted to encode a membrane protein of unknown function. The predicted products of these 12 genes fall into four functional categories. The majority (7/12) of these are sugar transferases presumably required for EPS assembly, two have response regulator domains (EpsF and EpsI), one is presumably involved in EPS export (EpsY), and two (EpsV and the product of MXAN7442) have as yet unknown functions. We did not recover magellan-4 insertions in the easA (MXAN2293) gene, which encodes an accessory protein essential for EPS biogenesis (Lu et al. 2005).
Figure 2.
magellan-4 insertions identify 10 different genes required for S motility in the M. xanthus eps gene cluster. The details are as in Figure 1; note that the central portion of the eps gene cluster, which does not contain genes required for S motility, is not shown.
Insertions of magellan-4 in genes required for LPS biosynthesis:
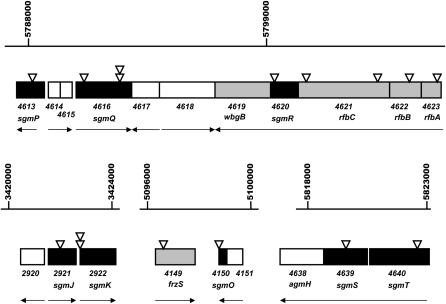
Among the independent magellan-4 insertions that disrupt S motility, four fall into a cluster of five adjacent genes. Four of these five genes, rfaA (MXAN4623), rfaB (MXAN4622), rfaC (MXAN4621), and wbgB (MXAN4619), are known to be required for LPS biosynthesis (Bowden and Kaplan 1998; Yang et al. 2000a) (Figure 3, top). Also, one magellan-4 insertion interrupts MXAN4620, predicted to encode a hypothetical protein with unknown function. Four magellan-4 insertions lie within the nearby genes MXAN4616, predicted to encode a glycosyl transferase, and MXAN4613, predicted to encode a second dTDP-glucose 4,6-dehydrogenase similar to that of the rfbB (MXAN4622) gene. These adjacent genes also are likely involved in the biosynthesis of LPS.
Figure 3.
magellan-4 insertions identify six different genes required for S motility in the M. xanthus rfb gene cluster. (Top) Shown are the positions of magellan-4 insertions in the rfb gene cluster, and adjacent genes, as in Figures 1 and 2. (Bottom) Three additional regions of the M. xanthus genome have adjacent genes with multiple insertions of magellan-4 that confer the loss of S motility. The sgmJ and sgmK genes define a new cluster of genes presumably required for LPS biogenesis, sgmO is adjacent to frzS, and the sgmT (histidine kinase/response regulator) and sgmS genes lie immediately upstream of agmH, required for A motility (Youderian et al. 2003). One or both of these genes may be required for both S and A motility.
Insertions of magellan-4 in additional genes known to be required for S motility:
Many additional genes have been characterized that encode products essential for S motility. These include the three genes known to be required for both S and A motility, mglA (MXAN1925), which is the second gene in an operon with its interacting partner, mglB (MXAN1926) (Hartzell and Kaiser 1991a,b; Hartzell 1997), agmA (MXAN3886), which encodes one of five different N-acetylmuramoyl l-alanine transamidases predicted to be made by M. xanthus (Youderian et al. 2003), and epsI/nla24 (MXAN7440), which encodes a response regulator essential for EPS biosynthesis (Caberoy et al. 2003; Li et al. 2003). Among our nonmotile derivatives of parent strain MxH1777, we recovered one mutant with an insertion of magellan-4 in the mglB gene; such mutations also were recovered in our reciprocal hunt for magellan-4 insertions that reduce the motility of a parental S− strain and are defective in A motility; these insertions have polar effects on the expression of mglA (Youderian et al. 2003). In the current hunt, we also recovered one magellan-4 insertion in the agmA gene, confirming that its function is required for both A and S motility (Youderian et al. 2003), but no insertions in the epsI/nla24 gene (Figure 2).
Genes identified previously that are essential for S motility also include tgl (MXAN3084), which encodes an outer membrane lipoprotein (Rodriguez-Soto and Kaiser 1997a,b) that can be transferred among cells upon contact (Nudleman et al. 2005); sglK (MXAN6671), which encodes a DnaK homolog essential for S motility (Weimer et al. 1998); frzS (MXAN4149), which encodes a cytoplasmic protein with a coiled-coil domain that can assemble into myosin-like lattices (Ward et al. 2000); nla1 (MXAN5853) and nla19 (MXAN1078), which encode two different response regulators (Caberoy et al. 2003); masK (MXAN1922), which encodes an essential tyrosine kinase (Thomasson et al. 2002); and three genes in the dif operon (MXAN6696–MXAN6691) encoding a methyl-accepting chemotaxis protein (difA, MXAN6696), a histidine kinase (difE, MXAN6692), and a CheW homolog (difC, MXAN6694) (Yang et al. 2000b; Bellenger et al. 2002). We recovered magellan-4 insertions in tgl, frzS, sglK, and nla23, but not in masK, nla1, nla19, difA, difC, or difE (Table 1).
Recently, Pham et al. (2005) showed that integrative disruption of the MXAN4150 (sgmO) gene, which they named rasA, results in defects in fibril production, S motility, and multicellular development. Our result that a magellan-4 insertion in this gene impairs S motility confirms their results independently. In addition, Kimura et al. (2004) found that the simultaneous disruption of the cotranscribed rppA (MXAN5907) and mmrA (MXAN5906) genes also results in an S motility defect. Therefore, we might predict that (polar) insertions of magellan-4 in the rppA gene would also disrupt S motility; however, we did not recover insertions in this possible target.
Insertions of magellan-4 in genes previously not known to be required for S motility:
Among the 132 magellan-4 insertions that we characterized, seven are from mutants that form colonies with distinct morphologies or display only partial S motility defects. One of these mutants, MxH1777 mor-115, forms bright orange colonies with a motility phenotype indistinguishable from that of the MxH1777 parent. Sequence analysis of this mutant shows that its magellan-4 insertion lies in the very 3′ end of the carR gene, which encodes a regulator of carotenoid biosynthetic gene expression, consistent with the observation that it overproduces the bright orange carotenoids made by M. xanthus (Hodgson 1993; Martinez-Laborda et al. 1990). A second morphology mutant forms colonies with a wrinkled appearance that are smaller than, and more than twice the height of, those formed by its parent. The insertion in this mutant, mor-105, lies within the stk (MXAN3474) gene, which encodes a DnaK homolog involved in cell-cell adhesion. Loss-of-function mutations in the stk gene suppress a subset of S motility defects (Dana and Shimkets 1993).
Five mutants were found to form colonies with sizes intermediate between those formed by their A− parent and its A−S− mutant derivatives. One of these carries an insertion in MXAN1641, predicted to encode a protein of unknown function. A second has an insertion in MXAN3840, predicted to encode a homolog of the ScpB protein, which regulates chromosome segregation in Gram-positive bacilli (Soppa et al. 2002). A third has an insertion in the M. xanthus homolog of the mutS gene (MXAN5134), required for mismatch repair. It is possible that defects in these three genes have pleiotropic effects on colony size and affect S motility indirectly by impairing central cell processes. In addition, we recovered two insertions in the cglB gene (MXAN3060), known to be involved in A motility, but not in S motility (Rodriguez and Spormann 1999), that confer partial motility defects. These results confirm that the ΔaglU cglB double mutants have a more severe defect than their ΔaglU parent (White and Hartzell 2000), consistent with our recent results that several other alleles in A motility genes, when paired with the ΔaglU mutation, result in more severe A motility defects (our unpublished results).
The remaining 41 insertions of magellan-4 confer profound defects in S motility; these likely affect the expression of genes whose products are directly involved in S motility. These insertions inactivate genes encoding proteins involved in central metabolic process, in both cytoplasmic and membrane proteins that may be components of the pilus motor, in regulators of transcription, and in polysaccharide metabolism. Three magellan-4 insertions inactivate genes MXAN0358, MXAN3759, and MXAN3797, predicted to encode central metabolic functions. The first of these genes encodes one of two isoleucine tRNA synthetases found on the M. xanthus genome, demonstrating that this ileS gene is not essential for growth. The effect of the magellan-4 insertion in this gene on S motility could be direct, but it is quite possible that this insertion affects the expression of the adjacent MXAN0359–MXAN0362 genes. These four genes are predicted to encode homologs of the Pseudomonas aeruginosa genes fimT, pilW, pilV, and pilY1, which contribute to the stability of type IV pili (Alm and Mattick 1995, 1997; Alm et al. 1996; Carbonnelle et al. 2005). The second of these is in a gene, MXAN3759, predicted to encode one of two propionyl-CoA carboxylases made by M. xanthus (Kimura et al. 1997). The third of these is in a gene, MXAN3797, predicted to encode a short-chain acyl-CoA dehydrogenase.
Fourteen magellan-4 insertions define 13 new genes predicted to encode proteins of unknown function (see Table 2). These include MXAN0440, predicted to encode an integral membrane exporter, and MXAN1795 and MXAN7360, predicted to encode lipoproteins; the insertion that affects MXAN7360 lies upstream of its potential start codons and likely inactivates its promoter. Three genes, MXAN1106, MXAN4639, and MXAN5766, are predicted to encode proteins with TPR motifs; the first among these is a protein that also has a DnaJ motif adjacent to its C-terminal TPR motif; the latter two have pairs of adjacent TPR motifs. These TPR repeat domain pairs compose a larger domain similar to that of the Pseudomonas aeruginosa PilF protein, which is required for the assembly of type IV pili (fimbrae) (Watson et al. 1996), a domain shared by the Tgl lipoprotein (Rodriguez-Soto and Kaiser 1997a,b). Four magellan-4 insertions define four new genes predicted to encode transcription regulators; three of these have response-regulator domains.
TABLE 2.
magellan-4 insertions identify 31 new genes required for S motility
| Gene | Name | Putative function |
|---|---|---|
| Central metabolic functions | ||
| MXAN0358 (1) | sgmA; ileS | Isoleucyl tRNA synthetase; downstream of fimT, pilW |
| MXAN3759 (1) | sgmM; pccB2 | Propionyl-CoA carboxylase |
| MXAN3797 (1) | sgmN | Short-chain acyl-CoA dehydrogenase |
| Cytoplasmic and membrane proteins of unknown function | ||
| MXAN0440 (1) | sgmB | Inner membrane protein; RND exporter |
| MXAN1106 (2) | sgmC | DnaJ/TPR repeat domain protein |
| MXAN1641 (1)a | sgmD | Hypothetical protein |
| MXAN1795 (1) | sgmE | Lipoprotein |
| MXAN2526 (1) | sgmH | Hypothetical protein |
| MXAN4150 (1) | sgmO | Conserved hypothetical protein; downstream of frzS |
| MXAN4620 (1) | sgmR | Hypothetical protein; downstream of rfbC; methyltransferase |
| MXAN4639 (1) | sgmS | TPR repeat protein; PilF domain |
| MXAN5766 (1) | sgmX | TPR repeat protein; PilF domain |
| MXAN5770 (1) | sgmY | Conserved hypothetical protein |
| MXAN6125 (1) | sgnA | Hypothetical protein; helicase? |
| MXAN6518 (1) | sgnB | Conserved hypothetical protein; ABC transporter |
| MXAN7360 (1) | sgnE | Lipoprotein |
| MXAN7442 (2) | sgnF | Membrane protein |
| Regulators of transcription | ||
| MXAN2128 (1) | sgmF | LysR family activator |
| MXAN4640 (1) | sgmT | Histidine kinase/response regulator |
| MXAN5592 (1) | sgmW | Response regulator |
| MXAN6627 (1) | sgnC | Response regulator |
| Polysaccharide metabolism | ||
| MXAN2203 (1) | sgmG | Asp box motif protein; glycosyl hydrolase? |
| MXAN2561 (1) | sgmI | Fibronectin type III domain protein; hydrolase? |
| MXAN2921 (1) | sgmJ; wbaZ | Glycosyl transferase group I domain |
| MXAN2922 (2) | sgmK | LPS biosynthesis; WcaJ C-terminal domain |
| MXAN3506 (4) | sgmL | Polysaccharide biosynthesis |
| MXAN4613 (1) | sgmP; rfbB2 | dTDP-glucose 4,6-dehydrogenase |
| MXAN4616 (3) | sgmQ | Glycosyl transferase group I and II domains |
| MXAN4707 (1) | sgmU; rfaF | Heptosyl transferase 9 domain |
| MXAN5333 (1) | sgmV; rfaG | Glycosyl transferase group I domains (2) |
| MXAN5831 (1) | sgmZ; glgP | Glycogen phosphorylase |
| MXAN6908 (3) | sgnD; pgi | Glucose-6-phosphate isomerase |
Genes are divided into four functional categories as shown and are numbered in accord with the M. xanthus genome sequence available at http://cmr.tigr.org/tigr-scripts/CMR/GenomePage.cgi?org=gmx. After each gene number, in parentheses is shown the number of magellan-4 insertions characterized in each gene; new genes involved in S motility were designated sgmA through sgnF; alternative suggestions for gene names are also listed. The predicted function of each gene is listed.
We have included MXAN1641 as an S gene although its direct role in motility has yet to be determined (see results).
Finally, the magellan-4 insertions define 12 new genes predicted to encode proteins involved in polysaccharide metabolism, 9 of which are likely critical for EPS biosynthesis, LPS biosynthesis, or both. Three magellan-4 insertions affect genes likely to encode products involved in polysaccharide catabolism; MXAN2203 is predicted to encode a glycosyl hydrolase with an Asp box motif (β hairpin), suggesting that its activity may be regulated by a complex phosphorelay system (Zhang and Shi 2005). The product of MXAN2561 has a fibronectin type III domain, found among several bacterial polysaccharide hydrolases (Folders et al. 2001; Kataeva et al. 2002). The glgP gene (MXAN5831) encodes glycogen phosphorylase.
DISCUSSION
Among 128 independent insertions of the defective transposon magellan-4 in genes required for S motility in M. xanthus, two-thirds lie within 27 of the 37 nonessential genes whose functions have been shown to be required for S motility. These include genes involved in the biogenesis of type IV pili, EPS and LPS. Our results show that the mechanism of S motility is more complex genetically than that of flagellar motility, which requires the products of <60 genes in Salmonella and E. coli (Chilcott and Hughes 2000). If we estimate that we have identified ∼75% of the nonessential genes involved in S motility by our mutant hunt, then we predict that there should be at least 80 nonessential genes whose products are required for S motility.
This number likely is an underestimate. Although the spectrum of magellan-4 insertions in S genes is very broad, it is not random and likely shows both site and regional preferences in M. xanthus, as does any other transposon and as is suggested by the results shown in Figures 1–3. Another indication that we have yet to reach saturation of the S motility genes with insertions of magellan-4 is that previously we found 12/115 nonmotile derivatives of an S mutant with insertions of magellan-4 in the mglBA genes (among 36 target genes) (Youderian et al. 2003), but only 1 of the 128 insertions in S motility genes described in this report falls within the mglBA operon (among 58 target genes). We note that some of the genes we have identified with insertions of magellan-4 may not be involved directly in S motility, due to polar and other effects that insertions may have on the expression of neighboring genes. This is certainly the case for insertions of magellan-4 in the mglB gene, which are polar in the expression of mglA (Youderian et al. 2003), and may also be the case for insertions in MXAN4620, upstream of wbgB (Figure 3, top), and in MXAN0358 (ileS), adjacent to homologs of the P. aeruginosa fimT, pilW, pilV, and pilY1 genes. These homologs may represent a new pil gene cluster.
Two of the new genes we have identified as social motility genes are likely involved in fatty acid metabolism. One of the magellan-4 insertions identifies a second M. xanthus gene, MXAN3759, predicted to encode a propionyl-CoA acyl carboxylase. A defect in its paralogue, pccB (MXAN1113), results in a reduction in the relative amount of long-chain fatty acids present in the membranes of vegetative and developing cells, but not in their abolition. Thus, although a pccB mutant can aggregate, it cannot form heat-resistant spores in response to carbon starvation (Kimura et al. 1997), a phenotype that can be masked partially by the addition of methylmalonyl-CoA to developing cells. The fact that a second propionyl-CoA acyl carboxylase isozyme also is required for social motility underscores the importance of long-chain fatty acids in the integrity of the M. xanthus membrane. The gene MXAN3797 is predicted to encode one of the >20 short-chain acyl-CoA dehydrogenases (SCADs) made by M. xanthus. Whether fatty acid metabolism plays a direct role in S motility or whether the magellan-4 insertions in MXAN3759 and MXAN3797 result in pleiotropic effects on membrane biogenesis remains to be determined.
magellan-4 insertions that impair S motility identify four new genes predicted to encode regulators of transcription. Three of these genes are predicted to encode products with response regulator domains. Among these, the product of one (MXAN4640) also is predicted to have a histidine kinase domain. This gene may be cotranscribed with MXAN4639, which also is required for S motility (Figure 3, bottom). The M. xanthus genome includes genes for 150 different proteins with response regulator domains. An earlier mutant hunt using integrative disruption to probe the functions of 27 of these genes showed that four are required for S motility (Caberoy et al. 2003). That we obtained magellan-4 insertions in only 2 of these 4 genes among the 27 again demonstrates that our magellan-4 insertions have not saturated the genes required for S motility. The fact that we obtained magellan-4 insertions in 3 additional genes among the 123 predicted to encode additional response regulators and that have yet to be tested for function shows that at least seven different response regulators are required for S motility. The systematic functional analysis of the 120 remaining genes predicted to encode response regulators may reveal additional genes required for S motility. We also obtained an insertion in gene MXAN2128, which encodes 1 of the 24 proteins in the LysR family of transcription regulators predicted to be encoded by genes present on the M. xanthus genome.
Among the genes of unknown function identified as social motility genes by magellan-4 insertions are three new genes predicted to encode proteins with TPR repeat motifs. We suspect that these proteins will play central roles in the mechanism of S motility for several reasons. The M. xanthus genome has >60 genes predicted to encode proteins with TPR repeat domains, at least three of which, agmK (MXAN4863), aglT (MXAN4869), and agmU (MXAN4870), are required for A motility (Youderian et al. 2003). The Tgl lipoprotein, which is transferred from cell to cell upon contact (Nudleman et al. 2005), also has a TPR repeat domain. Such domains are critical for the function of kinesin-dependent eukaryotic motors (Gindhart and Goldstein 1996). One or more of these proteins may play a structural role in the mechanism of pilus movement in M. xanthus.
During the mechanics of pilus extension and retraction, ATP is predicted to be hydrolyzed to energize the pilus motor, as is the case for the variety of motors that drive the mechanisms of eukaryotic motility involving the cycle of macromolecular sliding and/or polymerization/depolymerization. The analysis of S mutants suggests that the proteins involved in ATP hydrolysis may include not only PilT and PilB, the ATPases thought to be involved in pilus extrusion, but also the homologs of DnaJ and DnaK involved in S motility. Two of the M. xanthus genes predicted to encode >12 homologs of HSP70 (DnaK), sglK (Weimer et al. 1998; Yang et al. 1998), and stk (Dana and Shimkets 1993), play central roles in S motility. Also, one of the genes we have identified by magellan-4 insertions (MXAN1106) encodes a TPR repeat protein with a DnaJ domain.
Many of the new S motility genes that we have identified are involved in LPS and/or EPS polysaccharide biosynthesis. Three of these, MXAN4613, MXAN4616, and MXAN4620, are clustered near three genes previously shown to be involved in S motility (Figure 3, top); additional genes within this cluster are predicted to be involved in LPS production and may be required for S motility. The two adjacent genes MXAN2921 and MXAN2922 lie within an additional cluster of genes likely required for LPS biosynthesis and S motility (Figure 3, bottom). The role that LPS plays in S motility could be direct or indirect, because many bacteria regulate the balance between LPS and EPS biogenesis, and defects in LPS biosynthesis may cause dramatic changes in the structure or amount of EPS produced by cells. LPS mutants of M. xanthus produce more EPS antigen than do wild-type cells (Bowden and Kaplan 1998).
It is clear that EPS plays a direct role in the mechanism of S motility. Mutants defective in EPS production are hyperpiliated, a phenotype that can be rescued by the addition of EPS or chitin, a polymer of N-acetylglucosamine, but not by cellulose, a polymer of glucose (Li et al 2003). N-acetylglucosamine is a major component of M. xanthus EPS. The addition of chitin to wild-type M. xanthus cells results in pilus retraction. In contrast, the addition of N-acetylglucosamine to wild-type M. xanthus cells has the opposite effect and results in hyperpiliation. Glucose and galactose have no effect on pilus extension (Li et al. 2003). Our results suggest that the signals that govern pilus extension and retraction are the products of polysaccharide catabolism. The magellan-4 insertions identified three genes potentially involved in polysaccharide catabolism that are essential for S motility. These three genes include MXAN2561, predicted to encode a protein with a fibronectin type III domain, implicated in the hydrolysis of polysaccharides; MXAN2203, predicted to encode a glycosyl hydrolase; and, most important, the glgP gene (MXAN5831), predicted to encode glycogen phosphorylase.
Although social motility appears to be a mechanism conserved among diverse Gram-negative bacteria, very few genetic determinants of this mechanism are conserved in sequence among the bacteria that display social (or “twitching”) motility, M. xanthus and P. aeruginosa (Peabody et al. 2003). The gene encoding glycogen phosphorylase (EC 2.4.1.1), which cleaves polysaccharides with α(1 → 4) glucan linkages, is conserved among M. xanthus, P. aeruginosa, and Haemophilus influenzae, which has been shown recently to display S motility (Bakaletz et al. 2005). Glycogen phosphorylase is an economical enzyme that uses the cofactor PLP to carry out the phosphorylation of monosaccharides concomitant with their hydrolysis from polysaccharides, without consuming ATP to generate its monosaccharide phosphate products. We propose that the signal for the retraction of the M. xanthus pilus is likely N-acetylglucosamine phosphate, the predicted product of glycogen phosphorylase acting on chitin as a substrate. N-acetylglucosamine phosphate is an ideal signal for pilus retraction, because it can be cleaved to form N-acetylglucosamine by the phosphatases made by M. xanthus (Weinberg and Zusman 1990). Indeed, a mutation in the pph1 gene, encoding a Ser/Thr protein phosphatase, affects vegetative swarming on 0.3% agar but not on 1.5% agar (Treuner-Lange et al. 2001), a phenotype indicative of a defect in S motility. Currently, we are testing the hypothesis that glycogen phosphorylase generates N-acetylglucosamine phosphate from EPS as a signal for pilus retraction.
Acknowledgments
The authors thank Larry Shimkets for careful reading of the manuscript and helpful comments and Neal Burke for excellent technical assistance. This work was supported by grants MCB0242191 from the National Science Foundation to P.L.H. and GM59336 from the National Institutes of Health to P.Y.
References
- Alm, R. A., and J. S. Mattick, 1995. Identification of a gene, pilV, required for type-4 fimbrial biogenesis in Pseudomonas aeruginosa, whose product possesses a pre-pilin-like leader sequence. Mol. Microbiol. 16: 485–496. [DOI] [PubMed] [Google Scholar]
- Alm, R. A., and J. S. Mattick, 1997. Genes involved in the biogenesis and function of type-4 fimbriae in Pseudomonas aeruginosa. Gene 192: 89–98. [DOI] [PubMed] [Google Scholar]
- Alm, R. A., J. P. Hallinan, A. A. Watson and J. S. Mattick, 1996. Fimbrial biogenesis of Pseudomonas aeruginosa: pilW and pilX increase the similarity of type 4 fimbrae to the GSP protein-secretion systems and pilY1 encodes a gonococcal PilC homologue. Mol. Microbiol. 22: 161–173. [DOI] [PubMed] [Google Scholar]
- Altschul, S. F., W. Gish, W. Miller, E. W. Myers and D. J. Lipman, 1990. Basic local alignment search tool. J. Mol. Biol. 215: 403–410. [DOI] [PubMed] [Google Scholar]
- Bakaletz, L. O., B. D. Baker, J. A. Jurcisek, A. Harrison, L. A. Novotny et al., 2005. Demonstration of type IV pilus expression and a twitching phenotype by Haemophilus influenzae. Infect. Immun. 73: 1635–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsom, E. K., and G. F. Hatfull, 1996. Characterization of Mycobacterium smegmatis gene that confers resistance to phages L5 and D29 when overexpressed. Mol. Microbiol. 21:159–170. [DOI] [PubMed] [Google Scholar]
- Behmlander, R. M., and M. Dworkin, 1991. Extracellular fibrils and contact-mediated cell interactions in Myxococcus xanthus. J. Bacteriol. 173: 7810–7821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behmlander, R. M., and M. Dworkin, 1994. Integral proteins of the extracellular matrix fibrils of Myxococcus xanthus. J. Bacteriol. 176: 6304–6311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellenger, K., X. Ma, W. Shi and Z. Yang, 2002. A cheW homologue is required for Myxococcus xanthus fruiting body development, social gliding motility, and fibril biogenesis. J. Bacteriol. 184: 5654–5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden, M. G., and H. B. Kaplan, 1998. The Myxococcus xanthus lipopolysaccharide O-antigen is required for social motility and multicellular development. Mol. Microbiol. 30: 275–284. [DOI] [PubMed] [Google Scholar]
- Caberoy, N. B., R. D. Welch, J. S. Jakobsen, S. C. Slater and A. G. Garza, 2003. Global mutational analysis of NtrC-like activators in Myxococcus xanthus: identifying activator mutants defective for motility and fruiting body development. J. Bacteriol. 185: 6083–6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonnelle, E., S. Helaine, L. Prouvensier, X. Nassif and V. Pelicic, 2005. Type IV pilus biogenesis in Neisseria meningitidis: PilW is involved in a step occurring after pilus assembly, essential for fibre stability and function. Mol. Microbiol. 55: 54–64. [DOI] [PubMed] [Google Scholar]
- Chilcott, G. S., and K. T. Hughes, 2000. Coupling of flagellar gene expression to flagellar assembly in Salmonella enterica serovar typhimurium and Escherichia coli. Microbiol. Mol. Biol. Rev. 64: 694–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dana, J. R., and L. J. Shimkets, 1993. Regulation of cohesion-dependent cell interactions in Myxococcus xanthus. J. Bacteriol. 175: 3636–3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folders, J., J. Algra, M. S. Roelofs, L. C. Van Loon, J. Tommassen et al., 2001. Characterization of Pseudomonas aeruginosa chitinase, a gradually secreted protein. J. Bacteriol. 183: 7044–7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gindhart, Jr., J. G., and L. S. Goldstein, 1996. Tetratrico peptide repeats are present in the kinesin light chain. Trends Biochem. Sci. 21: 52–53. [PubMed] [Google Scholar]
- Hartl, D. L., A. R. Lohe and E. R. Lozovskaya, 1997. Modern thoughts on an ancyent marinere: function, evolution, regulation. Annu. Rev. Genet. 31: 337–358. [DOI] [PubMed] [Google Scholar]
- Hartzell, P. L., 1997. Complementation of Myxococcus xanthus sporulation and motility defects by a eukaryotic RAS homolog. Proc. Natl. Acad. Sci. USA 97: 9881–9886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell, P. L., and D. Kaiser, 1991. a Function of MglA, a 22-kilodalton protein essential for gliding in Myxococcus xanthus. J. Bacteriol. 173: 7615–7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell, P. L., and D. Kaiser, 1991. b Upstream gene of the mgl operon controls the level of MglA protein in M. xanthus. J. Bacteriol. 173: 7625–7635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin, J., and D. Kaiser, 1977. Cell-to-cell stimulation of movements in non-motile mutants of Myxococcus xanthus. Proc. Natl. Acad. Sci. USA 74: 2938–2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin, J., and D. Kaiser, 1979. a Genetics of gliding motility in Myxococcus xanthus (Myxobacterales): genes controlling movement of single cells. Mol. Gen. Genet. 171: 167–171. [Google Scholar]
- Hodgkin, J., and D. Kaiser, 1979. b Genetics of gliding motility in Myxococcus xanthus (Myxobactererales): two gene systems control movement. Mol. Gen. Genet. 171: 177–191. [Google Scholar]
- Hodgson, D. A., 1993. Light-induced carotenogenesis in Myxococcus xanthus: genetic analysis of the carR region. Mol. Microbiol. 7: 471–488. [DOI] [PubMed] [Google Scholar]
- Hughes, K. T., and J. R. Roth, 1988. Transitory cis complementation: a method for providing transposition functions to defective transposons. Genetics 119: 9–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashefi, K., and P. L. Hartzell, 1995. Genetic suppression and phenotypic masking of a Myxococcus xanthus frzF- defect. Mol. Microbiol. 15: 483–494. [DOI] [PubMed] [Google Scholar]
- Kataeva, I. A., R. D. Seidel, III, A. Shah, L. T. West, X. L. Li and L. G. Ljungdahl, 2002. The fibronectin type 3-like repeat from the Clostridium thermocellum cellobiohydrolase CbhA promotes hydrolysis of cellulose by modifying its surface. Appl. Environ. Microbiol. 68: 4292–4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura, Y., R. Sato, K. Mimura and M. Sato, 1997. Propionyl coenzyme A carboxylase is required for development of Myxococcus xanthus. J. Bacteriol. 179: 7098–7102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura, Y., S. Ishida, H. Matoba and N. Okahisa, 2004. RppA, a transducer homologue, and MmrA, a multidrug transporter homologue, are involved in the biogenesis and/or assembly of polysaccharide in Myxococcus xanthus. Microbiology 150: 631–639. [DOI] [PubMed] [Google Scholar]
- Lampe, D. J., M. E. Churchill and H. M. Robertson, 1996. A purified mariner transposase is sufficient to mediate transposition in vitro. EMBO J. 15: 5470–5479. [PMC free article] [PubMed] [Google Scholar]
- Lancero, H., N. B. Caberoy, S. Castaneda, Y. Li, A. Lu et al., 2004. Characterization of a Myxococcus xanthus mutant that is defective for adventurous motility and social motility. Microbiology 150: 4085–4093. [DOI] [PubMed] [Google Scholar]
- Li, Y., H. Sun, X. Ma, A. Lu, R. Lux et al., 2003. Extracellular polysaccharides mediate pilus retraction during social motility of Myxococcus xanthus. Proc. Natl. Acad. Sci. USA 100: 5443–5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, A., K. Cho, W. P. Black, X. Y. Duan, R. Lux et al., 2005. Exopolysaccharide biosynthesis genes required for social motility in Myxococcus xanthus. Mol. Microbiol. 55: 206–220. [DOI] [PubMed] [Google Scholar]
- Macneil, S. D., F. Calara and P. L. Hartzell, 1994. a New clusters of genes required for gliding motility in Myxococcus xanthus. Mol. Microbiol. 14: 61–71. [DOI] [PubMed] [Google Scholar]
- Macneil, S. D., A. Mouzeyan and P. L. Hartzell, 1994. b Genes required for both gliding motility and development in Myxococcus xanthus. Mol. Microbiol. 14: 785–795. [DOI] [PubMed] [Google Scholar]
- Magrini, V., D. Salmi, D. Thomas, S. K. Herbert, P. L. Hartzell et al., 1997. Temperate Myxococcus xanthus phage Mx8 encodes a DNA adenine methylase. Mox. J. Bacteriol. 179: 4254–4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Laborda, A., J. M. Balsalobre, M. Fontes and F. J. Murillo, 1990. Accumulation of carotenoids in structural and regulatory mutants of the bacterium Myxococcus xanthus. Mol. Gen. Genet. 223: 205–210. [DOI] [PubMed] [Google Scholar]
- Mattick, J. S., 2002. Type IV pili and twitching motility. Annu. Rev. Microbiol. 56: 289–314. [DOI] [PubMed] [Google Scholar]
- Merz, A. J., M. So and M. P. Sheetz, 2000. Pilus retraction powers bacterial twitching motility. Nature 407: 98–102. [DOI] [PubMed] [Google Scholar]
- Neff, N. F., J. H. Thomas, P. Grisafi and D. Botstein, 1983. Isolation of the beta-tubulin gene from yeast and demonstration of its essential function in vivo. Cell 33: 211–219. [DOI] [PubMed] [Google Scholar]
- Nudleman, E., D. Wall and D. Kaiser, 2005. Cell-to-cell transfer of bacterial outer membrane lipoproteins. Science. 309: 125–127. [DOI] [PubMed] [Google Scholar]
- Peabody, C. R., Y. J. Chung, M. R. Yen, D. Vidal-Ingigliardi, A. P. Pugsley et al., 2003. Type II protein secretion and its relationship to bacterial type IV pili and archaeal flagella. Microbiology 149: 3051–3072. [DOI] [PubMed] [Google Scholar]
- Pham, V. D., C. W. Shebelut, B. Mukherjee and M. Singer, 2005. RasA is required for Myxococcus xanthus development and social motility. J. Bacteriol. 187: 6845–6848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswamy, S., M. Dworkin and J. Downard, 1997. Identification and characterization of Myxococcus xanthus mutants deficient in calcofluor white binding. J. Bacteriol. 179: 2863–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez, A. M., and A. M. Spormann, 1999. Genetic and molecular analysis of cglB, a gene essential for single-cell gliding in Myxococcus xanthus. J. Bacteriol. 181: 4381–4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Soto, J. P., and D. Kaiser, 1997. a The tgl gene: social motility and stimulation in Myxococcus xanthus. J. Bacteriol. 179: 4361–4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Soto, J. P., and D. Kaiser, 1997. b Identification and localization of the Tgl protein, which is required for Myxococcus xanthus social motility. J. Bacteriol. 179: 4372–4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin, E. J., B. J. Akerley, V. N. Novik, D. J. Lampe, R. N. Husson et al., 1999. In vivo transposition of mariner-based elements in enteric bacteria and mycobacteria. Proc. Natl. Acad. Sci. USA 96: 1645–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skerker, J. M., and H. C. Berg, 2001. Direct observation of extension and retraction of type IV pili. Proc. Natl. Acad. Sci. USA 98: 6901–6904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soppa, J., K. Kobayashi, M. F. Noirot-Gros, D. Oesterhelt, S. D. Ehrlich et al., 2002. Discovery of two novel families of proteins that are proposed to interact with prokaryotic SMC proteins, and characterization of the Bacillus subtilis family members ScpA and ScpB. Mol. Microbiol. 45: 59–71. [DOI] [PubMed] [Google Scholar]
- Stephens, K., and D. Kaiser, 1987. Genetics of gliding in Myxococcus xanthus: molecular cloning of the mgl locus. Mol. Gen. Genet. 207: 256–266. [Google Scholar]
- Stephens, K., P. L. Hartzell and D. Kaiser, 1989. Gliding motility in Myxococcus xanthus: mgl locus, RNA, and predicted protein products. J. Bacteriol. 171: 819–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, H., D. R. Zusman and W. Shi, 2000. Type IV pilus of Myxococcus xanthus is a motility apparatus controlled by the frz chemosensory system. Curr. Biol. 10: 1143–1146. [DOI] [PubMed] [Google Scholar]
- Thomasson, B., J. Link, A. G. Stassinopoulos, N. Burke, L. Plamann et al., 2002. The GTPase, MglA, interacts with a tyrosine kinase to control type-IV pili-mediated motility of Myxococcus xanthus. Mol. Microbiol. 46: 1399–1413. [DOI] [PubMed] [Google Scholar]
- Treuner-Lange, A., M. J. Ward and D. Zusman, 2001. Pph1 from Myxococcus xanthus is a protein phosphatase involved in vegetative growth and development. Mol. Microbiol. 40: 126–140. [DOI] [PubMed] [Google Scholar]
- Wall, D., P. E. Kolenbrander and D. Kaiser, 1999. The Myxococcus xanthus pilQ (sglA) gene encodes a secretin homolog required for type IV pilus biogenesis, social motility and development. J. Bacteriol. 181: 24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward, M. J., H. Lew and D. R. Zusman, 2000. Social motility in Myxococcus xanthus requires FrzS, a protein with an extensive coiled-coli domain. Mol. Microbiol. 37: 1357–1371. [DOI] [PubMed] [Google Scholar]
- Watson, A. A., R. A. Alm and J. S. Mattick, 1996. Identification of a gene, pilF, required for type 4 fimbrial biogenesis and twitching motility in Pseudomonas aeruginosa. Gene 180: 49–56. [DOI] [PubMed] [Google Scholar]
- Weimer, R.M., C. Creighton, A. Stassinopoulos, P. Youderian and P. L. Hartzell, 1998. A chaperone in the HSP70 family controls production of extracellular fibrils in Myxococcus xanthus. J. Bacteriol. 180: 5357–5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg, R. A., and D. R. Zusman, 1990. Alkaline, acid, and neutral phosphatase activities are induced during development in Myxococcus xanthus. J. Bacteriol. 172: 2294–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, D. J., and P. L. Hartzell, 2000. AglU, a protein required for gliding motility and spore maturation of Myxococcus xanthus, is related to WD-repeat proteins. Mol. Microbiol. 36: 662–678. [DOI] [PubMed] [Google Scholar]
- Wolgemuth, C., E. Hoiczyk, D. Kaiser and G. Oster, 2002. How myxobacteria glide. Curr. Biol. 12: 869–877. [DOI] [PubMed] [Google Scholar]
- Wu, S. S., and D. Kaiser, 1995. Genetic and functional evidence that type IV pili are required for social gliding motility in Myxococcus xanthus. Mol. Microbiol. 18: 547–558. [DOI] [PubMed] [Google Scholar]
- Wu, S. S., and D. Kaiser, 1996. Markerless deletions of pil genes in Myxococcus xanthus generated by counterselection with the Bacillus subtilis sacB gene. J. Bacteriol. 178: 5817–5821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, S. S., J. Wu and D. Kaiser, 1997. The Myxococcus xanthus pilT locus is required for social gliding motility although pili are still produced. Mol. Microbiol. 23: 109–121. [DOI] [PubMed] [Google Scholar]
- Wu, S. S., J. Wu, Y. L. Cheng and D. Kaiser, 1998. The pilH gene encodes an ABC transporter homologue required for type IV pilus biogenesis and social gliding motility in Myxococcus xanthus. Mol. Microbiol. 29: 1249–1261. [DOI] [PubMed] [Google Scholar]
- Yang, Z., Y. Geng and W. Shi, 1998. A DnaK homolog in Myxococcus xanthus is involved in social motility and fruiting body development. J. Bacteriol. 180: 218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Z., X. Ma, L. Tong, H. B. Kaplan, L. J. Shimkets et al., 2000. a Myxococcus xanthus dif genes are required for biogenesis of cell surface fibrils essential for social gliding motility. J. Bacteriol. 182: 5793–5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Z., D. Guo, M. G. Bowden, H. Sun, L. Tong et al., 2000. b The Myxococcus xanthus wgbB gene encodes a glycosyltransferase homologue required for lipopolysaccharide O-antigen biogenesis. Arch. Microbiol. 174: 399–405. [DOI] [PubMed] [Google Scholar]
- Youderian, P., N. Burke, D. J. White and P. L. Hartzell, 2003. Identification of genes required for adventurous gliding motility in Myxococcus xanthus with the transposable element mariner. Mol. Microbiol. 49: 555–570. [DOI] [PubMed] [Google Scholar]
- Zhang, W., and L. Shi, 2005. Distribution and evolution of multiple-step phosphorelay in prokaryotes: lateral domain recruitment involved in the formation of hybrid-type histidine kinases. Microbiology 151: 2159–2173. [DOI] [PubMed] [Google Scholar]