Abstract
The Stubble-stubbloid (Sb-sbd) gene is required for ecdysone-regulated epithelial morphogenesis of imaginal tissues during Drosophila metamorphosis. Mutations in Sb-sbd are associated with defects in apical cell shape changes critical for the evagination of the leg imaginal disc and with defects in assembly and extension of parallel actin bundles in growing mechanosensory bristles. The Sb-sbd gene encodes a type II transmembrane serine protease (TTSP). Here we use a Sb-sbd transgenic construct to rescue both bristle and leg morphogenesis defects in Sb-sbd mutations. Molecular characterization of Sb-sbd mutations and rescue experiments with wild-type and modified Sb-sbd transgenic constructs show that the protease domain is required for both leg and bristle functions. Truncated proteins that express the noncatalytic domains without the protease have dominant effects in bristles but not in legs. Leg morphogenesis, but not bristle growth, is sensitive to Sb-sbd overexpression. Antibody localization of the Sb-sbd protein shows apical expression in elongating legs. Sb-sbd protein is found in the base and shaft in budding bristles and then concentrates at the growing tip when bristles are elongating rapidly. We propose a model whereby Sb-sbd helps coordinate proteolytic modification of extracellular matrix attachments with cytoskeletal changes in both legs and bristles.
ELABORATE changes in the dimensions and topology of epithelial sheets are required for the normal development of multicellular organisms; developmental events as basic as gastrulation and as specialized as the formation of the stereocilia of the mammalian inner ear are examples of epithelial morphogenesis. Cell division and death, cell rearrangement, and cell-shape change all contribute to different types of epithelial morphogenesis (reviewed in Fristrom 1988). These cell behaviors depend in part on changes in the cytoskeleton but occur in the context of neighboring cells, extracellular matrices (ECM), and hormonal milieus. Drosophila imaginal discs provide an attractive experimental system to study the complex interrelationships of the cytoskeleton, ECM, cell junctions, and extracellular signals during epithelial morphogenesis in a genetically tractable model organism.
During metamorphosis in Drosophila, the adult epidermis is pieced together from a collection of anlagen, the imaginal (adult) discs. Imaginal discs are simple, folded epithelial sacs which, in response to the metamorphic steroid hormone 20-hydroxyedcysone (ecdysone), undergo rapid and radical tissue reorganization to form specific structures of the adult integument. The thoracic imaginal discs give rise to the adult thoracic appendages (legs, wings, and halteres); their proximal parts fuse to form the epidermis of the thorax. The initial transformation from folded, undifferentiated imaginal discs to appendages with the basic shape of the adult structures takes place in the prepupal period, the first 12 hr after pupariation (AP). Following the ecdysone-triggered transition to the 84-hr pupal period, the appendage morphology is further refined, bristles and hairs form, and finally the adult cuticle is deposited.
Significant progress has been made in understanding how the ecdysone receptor and its partner ultraspirical interact with nuclear receptor cofactors and ecdysone-induced transcription factors to confer temporal and tissue specificity onto signals from this single hormone (reviewed in Thummel 1997, 2002). Less is known about products of the effector genes, molecules that have a direct function in cell and tissue morphogenesis. Genetic interaction screens, pioneered in our laboratory, have identified some of the genes that act in imaginal disc morphogenesis (Beaton et al. 1988; Gotwals and Fristrom 1991; Clark et al. 1995; Edwards and Kiehart 1996; Gates and Thummel 2000; Bayer et al. 2003; Ward et al. 2003; Chen et al. 2004). A role for the Stubble-stubbloid (Sb-sbd) gene in leg and wing morphogenesis was discovered in the first of these studies (Beaton et al. 1988).
Sb-sbd transcription is induced by ecdysone and is required both in prepupae, for the initial elongation of the leg disc to form a tubular leg, and in pupae (32 hr AP), for the apical extension of a single cell to form the mechanosensory bristle shaft (Appel et al. 1993). Bristle phenotypes are distinct in dominant (Sb) and recessive (sbd) mutations. Defects in leg morphogenesis are recessive in all Sb-sbd mutants (Table 1).
TABLE 1.
Leg and bristle phenotypes of Sb-sbd mutant alleles
| Phenotype
|
||||
|---|---|---|---|---|
| Allele/genotype | Bristle | Leg | Origin | Reference |
| Sb63b | Transposon | Appel et al. (1993) | ||
| Sb63b/+ | <1/2 wt length, thicker than wt, ends blunt | wt | Beaton et al. (1988) | |
| Sb63b/Sb63b | <1/4 wt length, thicker than wt, ends blunt | mlf | Beaton et al. (1988) | |
| Sb63b/Df (3R)sbd105 | <1/4 wt length, thicker than wt, ends blunt | mlf | Beaton et al. (1988) | |
| Sb1 | Transposon | Appel et al. (1993) | ||
| Sb1/+ | ∼1/2 wt length, thicker than wt, ends blunt | wt | Beaton et al. (1988) | |
| Sb1/Sb1 | (Lethal) | Dobzhansky (1929) | ||
| Sb1/Df (3R)sbd105 | (Lethal) | Beaton et al. (1988) | ||
| Sb1/Sb63b | <1/4 wt length, thicker than wt, ends blunt | mlf | Beaton et al. (1988) | |
| Sbspike | X ray | Moore (1935) | ||
| Sbspi/+ | <2/3 wt length, ends blunt | wt | Beaton et al. (1988) | |
| Sbspi/Sbspi | ∼1/4 wt length, ends blunt | mlf | R. Abu-Shumays and D. Fristrom (unpublished results) | |
| Sbspi/Df(3R)sbd105 | ∼1/4 wt length, ends blunt | mlf | Beaton et al. (1988) | |
| Sb70 | Transposon | Appel et al. (1993) | ||
| Sb70/+ | <1/2 wt length, thicker than wt, ends blunt | wt | Beaton et al. (1988) | |
| Sb70/Sb70 | <1/4 wt length, thicker than wt, ends blunt | mlf | Beaton et al. (1988) | |
| Sb70/Df(3R)sbd105 | <1/4 wt length, thicker than wt, ends blunt | mlf | Beaton et al. (1988) | |
| sbd2 | Spontaneous | Lindsley and Grell (1968) | ||
| sbd2/+ | wt | wt | Beaton et al. (1988) | |
| sbd2/sbd2 | ∼3/4 wt length, ends uneven | wt | Beaton et al. (1988) | |
| sbd2/Df(3R)sbd105 | ∼3/4 wt length, ends uneven | wt | Beaton et al. (1988) | |
| sbd201 | EMS | Beaton et al. (1988) | ||
| sbd201/+ | wt | wt | Beaton et al. (1988) | |
| sbd201/sbd201 | <1/2 wt length, ends uneven | mlf | Beaton et al. (1988) | |
| sbd201/Df(3R)sbd105 | <1/2 wt length, ends uneven | mlf | Beaton et al. (1988) | |
| Df(3R)sbd105 | X ray | Lewis (1948) | ||
| Df(3R)sbd105/wt | wt | wt | Beaton et al. (1988) | |
| Df(3R)sbd105/Df(3R)sbd105 | (Lethal) | Beaton et al. (1988) | ||
wt, wild type.
In prepupae the leg disc telescopes out of the concentrically folded epithelium to form a cylinder and everts to the outside of the developing imago. This change in tissue shape results primarily from changes in cell shape (Condic et al. 1991; Fristrom and Fristrom 1993). At the end of the third instar, cells that will form the basitarsis and distal tibia maintain an anisometric shape with the proximal-distal axis compressed and the circumferential axis elongated. By 6 hr AP, the leg has become tubular and the elongated cells have become isometric, the change in cell shape, longer in the proximal-distal direction and narrower in width, mediating the change in tissue shape. In Sb-sbd mutants, these cell-shape changes are limited and the legs of the adult exhibit the malformed (mlf) phenotype with leg segments that are short, thick, and often kinked or gnarled (see Beaton et al. 1988, Figure 1).
Figure 1.
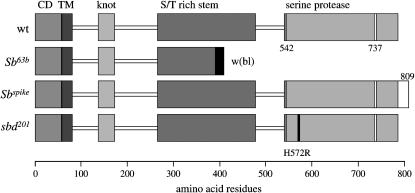
Sb-sbd genomic structure. Sizes of exons and introns are represented to scale and indicated in kilobases at the bottom. Exons are shown as solid bars with exon number below. Features from the cDNA (5′- and 3′-UTR, ATG translation start) and amino acid sequence are indicated above the exons in which they are found. The protease domain is split among the last four exons. Each of the amino acids forming the catalytic triad (H, D, S) is encoded in a separate exon, indicated by marks above the appropriate exon. The transmembrane signal/anchor (TMD), disulfide knot (knot), and the first part of the stem are in exon 4. See text and Figure 2 for further description of protein domains. Four polymorphisms that result in a change in amino acid sequence are found in exons 6 and 7 and are indicated by marks above the line. In exon 6, an in-frame deletion of CAG in the Oregon-R strain reduces a string of seven glutamines to six between amino acids 287 and 293. An A-to-T change at nucleotide 1910 (cDNA numbering) results in a serine-for-threonine substitution at amino acid 349 within a serine/threonine (S/T)-rich region. At nucleotide 1924, a C-to-T change replaces a proline with a serine at amino acid 354. In exon 7, a third wild-type strain (the progenitor for the sbd201 mutant) deletes nine nucleotides inframe, resulting in the deletion of one unit of a tandem repeat of threonine, threonine, serine (TTS), between amino acids 410 and 416 in an S/T-rich region. Nucleotide differences between the Oregon-R and y; cn bw sp strains that produce no amino acid changes are found in exon 4 (nt 1150), exon 6 (nt1859), and exon 10 (nt 2198) and are indicated by marks below the exons.
In pupae, each bristle develops as an apical cytoplasmic extension of a tricogen cell, one of the four cells that make up the mechanosensory bristle organ (Lees and Waddington 1942; Lees and Picken 1945; Hartenstein and Posakony 1989; Tilney et al. 1995). Within the extending bristle cell, a scaffold is formed by a core of microtubules surrounded by modules of membrane-associated actin microfilament bundles joined end to end (Overton 1967; Appel et al. 1993; Tilney et al. 1995, 1996, 1998; Wulfkuhle et al. 1998). The bristle grows as individual actin filaments continuously form at the bristle tip on the cytoplasmic side of the plasma membrane and are gathered together to form progressively larger and more tightly crosslinked bundles by the sequential action of two actin-binding proteins, forked and singed (Lees and Picken 1945; Tilney et al. 1996). Once the bristle is fully elongated, a chitinous cuticle is secreted and the actin bundle scaffolding is dismantled (Tilney et al. 1996, 2003; Guild et al. 2002). Bristles of Sb mutants are short, thick, and blunt ended. In these developing bristles the number of actin bundles is increased and in the most severe alleles, the bundles are also disorganized, with some bundles in the center of the bristle rather than regularly distributed around the cell perimeter (Lees and Picken 1945; Appel et al. 1993). Developing bristles in recessive sbd mutants start out with the normal number and distribution of actin bundles, but the bundles become deranged at the tip and stop prematurely and asynchronously (Appel et al. 1993). Reflecting this developmental defect, bristles of the homozygous sbd adults are short but with slightly tapered and frayed ends (see Appel et al. 1993, Figure 5).
Figure 5.
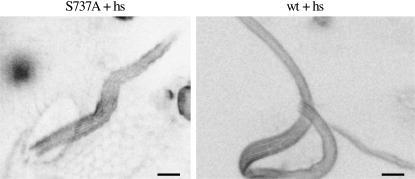
Sb-like bristle generated by expression of the S737A transgene. Confocal image (negative image) of phalloidin-stained bristles dissected from pupae at 37 hr AP following heat shocks at 25, 27, 29, and 31 hr AP. (Left) An Sb-like bristle from an S737A transgene-carrying pupa [S737A/S737A; Df(3R)sbd105/+]. (Right) A normal bristle from a wild-type control pupa (wt) also subjected to the same heat-shock regimen. The tip of the much longer wild-type bristle cannot be seen in this view. The entire S737A bristle is shown. Bars, 5 μm.
The Sb-sbd protein (which for convenience we will refer to as stubblin) is the first identified invertebrate member of the type II transmembrane serine proteases (TTSP), each containing a C-terminal extracellular serine protease domain, a short N-terminal intracellular domain, and a variety of structural motifs connecting the transmembrane domain with the catalytic domain (Hooper et al. 2001; Netzel-Arnett et al. 2003). The modular structure of these proteases suggests a possible role linking proteolysis of the ECM to cytoskeletal rearrangements. Like other members of the TTSP family, the predicted 786-aa stubblin (Figure 2) includes an N-terminal cytoplasmic domain, followed by a transmembrane signal/anchor domain and an extracellular stem region with a C-terminal serine protease domain (Appel et al. 1993; Hooper et al. 2001). The 244-aa protease domain shares extensive sequence similarity to the S1 family (clan SA) of trypsin-like proteases (Furie and Furie 1988; Rawlings et al. 2004) in the conserved regions of the substrate-binding pocket, cleavage activation site, and catalytic triad, indicating a preference for cleavage after arginine or lysine. Commonly, these proteases are zymogens that are activated by cleavage at a characteristic activation site motif. In some proteases with a long prodomain, the noncatalytic domain remains tethered via a disulfide bond to the catalytic domain after cleavage (Rawlings et al. 2004). In stubblin, Cys-531 and Cys-659 are appropriately placed to provide this function. In the extracellular region just beyond the transmembrane domain, a conserved pattern of three pairs of cysteines defines a disuflide knot domain (or CLIP domain) like that found in several other arthropod proteases, including Limulus proclotting enzyme and the Drosophila snake and easter proteins, both secreted proteases involved in embryonic dorsal/ventral patterning (Gay and Keith 1992; Smith and DeLotto 1992; Muta et al. 1993; Jiang and Kanost 2000). The disulfide knot has been proposed to act as a binding site for a protease activator (Muta et al. 1990). For successful transgenic rescue of snake mutants, the snake transgenic construct must include an intact knot domain (Smith et al. 1994). A long serine/threonine-rich stem (aa 260–480) connects the stubblin knot and the protease. A similar, although shorter, region is essential for snake function (Smith et al. 1994).
Figure 2.
Sequence changes in Sb-sbd mutants. Amino acid residue number is shown in the scale at the bottom. The top line shows protein domains of wild-type stubblin (wt): CD (cytoplasmic domain) aa 1–58, TM (transmembrane domain) aa 59–81, knot (disulfide-knotted domain) aa 138–173, S/T-rich (serine and threonine rich region of the stem) aa 260–480, and serine protease from activation cleavage site at aa 542 to the C terminus at aa 786. Position of the cleavage site is shown by a solid vertical bar and the catalytic serine at aa 737 is indicated by an open vertical bar. The truncated Sb63b cDNA sequence contains sequence from the whiteblood transposable element after the end of exon 6 in the stem at Sb-sbd cDNA nucleotide 2028 (corresponding to aa 386). A stop occurs 22 codons into the whiteblood sequence. The amino acid residues from the whiteblood sequence are indicated by a solid bar. Sb70 cDNA is similarly truncated (see text). In Sbspike, a frameshift mutation occurs in the C-terminal arginine codon (AGA to GAT), resulting in the substitution of an aspartic acid for the final arginine and the addition of 23 residues [(R786D)DDQKILTTADRLLLFVLIYQLYL]. The additional sequence extending the protein length to 809 residues is indicated by an open bar. sbd201 has a single base change (CAC to CGC) at nucleotide 2583, resulting in a substitution of arginine for histidine at aa 572, indicated by a solid vertical bar labeled H572R.
Initial molecular studies of the Sb-sbd gene showed that the most severe dominant mutants were characterized by DNA insertions between the stem and the protease domain (Appel et al. 1993). The dominant bristle phenotype in these mutants suggests that one or more of the noncatalytic domains have a function either independent of (neomorphic) or antagonistic to (antimorphic) the wild-type bristle function. The observation that this apparent defect in the extracellular portion of the protein has a specific effect (formation of additional bundles) on the assembly of actin filaments inside the cell raised the possibility that Sb-sbd may be involved in an outside-to-inside signal, perhaps coordinating bristle elongation with proteolytic modification of the surrounding apical ECM. The malformed leg phenotype, but not the bristle phenotype, in Sb-sbd mutants is enhanced in the presence of mutations in genes involved in myosin II-driven apical cell-shape change, including zipper (zip), the gene coding for nonmuscle myosin II heavy chain (Gotwals and Fristrom 1991; Bayer et al. 2003), reflecting the difference between apical cytoskeletal contraction in the elongating leg disc and formation of a long apical cytoplasmic extension by assembly and extension of parallel actin bundles in the developing bristle.
We took advantage of the differences in the bristle and leg roles for Sb-sbd to begin to connect biological functions to specific domains of stubblin. We have identified the molecular defects in three dominant Sb mutants and found that the most severe entirely lack a protease domain, while a mild allele is a frameshift mutation that adds a C-terminal hydrophobic sequence to the protease domain, arguably disabling the protease by interfering with folding and transmembrane processing. A severe recessive sbd mutant has a single base change likely to reduce but not eliminate protease function. Using transgenic lines expressing wild-type Sb-sbd, protease-disabled Sb-sbd, and a series of truncations, we show that a functional stubblin protease domain is required for both leg and bristle morphogenesis. Expression of truncated transgenes in a wild-type background generates Sb-like bristles, but not malformed legs. Antibody localization of stubblin in developing bristles of wild-type and Sb mutants indicates that the stubblin knot and/or stem domains help localize protease activity to the growing bristle tip. Surprisingly, overexpression of wild-type Sb-sbd has dominant effects on leg but not bristle morphogenesis during specific sensitive periods in both prepupal and pupal development. These results, taken in light of recently reported genetic interaction studies of Sb-sbd and Rho-signaling pathway mutations (Halsell et al. 2000; Bayer et al. 2003; Ward et al. 2003; Chen et al. 2004), suggest that Sb-sbd may coordinate ECM remodeling and cytoskeletal reorganization in both legs and bristles.
MATERIALS AND METHODS
Drosophila stocks:
Drosophila Sb-sbd stocks used in this study are described in Beaton et al. (1988) and Table 1. Wild-type strains used were Oregon-R and Canton-S lines that have been maintained in our laboratory since 1964 and 1979, respectively. Transgenic stocks are described below. Stocks were maintained on standard cornmeal–agar medium at either 25° or 18°.
Transgenic constructs:
See Figure 3. Generation of the wild-type Sb-sbd transgene (hs-Sb-sbd+) was described previously (Hammonds 2002; Bayer et al. 2003). Briefly, full-length Sb-sbd cDNA (Appel et al. 1993) was cloned into a pLitmus 29 Vector (New England Biolabs, Beverly, MA) and then into the heat-shock-inducible P-element transformation vector pCaSpeR-hs (Thummel and Pirrotta 1992) to enable expression of the transgene under heat-shock control. The pLitmus29 Sb-sbd cDNA clone was used as a mutagenesis template to construct modified Sb-sbd transgenes, which were subsequently cloned into pCaSpeR-hs and injected into w1118 embryos using standard methods (Rubin and Spradling 1982). The protease-disabled S737A construct, which substitutes an alanine for the catalytic serine at amino acid 737, was made from the wild-type template using QuikChange mutagenesis (Stratagene, La Jolla, CA) to change Ser (TCA) to Ala (GCA) using the primer 5′-TGTCAGGGCGATGCAGGAGGTCC-3′. The truncated Sb transgene hs-Sb-Δprotease deletes the wild-type Sb-sbd cDNA coding for the protein sequence C-terminal to amino acid 517 and so lacks the entire protease domain. The truncated Sb-transgene hs-SbCD-TM-knot deletes the DNA coding for the protein sequence C-terminal of residue 257, so that only the cytoplasmic domain, the transmembrane domain, and the disulfide knot remain. The hs-SbCD and hs-SbCD-TM stocks were gifts from C. Bayer. The hs-SbCD construct includes only the cytoplasmic domain while hs-SbCD-TM retains the cytoplasmic domain with the transmembrane domain and some extracellular sequence but excludes the disulfide knot. Expression of transgenic protein products was confirmed by Western blotting for the hs-Sb-sbd+, hs-Sb-Δprotease, and hs-Sb-S737A constructs. The shorter truncations do not contain the epitope against which our antibody was generated so their expression cannot be verified and results with these constructs must be considered preliminary.
Figure 3.
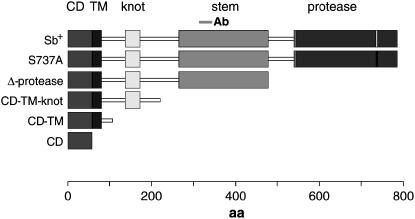
Sb-sbd constructs for transgenic lines. Also shown is the location of the epitope for the stubblin stem (stem Ab) antiserum. The wild-type Sb-sbd (Sb+) construct includes the full-length Sb-sbd cDNA. The protease-disabled construct (S737A) substitutes an alanine for the catalytic serine at aa 737. The Δ-protease construct deletes the wild-type cDNA coding for the protein sequence C-terminal of aa 517 and is therefore missing the entire protease domain. The CD–TM–knot construct deletes the wild-type cDNA coding for the protein sequence C-terminal to residue 257 and thus retains the cytoplasmic domain and extracellular sequence through the disulfide knot (knot), but deletes most of the S/T-rich stem and all of the protease domain. CD–TM also deletes the disulfide knot, and CD expresses only the intracellular domain. CD–TM and CD constructs were gifts from C. Bayer.
Heat-shock protocol:
Animals were staged from white prepupae (WPP) and heat-shocked immediately for the 0-hr time point or kept at 25° until the desired age, described in hours AP. The collected WPP were placed onto the walls of standard plastic food vials. For heat shocks the vials were immersed in a 37° water bath for 60 min. After heat shock the vials were returned to 25° until the adults eclosed. Adults were then scored for bristle or leg phenotype.
Sequence analysis of Sb-sbd mutants:
To identify sequence defects in Sb-sbd mutants, genomic DNA from each homozygote mutant (Sbspike, sbd2, sbd201, and the sbd201 progenitor br1) was amplified using Sb-sbd exon-specific primers. All sequences were determined in both directions. Mutations and polymorphisms were verified by sequencing independent PCR products using at least two different primer pairs. The 3′-ends of Sb63b and Sb70 cDNA were isolated from homozygote WPP total RNA by 3′-RACE PCR (Frohman 1993) using reagents from the Invitrogen (Carlsbad, CA) 3′-RACE kit. First-strand cDNA synthesis was primed with an oligo(dT) primer containing an adaptor sequence at its 5′-end. The PCR step used a 5′ primer from exon 5 in the Sb-sbd stem region (nt 1558–1773) and the adaptor sequence as the reverse primer. This PCR product was then cloned into a PCRII vector (Invitrogen) and sequenced. Manual double-stranded sequencing was done with the dideoxy chain termination method using Sequenase version 2 kit (USB, Cleveland). Automated sequencing was done at the University of California DNA sequencing facility. PCR and sequencing primers were made by GIBCO–BRL/Invitrogen or the University of California Cancer Research Laboratory Microchemical Facility. DNA sequence analysis was facilitated by the use of MacVector sequence analysis software (Oxford Molecular Group/Accelrys, San Diego) and the NCBI BLAST server.
Preparation of antibodies:
A 26-amino-acid synthetic peptide from a nonrepetitive region in the stubblin stem was used to generate polyclonal antisera in guinea pigs (see Figure 3). The stem peptide was a generous gift of D. King. Antisera were prepared by Covance Research Services (Richmond, CA).
Dissection and antibody staining of imaginal discs:
White prepupae were collected and either dissected immediately or aged for 1–4 hr at 25°. The disc–brain complex was dissected in Drosophila Ringers (130 mm NaCl, 5 mm KCl, 1.5 mm CaCl2) and fixed for 30 min in 4% formaldehyde in TBS pH 7.6 (20 mm Tris, 137 mm NaCl) at room temperature on a rocker. The fixed tissue was washed three times in TBS and then transferred to blocking solution (2% goat serum, 1% BSA in TBS). In the block, the discs were separated from the brain and any other adhering tissues. The cleaned discs were transferred to a fresh block and incubated, rocking, at room temperature for a total of 2 hr. Discs were then incubated overnight at 4° in stubblin antibody diluted 1:400 in blocking solution. Primary antibody was washed out with five washes of TBS. After a 15-min blocking step, discs were incubated for 1.5 hr in secondary antibody, fluorescein-conjugated anti-guinea pig IgG (H + L), affinity purified (Vector Laboratories, Burlingame, CA), and used at 20 μg/ml. Discs were then washed five times with TBS. After the last wash, TBS was replaced with 25% glycerol in TBS and the discs were allowed to equilibrate for 5 min. Discs were mounted on slides with Vectashield mounting medium (Vector Laboratories, Burlingame, CA).
Dissection and antibody staining of developing bristles:
Pupae were staged from WPP and aged at 25° for 32–48 hr or at 18° to an equivalent developmental stage. Thoraces were dissected using a modification of the method described by Tilney et al. (1996). Pupae were placed on double-stick tape and pupal cases were removed. Dorsal thoraces were collected as described (Tilney et al. 1996) except that cleaning of the tissue was delayed until after a longer initial fixation step: 30 min at room temperature and then 2 hr to overnight at 4°. Following fixation, the large tracheoles and fat body were removed, and the pupal cuticle was gently peeled away from the epidermis. The fixed, cleaned thoraces were incubated in 0.1% Triton X-100, 4% formaldehyde in TBS for 20 min and then washed five times in TBS, 0.1% Triton. For antibody staining, thoraces were incubated in blocking solution (1% BSA, 2% goat serum in TBS) for 1.5 hr and then in antibody solution overnight at 4° at the same dilutions as used for disc staining. Secondary antibody incubations and subsequent washes were done as for disc staining. For mounting, a final 40% glycerol equilibration step was added before mounting in Vectashield mounting medium diluted 1:1 in TBS. For phalloidin staining of actin, fixed and permeablized thoraces were incubated 2 hr at room temperature or overnight at 4° in phalloidin conjugated to either Texas Red or fluorescein (Molecular Probes, Eugene, OR) and diluted into 4% formaldehyde in TBS and then washed and mounted as for antibody-stained tissues.
Conventional fluorescence and confocal microscopy:
Slides were examined with both conventional fluorescence microscopy on a Zeiss Axiophot microscope and scanning laser confocal microscopy using a Bio-Rad (Hercules, CA) 1024 confocal microscope and COMOS software. Confocal images were processed and analyzed using either NIH Image 1.62 or Photoshop 3.0.5 (Adobe, San Jose, CA).
RESULTS
Sb-sbd genomic structure and polymorphisms:
The sequence of the Sb-sbd cDNA and the preliminary genomic structure were published previously (Appel et al. 1993). These results have been revised and extended (Figure 1). Several polymorphisms were found when the cDNA sequence (Appel et al. 1993; gi 158511) from a wild-type Oregon-R strain was compared to the published genomic sequence from a y; cn bw sp strain (Adams et al. 2000; gi 7300108). Four changes, all in exons 6 or 7 of the repetitive stem region, result in amino acid sequence differences (Figure 1). The concentration of polymorphisms in the stem exons, including nonconservative substitutions (e.g., a proline to serine at aa 349), suggests that the function of the stem tolerates sequence and secondary structure variation.
Molecular characterization of Sb-sbd mutations:
To address specifically the basis of the differences between the effects of dominant and recessive mutants on leg morphogenesis and bristle development, we sequenced dominant (Sb) and recessive (sbd) alleles (Figure 2). The dominant alleles form an allelic series with respect to both bristle length and leg morphology. Sb63b and Sb70 are equivalent and stronger than Sb1, which in turn is more severe than Sbspike (Beaton et al. 1988). Deficiencies for the locus (e.g., sbd105) have no dominant phenotype and have sbd bristles but are not malformed in heteroallelic combination with the mildest sbd allele, sbd2. A more severe sbd allele, sbd201, isolated on the basis of its malfomed leg phenotype in combination with br1, is both sbd and malformed when heterozygous with a Sb-sbd deficiency (Beaton et al. 1988). Although it is a severe recessive allele, several genetic observations support the conclusion that sbd201 is not a null but an antimorph. First, compared to deficiencies for the Sb-sbd locus, sbd201 interacts with (broad) br and zip mutants with greater penetrance to produce severely mlf legs (J. Fristrom, unpublished results). If it were a null, sbd201 would behave similarly to the deficiency. Similarly, a null sbd201 homozygote would be expected to have the same phenotype as sbd201 in heteroallelic combination with a Sb-sbd deficiency. Both of these combinations have a severe mlf leg phenotype like that of Sb dominant mutants either homozygous or in combination with a deficiency (Beaton et al. 1988). The bristle phenotype, however, is more severe in the sbd201 homozygote than in the deficiency trans-heterozygote (our unpublished observation), and unlike Sb-sbd deficiencies, sbd201 does not enhance the bristle phenotype of the mild hypomorph sbd2. Thus, although the bristle phenotype in sbd201 mutants is recessive, sbd201 effects on bristles are different from those of a sbd deficiency and, with respect to the leg phenotype, sbd201 behaves more like the Sb alleles. For this reason, sbd201 was chosen for further sequence analysis along with sbd2 and the dominant mutants Sb63b, Sb70, and Sbspike.
sbd201:
Genomic DNA from sbd201 homozygotes and the progenitor stock for sbd201 was sequenced in both directions through the entire protein-coding region and the two 5′ untranslated exons. Two differences from the published wild-type Sb-sbd sequence were found. In exon 7, in an ST-rich region of the stem, sbd201 DNA contains an in-frame 9-bp deletion, which eliminates one STT repeat. This variation is also in the progenitor stock and so does not itself cause the sbd phenotype. The second and more significant difference is a single base change (A to G) at cDNA nucleotide 2583, resulting in an amino acid substitution of arginine (CGC) for histidine (CAC) at aa 572 (Figure 2). The progenitor stock for sbd201 has the wild-type sequence at this position. This amino acid is within a conserved region of the catalytic domain that forms the side of the P1′ pocket. This residue is equivalent to chymotrypsinogen His40, which has been proposed to have a role in stabilization of the zymogen by forming a hydrogen bond with Asp194 (Bode et al. 1978; Madison et al. 1993). Cleavage of the prodomain at the activation cleavage site disrupts this bond and leads to a conformational change that completes formation of the oxyanion hole and substrate-binding pockets. Alignment of all 29 mammalian TTSPs characterized to date (reviewed in Netzel-Arnett et al. 2003) shows that histidine 40 is conserved in 25 of these. Mouse and human matriptase substitute alanine and the enteropeptidases substitute leucine at this position. The histidine-to-arginine substitution in sbd201 may prevent the formation of a stable zymogen, resulting in nonspecific activation of the sbd201 protease, degradation of the protease, or both, or may disrupt prime-side substrate interactions to reduce effective substrate binding. Therefore, this mutation is likely to severely reduce but not completely eliminate stubblin protease activity.
sbd2:
All 11 exons of the Sb-sbd cDNA, including the 9 protein-coding exons and both 5′ untranslated exons, were sequenced from sbd2/sbd2 genomic DNA. The only detected coding sequence difference between sbd2 and the two published wild-type sequences (Oregon-R and y; cn bw sp) is the same STT deletion in exon 7 seen with sbd201 and its wild-type progenitor. Because it also occurs in wild-type flies, this deletion is not the cause of the mutant phenotype of sbd2. No other alterations were found in the coding sequence. In the 697-bp intron between exons 6 and 7 in the stem, there is a tandem duplication of ggttctg not found in any sequenced wild-type strain; it is conceivable that this duplication may have a regulatory effect, such as disruption of an intronic enhancer binding site, although no specific regulatory function in this intron has been established. The possibility remains that there are changes in an as-yet-unidentified promoter region, splice junction, or other regulatory region. Considering that Sb-sbd deficiencies have a sbd phenotype, a regulatory mutation, particularly one that results in Sb-sbd underexpression, would be consistent with the mild hypomorphic phenotype of sbd2/sbd2.
Sb dominant mutations:
Three Sb-sbd mutations with dominant bristle phenotypes, Sb1, Sb70, and Sb63b, are associated with insertions in the 3′ region of the Sb-sbd gene, in front of the catalytic domain (Appel 1992; Appel et al. 1993). Northern blots show that all three mutations produce truncated transcripts of ∼2.8 kb instead of the wild-type 3.7-kb transcript (Abu-Shumays 1995). To identify the specific defect in the most severe mutation, Sb63b, the 3′-end of the cDNA, isolated by RT–PCR from homozygote Sb63b/Sb63b white prepupal RNA, was sequenced. The shortened transcript matches Sb cDNA up to nucleotide 2028 (the end of exon 6, stem region) with 647 nucleotides from a whiteblood retroviral-like transposable element (Bingham and Chapman 1986) joined to the 3′-end. The combined Sb:whiteblood transcript is 2.7 kb, consistent with the ∼2.8 kb-band seen on Northern blots (Appel et al. 1993; Abu-Shumays 1995). A stop codon occurs after 66 nucleotides of whiteblood sequence so that the protein product from this transcript could include up to 22 amino acids derived from the whiteblood insertion (Figure 2). The transition from Sb-sbd to whiteblood occurs at a splice junction, indicating that the transposable element is likely to be inserted in the intron preceding exon 7. Sequencing of Sb70, which has a severe bristle phenotype like that of Sb63b, identified a whiteblood insertion producing a similarly truncated Sb:whiteblood transcript. Unlike Sb63b, however, Sb70 does not have a second insertion at the 5′-end of the gene (Appel 1992). The second insertion in Sb63b has not been identified. It is successfully spliced out in white prepupal RNA detectable by Northern or RT–PCR (Abu-Shumays 1995). It should be noted that the Sb:whiteblood transcript is overexpressed approximately fourfold compared to the wild-type transcript in both Sb63/+ and Sb70/+ white prepupae (Abu-Shumays 1995).
Sb1 genomic DNA also contains an insertion before the beginning of the catalytic domain (Appel et al. 1993). Sb1/+ flies have a milder bristle phenotype than Sb63b or Sb70 heterozygotes and a weaker mlf leg phenotype in trans-heterozygote combinations. Sb1/Sb1 homozygotes are lethal as early first instar larvae (data not shown). There is evidence that Sb1 lethality may be caused by closely linked but functionally independent loci (Appel 1992; Nelson and Szauter 1992; Hammonds 2002). Because the lethality of Sb1 homozygotes and the poor viability of the deficiency trans-heterozygotes make isolation of Sb1 RNA problematical, cDNA sequence analysis was not pursued for this mutant. However, the trans-heterozygote Sb1/Sb63b is weakly viable. Amplification of genomic DNA from these flies adjacent to and across the insertion site of the Sb63b whiteblood element between exons 6 and 7 indicates that the Sb1 insertion also disrupts this region of the gene (data not shown), consistent with Southern analysis (Appel et al. 1993) and the 2.8-kb transcript size (Abu-Shumays 1995).
Sbspike produces the wild-type-size 3.7-kb transcript (Abu-Shumays 1995). Both Sbspike/Df (3R)sbd105 trans-heterozygotes and Sbspike homozygotes are viable and are mlf with a more severe bristle phenotype than Sbspike/+. Exon-specific primers were used to amplify the Sb-sbd coding exons and the 3′ and 5′ untranslated regions from Sbspike/Sbspike genomic DNA . Three differences between the Sbspike/Sbspike DNA and the published wild-type sequences were detected. In exon 11, the Sbspike/Sbspike DNA sequence drops the A in the first position of the C-terminal arginine codon, resulting in a frameshift with the substitution of aspartic acid (GAT) for the arginine (AGA), substitution of another aspartic acid for the stop codon, and addition of 23 amino acids to the highly conserved protease domain before a new stop codon occurs (Figure 2). This appended sequence seems unlikely to be benign and may result in a complete loss of normal stubblin function. Of the first five residues, four are highly charged (DDDQK) and 11 of 12 of the last residues are hydrophobic (LLLFVLIYQLYL), suggesting that this sequence may interfere with processing of the protein through the membrane. In addition, the extra residues in the Sbspike protease domain could disrupt effective folding, resulting in a change in the structure that alters or abolishes the protease function. Sbspike DNA also contains an A-to-T substitution in the repetitive ST-rich region of exon 7 (stem), resulting in a threonine (ACA) being replaced by isoleucine (ATA) at aa 428. Because there are several differences in this region of the stem among the sequences of three wild-type strains, the additional residues at the end of the protease domain are more likely to be significant to the function of the protein. As well as these changes in the coding sequence, the intron between exons 6 and 7 contains the same 7-bp tandem repeat that is found in sbd2. We have not ruled out the possibility that this duplication contributes to the hypomorphic phenotype of sbd2 and therefore also could be a factor in Sbspike, for example, by reducing expression levels of the Sbspike transcript and contributing to the observed milder (compared to Sb63b and Sb70) phenotype.
Rescue of Sb-sbd phenotypes with heat-shock-inducible Sb-sbd transgenes:
Although the abundance of genomic mutational evidence supports the view that we have identified the structural Sb-sbd gene (Appel et al. 1993), the ultimate demonstration of gene identity is rescue of mutant phenotypes with the wild-type gene. Rescue of hypomorphic mutations and antimorphic mutations should be achievable with sufficient quantities of the wild-type gene product. Neomorphic mutations, with a qualitatively different function, should be resistant to rescue by the wild-type gene. To confirm by transgenic rescue that the phenotypes of the Sb-sbd recessive mutations result from insufficient wild-type Sb-sbd gene product, to replicate the bristle phenotypes of the Sb dominant mutations, and to initiate a structure/function analysis of the Sb-sbd gene, we constructed a series of Sb-sbd transgenes, shown in Figure 3. Wild-type Sb-sbd cDNA and a set of modified Sb-sbd cDNAs were cloned into a heat-shock-inducible transformation vector (see materials and methods) to allow expression of the transgenes during either leg morphogenesis (0–6 hr AP) or bristle formation (24–48 hr AP).
Rescue of bristle phenotypes:
Table 2 shows rescue with a wild-type transgene of the bristle phenotype of the mild hypomorph sbd2 and the more severe mutant sbd201 with a single 1-hr heat shock at 27.5 hr AP, shortly before bristle nubs appear. With two copies of the transgene, 100% of the treated sbd2 flies had wild-type bristles. A single copy of the hs-Sb-sbd+ transgene typically produced bristles intermediate between sbd2 and wild-type length (data not shown). Two copies of the hs-Sb+ transgene induced at 27.5 hr AP also rescued the more severe recessive bristle mutation, sbd201, with 90% rescue of bristles to wild type and 10% to bristles intermediate between sbd201 and wild-type length. Because of the high incidence of lethality in the sbd201 homozygotes, sbd201/Df sbd105 animals were used for these experiments. Bristles of the dominant mutant Sb63/+ were not rescued by the wild-type transgene even with multiple heat shocks throughout early bristle development (24–30 hr AP). A single heat shock at 27.5 hr AP rescued bristles of the milder dominant mutant Sbspike/+ to wild type in 19% of the treated flies, while another 27% had bristles intermediate between Sbspike and wild type. No rescue was evident in 38% of the Sbspike/+ heat-shocked flies. These results confirm the identity of the Sb-sbd gene and are consistent with genetic predictions that sbd2 is a hypomorph, that sbd201, although recessive, is an antimorph, and that Sb dominant mutants are neomorphs (Sb63b) or antimorphs (Sbspike).
TABLE 2.
Rescue of bristle phenotype with heat-shock induction of wild-type Sb-sbd transgene
| Bristle phenotype
|
||||||
|---|---|---|---|---|---|---|
| Genotype | Treatment | n | % sbd | % Sb | % int | % wt |
| hs-Sb+/hs-Sb+;sbd2/sbd2 | hs1 | 87 | 0 | 0 | 0 | 100 |
| No hs | 53 | 100 | 0 | 0 | 0 | |
| hs-Sb+/hs-Sb+;sbd201/Df(3R)sbd105 | hs1 | 50 | 0 | 0 | 10 | 90 |
| No hs | 64 | 100 | 0 | 0 | 0 | |
| S737A/S737A; sbd2/sbd2 | hs1 | 65 | 100 | 0 | 0 | 0 |
| No hs | 86 | 100 | 0 | 0 | 0 | |
| hs-Sb+/hs-Sb+; Sbspike/+ | hs1 | 63 | 0 | 38 | 43 | 19 |
| No hs | 72 | 0 | 100 | 0 | 0 | |
| hs-Sb+/hs-Sb+; Sb63b/+ | hs1 | 55 | 0 | 96 | 4 | 0 |
| hs2 | 58 | 0 | 100 | 0 | 0 | |
| No hs | 84 | 0 | 100 | 0 | 0 | |
Homozygote sbd2/sbd2, deficiency trans-heterozygote sbd201/Df(3R)sbd105 or heterozygote Sb63b/+, and Sbspike/+ stocks carrying two copies of the wild-type Sb-sbd transgene (hs-Sb+/hs-Sb+), and sbd2/sbd2 carrying two copies of the protease-disabled S737A transgene were collected as white prepupae, aged at 25° until 27.5 hr AP, and then either heat shocked at 37° for 1 hr (hs1) or maintained at 25° (no hs). Another set of Sb63b pupae carrying the wild-type transgene was heat shocked at 24, 26, and 28 hr AP (hs2). After heat shock, pupae were returned to 25° until eclosion. Adults were scored for wt, sbd, Sb, or intermediate (int) phenotype bristles (n: number of adults; % wt, sbd, Sb, or int: percentage of each phenotype observed).
Rescue of leg phenotypes:
To rescue mlf legs in Sb-sbd mutants, the wild-type Sb-sbd transgene was induced by a 1 hr 37° heat shock at the white prepupal stage (0 hr AP), corresponding to the time when the Sb-sbd gene is normally expressed in prepupal development. Results from these experiments are summarized in Table 3. Induction of the wild-type transgene increased the occurrence of wild-type legs in both sbd201 homozygotes and deficiency heterozygotes [sbd201/Df(3R)sbd105] carrying two copies of the wild-type transgene. A single copy of the wild-type Sb-sbd transgene in sbd201 deficiency heterozygotes [hs-Sb-sbd+/+; sbd201/Df(3R)sbd105] rescued mlf legs more effectively than two copies did, suggesting that leg morphogenesis is sensitive to overexpression of wild-type Sb-sbd. In preliminary experiments no rescue of malformed legs in the dominant Sb mutants Sb1/Df(3R)sbd105 or Sbspike/Df(3R)sbd105 was produced by induction of the wild-type Sb-sbd transgene at 0 hr AP using two copies of the transgene or in Sb63b/Df(3R)sbd105 with either one or two copies (data not shown). This was unexpected, given that the mlf syndrome is recessive, and indicates a functional difference between the defect in the sbd201 stubblin and the Sb mutant stubblins despite similar leg phenotypes in these mutants. It is possible that some endogenous Sb-sbd transcription precedes the 0-hr-AP induction of the wild-type transgene, so that mutant stubblin is already present and the heat-shock-induced wild-type stubblin is too late to rescue mlf legs in the Sb mutants. Alternatively, or additionally, “collateral damage” of overexpression of Sb-sbd could mask rescue of the specific Sb leg morphogenesis defect. The improved rescue of sbd201/Df(3R)sbd105 with one compared to two copies of the wild-type transgene supports the argument that there are deleterious effects of overexpression of Sb-sbd.
TABLE 3.
Rescue of mlf legs by heat-shock induction of the wild-type Sb-sbd transgene
| Leg phenotypes
|
||||
|---|---|---|---|---|
| Genotype | Treatment | n | % mlf | % wt |
| hs-Sb+/hs-Sb+; sbd201/sbd201 | +hs | 63 | 68 | 32 |
| −hs | 85 | 89 | 11 | |
| hs-Sb+/hs-Sb+; sbd201/Df(3R)sbd105 | +hs | 72 | 67 | 33 |
| −hs | 58 | 93 | 7 | |
| hs-Sb+/+; sbd201/Df(3R)sbd105 | +hs | 117 | 54 | 46 |
| −hs | 118 | 91 | 9 | |
| +/+; sbd201/sbd201 | +hs | 52 | 94 | 6 |
| −hs | 88 | 96 | 4 | |
| S737A/+; sbd201/Df(3R)sbd105 | +hs | 121 | 100 | 0 |
| −hs | 122 | 93 | 7 | |
White prepupae from sbd201/sbd201 or sbd201/Df (3R) sbd105 stocks carrying zero (+/+), one (hs-Sb+/+), or two (hs-Sb+/hs-Sb+) copies of the wild-type Sb-sbd transgene or one copy of the S737A protease-disabled transgene were either heat shocked for 1 hr at 37° immediately after collection at 0 hr AP (+hs) or maintained at 25° (−hs). Adults were scored for the mlf leg phenotype. The number scored (n) and percentage with mlf (% mlf) and wt (% wt) phenotypes are shown.
Overexpression of hs-Sb-sbd+ in wild-type flies:
To investigate the effects of overexpression of wild-type Sb-sbd, the hs-Sb-sbd+ transgene was induced at other times throughout prepupal and pupal development in wild-type animals (Table 4). Induction of hs-Sb-sbd+ at 0 hr AP or 3 hr AP produced malformed legs. After a 3-hr-AP heat shock, only 50% of the treated flies developed wild-type legs (the other 50% developed mlf) compared to 96% of the no-transgene control animals. With a 0-hr-AP-only heat shock, 41% of the treated flies had wild-type legs compared to 93% of the no-transgene controls. These observations are similar to those made by Bayer et al. (2003). This effect was not seen in prepupal heat shocks after 4 hr AP. Induction of hs-Sb-sbd+ during pupal development with a double heat shock at 30 hr AP and 33 hr AP produced a different and unanticipated phenotype (Figure 4). All segments of all legs in these flies are shortened, with severity increasing on a distal-to-proximal axis. First and third legs are more severely affected than second legs. In addition to the leg phenotype, there are defects in cuticle on the dorsal thorax (not shown), including a mild cleft and disorientation of the bristles similar to the phenotypes of mutations that affect thoracic closure (Usui-Ishihara et al. 2000; Pena-Rangel et al. 2002). The bristle morphology and length are normal. A single heat shock at 27.5 hr AP, which rescued sbd bristles, produced a slight shortening of prothoracic femurs, but otherwise wild-type flies. Heat shocks after 42 hr AP, after secretion of the adult cuticle, produced no leg or thoracic phenotype.
TABLE 4.
Overexpression of wild-type Sb-sbd causes defects in leg morphogenesis
| Genotype | Treatment | n | % wt |
|---|---|---|---|
| hs-Sb+/hs-Sb+; +/+ | 0 hr AP hs | 118 | 54 |
| 3 hr AP hs | 58 | 50 | |
| 30 and 33 hr AP hs | 55 | 0 | |
| No hs | 120 | 100 | |
| No transgene | 0 hr AP hs | 88 | 96 |
| 3 hr AP hs | 56 | 93 | |
| 30 and 33 hr AP hs | 70 | 100 | |
| No hs | 70 | 100 |
White prepupae from wild-type flies carrying two copies of the wild-type Sb-sbd transgene (hs-Sb+) or no transgene were heat shocked immediately (0 hr AP hs) or aged at 25° until 3 hr AP and then heat shocked (3 hr AP). A third group was heat shocked at 30 hr and again at 33 hr AP (30 and 33 hr AP). All heat shocks were at 37° for 1 hr. Adults (n) were scored for leg phenotype. The percentage of individuals with all wild-type legs (no legs malformed) is shown (% wt). See text for a description of differences between malformed leg phenotypes resulting from early and late heat shock.
Figure 4.
Leg effects of Sb-sbd overexpression in pupae in the first (1st), second (2nd), and third (3rd) legs of adults from pupae carrying two copies of the hs-Sb-sbd+ transgene either with (+hs) or without (−hs) a double heat shock at 30 and 33 hr AP. Note short proximal segments decreasing in severity from femur to basitarsis in the legs from heat-shocked pupae. First legs show the most extreme effect.
Expression of the protease-disabled transgene in wild-type and Sb-sbd mutant flies:
To determine if an intact protease domain is required for rescue of Sb-sbd loss-of-function mutations or overexpression effects, we repeated the rescue and overexpression experiments using a transgene with alanine replacing the critical catalytic serine at aa 737 (S737A) to disable the proteolytic function (see Figure 3). This transgene (hs-Sb-sbd S737A) was unable to rescue sbd2 bristles (Table 2) or sbd201 mlf legs (Table 3). Induction of hs-Sb-sbd S737A in wild-type prepupae at 0 hr AP, 3 hr AP, or with a double heat shock at 0 and 3 hr produced no effect on leg morphogenesis (data not shown). Similarly, the leg and cuticle defects seen with the wild-type Sb-sbd transgene induced at 30 hr and 33 hr AP were not seen with the protease-disabled transgene, implying a requirement for a functional protease for the overexpression effects as well as for normal leg and bristle morphogenesis.
Generation of Sb bristles by expression the Sb-sbd truncation and S737A transgenes:
To generate dominant bristle phenotypes similar to those seen with the dominant mutations that make truncated transcripts, we made a series of truncated Sb-sbd transgenes (Figure 3). Three transgenic constructs—the truncation deleting the protease domain (Δ-protease) and the truncation deleting the protease and most of the stem, but including the disulfide knot (CD–TM–knot), as well as the S737A mutation—all produced at least some short, thick Sb-like bristles when expressed throughout early bristle development. A single heat shock at 27.5 hr did not produce a detectable phenotype. Table 5 shows the results of a series of four heat shocks at 25, 27, 29, and 31 hr AP with two copies of the transgene and a single copy of the endogenous Sb-sbd gene. In all cases there was some variation in the bristle phenotype, with the dorsocentral bristles most consistently short. The hs-Sb-sbd S737A transgene produced short-bristle phenotypes with highest penetrance and severity; 97% of the heat-shocked pupae produced adults with predominantly short bristles, many shorter than those of Sb1. While some of these bristles are both short and thick as in Sb63b, some have thick bases with thin extensions, a combination of the Sb and sbd bristle phenotypes. Figure 5 shows a confocal image of an actin-stained bristle dissected at 37 hr AP from a pupa carrying the S737A transgene induced by heat shock at 25, 27, 29, and 31 hr AP. Also shown is a bristle dissected from a wild-type pupa (no transgene) heat shocked in parallel. Although there is a dramatic difference in bristle length between these two 37-hr-AP bristles, the short bristle does not show the consistent actin bundle mislocalization and increase in the number of bundles seen in a Sb63b bristle, i.e., 25–30 bundles not restricted to the cell periphery in Sb63b homozyogtes compared to 12–15 solely peripheral bundles in wild-type bristles (Appel et al. 1993). The more consistent and severe phenotype may require the constant and abundant presence of the truncated protein in bristles as suggested by the difference in bristle phenotype between Sb1, in which the truncated Sb1 transcript is expressed in amounts comparable to the wild-type transcript, and Sb63b, in which the similarly truncated transcript is overexpressed compared to the wild-type transcript. Sb1 bristles are longer than those of Sb63b and while Sb1 bristles often have extra actin bundles, the number of bundles (11–18) overlaps with that of wild type (Lees and Picken 1945; Overton 1967).
TABLE 5.
Transgenic expression of Sb-Sbd truncations or a disabled protease in pupae produces Sb-like bristles
| Genotype | Treatment | n | % Sb |
|---|---|---|---|
| S737A/S737A; Df(3R)sbd105/+ | +hs | 74 | 97 |
| −hs | 111 | 0 | |
| Δ-protease/Δ-protease; Df(3R)sbd105/+ | +hs | 68 | 44 |
| −hs | 53 | 4 | |
| CD-TM-knot/CD-TM-knot; Df(3R)sbd105/+ | +hs | 75 | 35 |
| −hs | 89 | 0 | |
| CD-TM/CD-TM; Df(3R)sbd105/+ | +hs | 115 | 13 |
| −hs | 89 | 0 | |
| +/+; Df(3R)sbd105/+ | +hs | 73 | 8 |
| −hs | 59 | 0 |
Sb-sbd deficiency trans-heterozygotes [Df(3R)sbd105/+] carrying two copies of the S737A transgene or two copies of one of the truncation transgenes [Δ-protease, CD-TM-knot, CD-TM] or no transgene [+/+; Df(3R)sbd105/+] were collected and aged at 25° to 25 hr AP. These pupae were either subjected to four 1-hr heat shocks at 37° (25, 27, 29, and 31 hr AP) with a 1-hr recovery period at 25° between heat shocks (+hs) or maintained at 25° (−hs). Adults (n) were scored (% Sb) for bristle phenotype (at least three Sb dorsocentral or scutellar bristles, i.e., blunt-ended bristles <2/3 wt length).
The bristle effects of transgenes expressing only the cytoplasmic domain with the transmembrane domain (CD–TM) were indistinguishable from heat-shocked lines without any transgene (w; sbd 105/+), even when the copy number of the transgene was increased to 3 (w/hs-Sb CD-TM;hs-Sb CD-TM/hs-Sb CD-TM: sbd105/+). However, because we cannot confirm expression of these shorter constructs with our antibody to the stubblin stem, these results must be considered preliminary. Pupal heat shocks with the wild-type transgene resulted in adults with wild-type bristle length but with severe leg and thorax malformations (Table 4 and data not shown). No leg or thorax effects were seen with any of the truncations or the S737A substitution.
Localization of stubblin in discs:
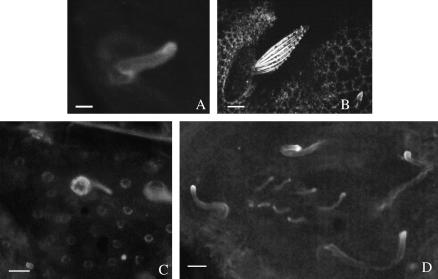
Cell-shape change occurring at the apical surface during prepupal morphogenesis is driven by actin–myosin contractility (Condic et al. 1991). Previously, we reported that an antibody to the juxtamembrane region of stubblin localized to the apical surface in prepupal discs, consistent with a direct role for stubblin in apical cell-shape change (von Kalm et al. 1995). We repeated this localization using a new antiserum made against a nonrepetitive region of the stem (see Figure 3) with similar results (Figure 6, A, B, and E). This antiserum also stains prepupal discs from Sb63b homozygotes, but more intensely, as predictable from the overexpression of the Sb63b transcript (Figure 6D). At 3.5 hr AP, the Sb63b discs already are clearly shorter than their wild-type counterparts, although elongation has started. Leg discs from heat-shocked third instar wild-type larvae carrying two copies of the wild-type Sb-sbd transgene show precocious apical localization, visible as concentric rings in the unevaginated disc (Figure 6F). Non-heat-shocked controls show no stubblin expression as expected for larval discs prior to the rise in ecdysone titer (Figure 6C).
Figure 6.
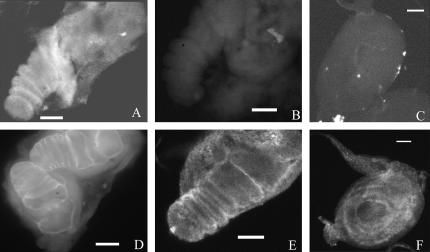
Larval and prepupal leg discs stained with stubblin antistem antiserum. (A and E) Wild-type leg discs dissected at 3.5 hr AP show staining at the apical surface. (E) A confocal image; (A–D and F) conventional epifluorescence. (B) Negative control (no primary antibody). (D) Sb63b/Sb63b leg discs dissected at 3.5 hr AP show a similar pattern but more intense staining, consistent with the overexpression of the Sb63b transcript. Sb63b/Sb63b discs have not elongated as far as their wild-type counterparts. (C and F) Larval discs dissected prior to normal endogenous Sb-sbd expression from animals carrying two copies of the wild-type Sb-sbd transgene: (C) larvae with no heat shock and (F) larvae heat shocked for 60 min at 37°. Heat-shocked larvae show precocious apical expression, visible as concentric rings in the uneverted disc. Bars, 50 μm.
Localization of stubblin in developing bristles:
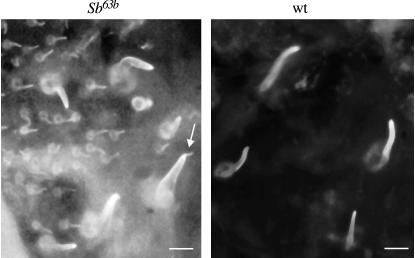
Because Sb-sbd mutants affect the actin bundle scaffolding in growing bristles, it was of interest to determine the localization of stubblin during bristle development when actin bundles are forming. We used the stubblin stem antiserum for immunolocalization studies in bristles from 32 hr AP, when bristle buds first appear, through 38 hr AP, about midway through bristle extrusion. Actin staining of early bristles shows the beginning of actin bundles as punctate staining on the surface of the bristle bud with discrete bundles extending to the base of the shaft (Tilney et al. 1996; Wulfkuhle et al. 1998; Figure 7B). In contrast, stubblin staining at 34–35 hr AP shows cytoplasmic staining of the cell body of the bristle shaft cell with diffuse staining of the budding shaft and tip (Figure 7, A and C). By 37 hr AP, stubblin staining is more concentrated at the tips, indicating that stubblin is transferred to the tip (Figure 7D). From our micrographs, we cannot rule out that the early staining seen at the bristle base is in the socket cell, but because extruding bristle shafts clearly show stubblin staining, we argue by parsimony that expression is restricted to the shaft cell. Sb63b bristles at 37 hr AP are shorter than their wild-type counterparts but stain in a similar pattern except the staining is more intense, with the appearance of heavily staining cap-like extensions on the tips (Figure 8). These localization patterns of stubblin in growing bristles, distinct from those of actin, particularly in sprouting bristles when actin bundles are first detectable, indicate that the actin bundle defects seen in Sb-sbd mutants do not result from a direct interaction of stubblin with actin bundles. The cap of stubblin that appears at the ends of Sb63b bristles suggests the possibility that an accumulation of defective stubblin at the bristle tips may block bristle extension, consistent with the Sb bristle phenotype of abruptly terminated bristles.
Figure 7.
Stubblin localization in developing bristles. Bristles from 34- to 37-hr-AP pupae stained with stubblin stem antiserum (A, C, and D) or phalloidin (B). Confocal images of individual early bristles (∼34 hr AP) show distinct staining patterns for stubblin (A) compared to actin (B). (C) A field of bristles from a 36-hr-AP pupa (conventional epifluorescence) shows bristle base, shaft, and tip stubblin staining of macrochaetes. Smaller microchaetes, which develop slightly after macrochaetes, still show predominate bristle base staining, visible as rings. Confocal image of a field of bristles from a 37-hr-AP pupa shows an increased concentration of stubblin at the growing bristle tips (D). Image in B from Dianne Fristrom. Bars: A and B, 5 μm; C and D, 20 μm.
Figure 8.
Wild-type and Sb63b/+ bristles from 36-hr-AP pupae stained with stubblin stem antiserum. Both show stubblin protein localized to bristle cell base, shaft, and tip, but Sb63b staining is more intense, with heavily staining tip extensions (arrow) not seen in the wild-type bristles. Bars, 20 μm.
DISCUSSION
The ecdysone-induced Sb-sbd gene is required for normal morphogenesis of imaginal discs and formation of bristle shafts, both processes involving cytoskeletal changes and extracellular proteolytic activity. The morphogenesis of the leg imaginal disc to form a tubular leg depends on myosin-driven contraction leading to apical cell-shape change. In contrast, formation of bristles is driven by polymerization of membrane-associated, parallel actin filaments. We discuss here the possible roles of Sb-sbd in imaginal disc morphogenesis and bristle extrusion and speculate that the stubblin protease modifies apical ECM and contributes, directly or indirectly, to activation of a Rho-signaling pathway.
The role of Sb-sbd in leg disc morphogenesis:
Morphogenesis of leg imaginal discs requires both contraction of the apical actin–myosin contractile ring and proteolysis. In rapid response to the ecdysone spike that triggers metamorphosis, the shapes of cells in the distal femur, tibia, and basitarsis change from elongated to isometric (Condic et al. 1991). If these changes are blocked, the resulting legs in the adult fly are malformed with short and thick proximal segments. Genetical and cell biological observations lead to the view that leg morphogenesis depends on myosin-driven contractility. Both actin and nonmuscle myosin II localize to the apical belt in leg discs (von Kalm et al. 1995). Cytochalasins, which disrupt actin filaments, reversibly inhibit leg disc elongation (Fristrom and Fristrom 1975). Mutations in the zip gene, which encodes the nonmuscle myosin II heavy chain, cause the mlf phenotype as do those of the myosin regulatory light chain gene, spaghetti squash (sqh) (Edwards and Kiehart 1996).
Contractility alone is insufficient for leg morphogenesis. The leg disc epithelium is covered by a basal ECM similar to those of vertebrates, containing collagen IV, laminin, and sulfated proteoglycans (Fessler and Fessler 1989; Fristrom and Fristrom 1993). The disc apical surface secretes a chitinous cuticle (exoskeleton) during metamorphosis. Epidermal morphogenesis, including leg elongation during prepupal morphogenesis and bristle formation during pupal morphogenesis, must occur when the epidermis is unconstrained by the exoskeleton. Prepupal leg elongation occurs between pupariation and the formation of the pupal chitinous exoskeleton (Fristrom and Fristrom 1993). Apolysis of the pupal cuticle at ∼18 hr AP allows further leg morphogenesis and formation of bristles and hairs before the adult exoskeleton is deposited between 36 to 70 hr AP. After apolysis, the apical surface of the disc secretes a poorly characterized, fibrous, nonchitinous ECM (Brower et al. 1987; Fristrom and Fristrom 1993). A role for proteolysis in disc morphogenesis is well documented. In addition to Sb-sbd, other proteases known to be regulated by ecdysone and potentially involved in disc morphogenesis include an unidentified extracellular protease secreted by cultured discs in response to ecdysone (Pino-Heiss and Schubiger 1989) and a collagenase that cleaves type IV collagen (Birr et al. 1990; Fessler et al. 1993). Disc morphogenesis is restricted by protease inhibitors (Pino-Heiss and Schubiger 1989). It is accelerated in vitro by exogenous trypsin or chymotrypsin (Poodry and Schneiderman 1971; Fekete et al. 1975). Addition of 0.1% trypsin to the in vitro medium decreases the time of leg elongation of dissected discs from 2–3 hr to 10 min, but only in discs that have been exposed to ecdysone (Fekete et al. 1975). Protease treatment does not passively lead to leg elongation; trypsin acceleration of leg elongation is inhibited under conditions that reduce ATP levels and does not overcome cytochalasin B inhibition of disc elongation (Fekete et al. 1975). Thus, contractility and proteolysis must be coordinated for normal morphogenesis to occur. Stubblin, the product of ecdysone-dependent Sb-sbd and a TTSP that localizes to the apical surface of leg discs, is a candidate for one of the coordinators.
A role for Sb-sbd in leg morphogenesis was first described by Dobzhansky (1929) and rediscovered because of the genetic interaction between Sb-sbd and the metamorphic transcriptional regulator, br, to produce mlf legs (Beaton et al. 1988). The legs of some Sb-sbd homozyogtes, Sb-sbd trans-hetereozygotes, or br; Sb-sbd/+ double mutants are mlf. Leg morphogenesis in these mutants can be rescued in vitro by exposure to exogenous trypsin in prepupal discs (Appel et al. 1993). Our current studies show that induction of the wild-type Sb-sbd transgene early in prepupal development (0 hr AP) partially rescued severe sbd201/sbd201 mlf legs, but not alleles with dominant bristle phenotypes (Sb1, Sb63b, Sbspike). Rescue depends on the presence of a functional protease catalytic domain; the catalytically disabled S737A transgene did not rescue mlf legs. One copy of the wild-type transgene rescued more animals than two copies (50% rescue vs. 30%), demonstrating that overexpression of wild-type stubblin has deleterious effects on leg development. Expression of the wild-type transgene in wild-type animals during prepupal development resulted in adults with mlf legs, confirming the sensitivity of leg morphogenesis to overexpression of stubblin. Overexpression of protease in vivo may result in excess detachment of epidermis from the apical ECM, compromising the structural integrity of the elongated leg and causing malformations even if cell-shape changes are completed. Relevant here is our observation that some stubblin overexpression phenotypes resulting from induction of the wild-type Sb-sbd transgene in wild-type prepupae (0 and 3 hr AP) were not identical to “classic” mlf. While “classic” mlf legs associated with limited cell-shape changes have short, kinked femurs, primarily on the metathoracic legs (see Beaton et al. 1988, Figure 1), prepupal overexpression of stubblin also produced some longer, fragile mesothoracic and metathoracic legs that arguably could result from excessive leg elongation like that seen in vitro with protease exposure (Fekete et al. 1975).
Myosin II contractility depends on the phosphorylation state of myosin regulatory light chain (MLC) (Tan et al. 1992; Amano et al. 1996). Numerous studies point to the Rho subfamily of small GTPases as upstream regulators of the actin cytoskeletal rearrangements and control of MLC phosphorylation (Tapon and Hall 1997; Van Aelst and D'Souza-Schorey 1997; Ridley 2001). Recent genetic interaction studies have identified members of a potential Rho-signaling pathway that may lead to the contraction of the actin–myosin apical belt and the cell-shape changes that drive prepupal leg disc morphogenesis. Rho-signaling pathway components implicated in Drosophila disc morphogenesis include RhoGEF2 and RhoA (Halsell et al. 2000; Ward et al. 2003) and Drosophila Rho kinase (drok) (Winter et al. 2001). Mutations in these genes interact with zip mutations to produce mlf legs (Gotwals and Fristrom 1991; Halsell et al. 2000; Bayer et al. 2003). Activators upstream of RhoA in leg morphogenesis have not been identified. The possibility that Sb-sbd is involved in this or a parallel pathway is suggested by the mlf leg phenotype of Sb-sbd mutations in combination with zip mutations (Gotwals and Fristrom 1991) or with mutations of RhoA, RhoGEF2, or drok genes (Bayer et al. 2003; Ward et al. 2003).
Any consideration of the mechanism of action of the Sb-sbd gene in imaginal disc morphogenesis must take into account the nature of the morphogenetic defects associated with Sb-sbd mutations. The proteolytic domain of stubblin is essential for normal morphogenesis. Stubblin in sbd201/sbd201, which has an arguably impaired catalytic domain, and stubblin molecules lacking the proteolytic domain (in Sb63b, Sb70, and Sb1) are associated with abnormalities in disc morphogenesis. Models for roles of the proteolytic domain are all speculative because the stubblin substrate is unknown. The possibility that stubblin may directly or indirectly modify the apical ECM has been suggested often (Beaton et al. 1988; Appel et al. 1993; von Kalm et al. 1995; Hooper et al. 2001; Bayer et al. 2003) and is supported by the acceleration of leg elongation caused by exogenous trypsin, the in vitro rescue of partially elongated Sb63b/Sb63b leg discs by trypsin, and the apical localization of stubblin in leg discs. However, stubblin may have a dual role both in modifying the ECM to permit cell-shape change and in stimulating the contractility of the apical contractile belt, for example, by activating a Rho-signaling pathway. Because morphogenesis begins in Sb mutants that are missing the protease domain, stubblin cannot be acting alone to initiate contractility, but may be required to amplify a signal to complete morphogenesis.
As a transmembrane protease, stubblin is a candidate for a membrane-associated activator of RhoA, transducing an outside-to-inside signal either directly, through its own intracellular domain, or indirectly, through cleavage of another cell-surface protein that activates Rho signaling (see also Bayer et al. 2003). The first possibility is unlikely because the stubblin cytoplasmic domain lacks identified protein interaction motifs, and our preliminary studies suggest that overexpression of the SbCD or SbCD-TM transgenes has no effect on leg morphogenesis. Regarding the second possibility, serine proteases that, like stubblin, use the Ser-His-Asp catalytic triad can be classified according to highly conserved evolutionary markers on the basis of codon usage at Ser195 and Ser214 and the presence of Pro vs. Tyr at residue 225 (chymotrypsin numbering) (Krem and Di Cera 2001). According to this classification system, stubblin belongs to the oldest lineage and therefore is more likely, on the basis of analysis of other proteolytic cascades, to be the terminal or penultimate protease in an activation cascade and less likely to be an initiator (Krem and Di Cera 2001). To speculate briefly, a protease-activated receptor (PAR) could be a stubblin substrate. PARs belong to a family of G-protein-coupled receptors that are activated by site-specific proteolysis (Dery et al. 1998). Several other TTSP proteases have been demonstrated to activate protease-activated receptor-2 (PAR-2) (Lin et al. 1999; Takeuchi et al. 2000; Friedrich et al. 2002; Iwakiri et al. 2004; Wilson et al. 2005). Proteolytic modification of ECM attachments not only may be permissive, but also may actively contribute to Rho-mediated signaling. Future studies must focus on the identification of stubblin substrates.
The role of Sb-sbd in bristle morphogenesis:
Bristle formation involves actin polymerization but not myosin II contractility. The first bristle nubs appear at ∼32 hr AP, during a period of high ecdysone titer, and extend to their full length by 48 hr AP (Lees and Waddington 1942; Fristrom and Fristrom 1993). Bristles grow from the tip (Lees and Picken 1945). The bristle elongates by the assembly of membrane-associated, crosslinked actin filament bundles formed in short modules, new bundles being joined end to end with the preceding more basal bundle (Tilney et al. 1996; Guild et al. 2003). From observations of bristle growth in cultured thoraces in the presence of compounds that effect actin polymerization or microtubule dynamics, Tilney et al. (2000, 2003) concluded that actin polymerization drives bristle elongation. Unlike leg disc morphogenesis, bristle development is not affected by mutations in zip and these mutations do not interact with Sb-sbd mutations to affect bristle elongation (Bayer et al. 2003). In the bristles of recessive sbd animals, the actin bundles are normal at first, but stop prematurely and asynchronously, so that some bundles continue to extend, while others stop. The resulting bristles are shorter than wild-type bristles and have ragged ends instead of smooth, tapered ends. This defect was rescued by the wild-type Sb-sbd transgene in both the mild allele sbd2 and the more severe allele sbd201. The molecular defect in sbd201, a histidine-to-arginine substitution at aa 571 in the catalytic domain, is likely to compromise but not abolish protease activity (see results). In contrast to the results with mlf leg rescue, complete rescue of sbd201 or sbd2 bristles required two copies of the hs-Sb-sbd+ transgene, a single copy resulting in partial rescue (intermediate length bristles). Only the transgene with an intact protease domain rescued bristles. Unlike legs, bristles are not sensitive to overexpression of wild-type stubblin.
Identification of molecular defects in Sb dominant mutations, Sb63b and Sb70, showed that truncated, protease-absent stubblin causes the severe dominant bristle phenotypes. The bristles in these mutants and those in Sb1, another dominant mutant associated with a transposable element eliminating the protease domain, were not rescued by the wild-type transgene, and so these three mutations are likely to be neomorphs, acting in some novel way not characteristic of the wild-type protein. The bristle phenotype of the milder allele Sbspike, a frameshift mutation that adds sequence to the C terminus of the protease domain and likely disrupts normal stubblin function, was ameliorated by the wild-type transgene, although not rescued completely to wild-type length. Induction of transgenes truncated to remove the protease domain and then progressively larger portions of the noncatalytic region showed dominant effects from expression of truncated protein as short as the CD–TM–knot construct. The most penetrant and severe phenotypes were produced by the S737A construct, which changes only the catalytic serine. Consistent with the difference in the dominant and recessive character of Sb and sbd mutations in bristle and legs, none of the truncation transgenes had any effects on leg morphogenesis. Taken together, these observations indicate a role for the stubblin noncatalytic domains that is specific to bristle elongation. The localization pattern of stubblin in bristles brings up the possibility that the stem or knot may be involved in localization of the protease to the bristle tip, although this is not directly testable with our antiserum to the C-terminal region of the stem. Stubblin appears first in the budding bristle cell body (and perhaps in the socket cell) and then along the shaft to the bristle tip where it concentrates. Immunolocalization with the stubblin stem antiserum in Sb63b/+ developing bristles showed excessive accumulation of protein at the bristle tips compared to the normal protein in wild-type bristles. If the noncatalytic domains direct stubblin localization, expression of a truncated Sb63b or Sb1 protein may block wild-type stubblin proteolytic function. The observation that overexpression of the wild-type Sb-sbd transgene fails to rescue the bristle phenotype of these mutants suggests that either the transgenic stubblin is inefficiently transported or the interference is irreversible. The requirement for two copies of the wild-type transgene for bristle rescue of even the mildest sbd mutation indicates that bristle elongation depends on a sufficient concentration of protease. This extremely long (400-μm) cytoplasmic projection may require concentration of a protease at the bristle tip to facilitate bristle extrusion through the apical ECM. If so, it would suggest that stubblin protease either directly cleaves an apical ECM component or activates another protease that does so. A role for stubblin in relieving ECM constraint is consistent with the accumulation of stubblin at the bristle tips and the increasing growth rate as bristles get longer (Tilney et al. 2000).
The extra and mislocated actin bundles in Sb63b bristles suggest a possible role for neomorphic stubblin in regulation of actin bundle initiation (Appel et al. 1993). The actin filament membrane connector in bristles is unknown. On the basis of the localization pattern of stubblin in bristles, which is distinctly different from that of actin, it is unlikely that stubblin interacts directly with actin. Stubblin could, however, activate another more spatially restricted signal similarly to the mechanism proposed for the TTSP enteropeptidase (see Kong et al. 1997). Recently, Tilney et al. (2004) reported evidence for the influence of external forces (pupal cuticle and placement of the dendrite) and internal forces (actin bundling, actin assembly, and actin bundle positioning) on bristle shape and length. We propose that ECM constraint is an additional external force in bristle morphogenesis. Lees and Picken (1945), Overton (1967), and Tilney et al. (2003) all found that the total bristle volume is the same in wild-type and Sb mutants; the increased bundle number and thickness in the mutants corresponds to the decrease in length. The extra bundles are most numerous in severe dominant mutants that produce a truncated protein entirely lacking the stubblin protease, e.g., Sb63b (Appel et al. 1993). One interpretation of these observations is that bundle formation continues in Sb bristles, but the bristle cannot extend without the action of the protease on the restricting apical ECM so the modules accumulate within the stunted bristle cell. In addition, neomorphic stubblin in severe Sb mutants indirectly causes, through the acquisition of a new function mediated by the stem domains, increased assembly of actin bundles. In sbd mutants that retain any protease function, e.g., sbd2 or sbd201 homozygotes, actin bundles can extend in “holes” where stubblin is active, resulting in the observed nonuniform termination of the actin bundles (Appel et al. 1993). Hypothetically, in sbd nulls, bristle growth would be severely restrained but extra bundles would not form.
By what mechanisms could wild-type stubblin have roles in regulating the internal force of actin assembly as well as in modifying the ECM to permit extension? Like leg morphogenesis, bristle extension requires the coordinated action of cytoskeletal change and ECM proteolysis. However, other than the requirement for stubblin, the molecular mechanisms in leg and bristle development differ. Studies on the formation of yeast cables have identified formin homology (FH) proteins as nucleators specific to the assembly of parallel, unbranched actin filaments, like those that drive bristle extension (Evangelista et al. 2002; Pruyne et al. 2002; Sagot et al. 2002). Several FH proteins have been found to interact with the Rho GTPases, suggesting that FH proteins may act as Rho effectors that communicate RhoGTPase signals to the cytoskeleton (Kohno et al. 1996; Imamura et al. 1997; Watanabe et al. 1997). To extend the model proposed above for the function of stubblin in legs, one can speculate that in bristle development regulation of actin filament polymerization requires an FH protein and a Rho GTPase that is activated by a PAR receptor. Expressed at the tips of growing bristles, stubblin could activate a more spatially restricted PAR or PAR activator and proteases that release the apical ECM attachments around the bristle tip, again linking cytoskeletal changes to ECM modification. Clarification of the roles of stubblin will require the identification of stubblin substrates and other interacting proteins, while keeping in mind that stubblin substrates may differ during disc morphogenesis and bristle elongation. Recent genetic screens (Bayer et al. 2003; Ward et al. 2003), which have identified several new Sb-sbd alleles and genetic interactors, could facilitate this search.
Acknowledgments
We thank Cindy Bayer and Laurie von Kalm for helpful discussions, for their collaboration in construction of the transgenic stocks, and for their critical comments on the manuscript. We are grateful to Dianne Fristrom for sharing her expertise in disc dissection, immunohistochemistry, and microscopy, and for the image in Figure 7B. We are indebted to David King for his advice on peptide antigens and for synthesis of the peptide used to generate the stubblin stem antiserum. We thank Charles Craik for his insightful comments on the potential impact of the sbd201 mutation on stubblin protease function. We also appreciate the efforts of two anonymous reviewers whose comments improved the manuscript. This work was supported in part by grants from the National Science Foundation (IBN-9630783) and the National Institutes of Health (GM50264).
References
- Abu-Shumays, R., 1995. The characterization of two 20 hydroxyecdysone regulated genes in Drosophila imaginal discs. Ph.D. Thesis, University of California, Berkeley, CA.
- Adams, M. D., S. E. Celniker, R. A. Holt, C. A. Evans, J. D. Gocayne et al., 2000. The genome sequence of Drosophila melanogaster. Science 287: 2185–2195. [DOI] [PubMed] [Google Scholar]
- Amano, M., M. Ito, K. Kimura, Y. Fukata, K. Chihara et al., 1996. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J. Biol. Chem. 271: 20246–20249. [DOI] [PubMed] [Google Scholar]
- Appel, L. F., 1992. The cloning and characterization of Stubble-stubbloid, a morphogenetic locus in Drosophila. Ph.D. Thesis, University of California, Berkeley, CA.
- Appel, L. F., M. Prout, R. Abu-Shumays, A. Hammonds, J. C. Garbe et al., 1993. The Drosophila Stubble-stubbloid gene encodes an apparent transmembrane serine protease required for epithelial morphogenesis. Proc. Natl. Acad. Sci. USA 90: 4937–4941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer, C. A., S. R. Halsell, J. W. Fristrom, D. P. Kiehart and L. von Kalm, 2003. Genetic interactions between the RhoA and Stubble-stubbloid loci suggest a role for a type II transmembrane serine protease in intracellular signaling during Drosophila imaginal disc morphogenesis. Genetics 165: 1417–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaton, A. H., I. Kiss, D. Fristrom and J. W. Fristrom, 1988. Interaction of the Stubble-stubbloid locus and the Broad-complex of Drosophila melanogaster. Genetics 120: 453–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham, P. M., and C. H. Chapman, 1986. Evidence that white-blood is a novel type of temperature-sensitive mutation resulting from temperature-dependent effects of a transposon insertion on formation of white transcripts. EMBO J. 5: 3343–3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birr, C. A., D. Fristrom, D. S. King and J. W. Fristrom, 1990. Ecdysone-dependent proteolysis of an apical surface glycoprotein may play a role in imaginal disc morphogenesis in Drosophila. Development 110: 239–248. [DOI] [PubMed] [Google Scholar]
- Bode, W., P. Schwager and R. Huber, 1978. The transition of bovine trypsinogen to a trypsin-like state upon strong ligand binding. The refined crystal structures of the bovine trypsinogen-pancreatic trypsin inhibitor complex and of its ternary complex with Ile-Val at 1.9 A resolution. J. Mol. Biol. 118: 99–112. [DOI] [PubMed] [Google Scholar]
- Brower, D. L., M. Piovant, R. Salatino, J. Brailey and M. J. Hendrix, 1987. Identification of a specialized extracellular matrix component in Drosophila imaginal discs. Dev. Biol. 119: 373–381. [DOI] [PubMed] [Google Scholar]
- Chen, G. C., P. Gajowniczek and J. Settleman, 2004. Rho-LIM kinase signaling regulates ecdysone-induced gene expression and morphogenesis during Drosophila metamorphosis. Curr. Biol. 14: 309–313. [DOI] [PubMed] [Google Scholar]
- Clark, H. F., D. Brentrup, K. Schneitz, A. Bieber, C. Goodman et al., 1995. Dachsous encodes a member of the cadherin superfamily that controls imaginal disc morphogenesis in Drosophila. Genes Dev. 9: 1530–1542. [DOI] [PubMed] [Google Scholar]
- Condic, M. L., D. Fristrom and J. W. Fristrom, 1991. Apical cell shape changes during Drosophila imaginal leg disc elongation: a novel morphogenetic mechanism. Development 111: 23–33. [DOI] [PubMed] [Google Scholar]
- Dery, O., C. U. Corvera, M. Steinhoff and N. W. Bunnett, 1998. Proteinase-activated receptors: novel mechanisms of signaling by serine proteases. Am. J. Physiol. 274: C1429–C1452. [DOI] [PubMed] [Google Scholar]
- Dobzhansky, T., 1929. The manifold effects of the genes Stubble and stubbloid in Drosophila melanogaster. Z. Indukt. Abstamm. Vererbungsl. 54: 427–457. [Google Scholar]
- Edwards, K. A., and D. P. Kiehart, 1996. Drosophila nonmuscle myosin II has multiple essential roles in imaginal disc and egg chamber morphogenesis. Development 122: 1499–1511. [DOI] [PubMed] [Google Scholar]
- Evangelista, M., D. Pruyne, D. C. Amberg, C. Boone and A. Bretscher, 2002. Formins direct Arp2/3-independent actin filament assembly to polarize cell growth in yeast. Nat. Cell Biol. 4: 260–269. [DOI] [PubMed] [Google Scholar]
- Fekete, E., D. Fristrom, I. Kiss and J. W. Fristrom, 1975. The mechanism of evagination of imaginal discs of Drosophila melanogaster II. Studies on trypsin-accelerated evagination. Roux's Arch. Dev. Biol. 178: 123–138. [DOI] [PubMed] [Google Scholar]
- Fessler, J. H., and L. I. Fessler, 1989. Drosophila extracellular matrix. Annu. Rev. Cell Biol. 5: 309–339. [DOI] [PubMed] [Google Scholar]
- Fessler, L. I., M. L. Condic, R. E. Nelson, J. H. Fessler and J. W. Fristrom, 1993. Site-specific cleavage of basement membrane collagen IV during Drosophila metamorphosis. Development 117: 1061–1069. [DOI] [PubMed] [Google Scholar]
- Friedrich, R., P. Fuentes-Prior, E. Ong, G. Coombs, M. Hunter et al., 2002. Catalytic domain structures of MT-SP1/matriptase, a matrix-degrading transmembrane serine proteinase. J. Biol. Chem. 277: 2160–2168. [DOI] [PubMed] [Google Scholar]
- Fristrom, D., 1988. The cellular basis of epithelial morphogenesis. A review. Tissue Cell 20: 645–690. [DOI] [PubMed] [Google Scholar]
- Fristrom, D., and J. W. Fristrom, 1975. The mechanism of evagination of imaginal discs of Drosophila melanogaster. 1. General considerations. Dev. Biol. 43: 1–23. [DOI] [PubMed] [Google Scholar]
- Fristrom, D., and J. Fristrom, 1993. The metamorphic development of the adult epidermis, pp. 843–897 in The Development of Drosophila melanogaster, edited by M. Bate and A. Martinez-Arias. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Frohman, M. A., 1993. Rapid amplification of complementary DNA ends for generation of full-length complementary DNAs: thermal RACE. Methods Enzymol. 218: 340–356. [DOI] [PubMed] [Google Scholar]
- Furie, B., and B. C. Furie, 1988. The molecular basis of blood coagulation. Cell 53: 505–518. [DOI] [PubMed] [Google Scholar]
- Gates, J., and C. S. Thummel, 2000. An enhancer trap screen for ecdysone-inducible genes required for Drosophila adult leg morphogenesis. Genetics 156: 1765–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay, N. J., and F. J. Keith, 1992. Regulation of translation and proteolysis during the development of embryonic dorso-ventral polarity in Drosophila. Homology of easter proteinase with Limulus proclotting enzyme and translational activation of Toll receptor synthesis. Biochim. Biophys. Acta 1132: 290–296. [DOI] [PubMed] [Google Scholar]
- Gotwals, P. J., and J. W. Fristrom, 1991. Three neighboring genes interact with the Broad-Complex and the Stubble-stubbloid locus to affect imaginal disc morphogenesis in Drosophila. Genetics 127: 747–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guild, G. M., P. S. Connelly, K. A. Vranich, M. K. Shaw and L. G. Tilney, 2002. Actin filament turnover removes bundles from Drosophila bristle cells. J. Cell Sci. 115: 641–653. [DOI] [PubMed] [Google Scholar]
- Guild, G. M., P. S. Connelly, L. Ruggiero, K. A. Vranich and L. G. Tilney, 2003. Long continuous actin bundles in Drosophila bristles are constructed by overlapping short filaments. J. Cell Biol. 162: 1069–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halsell, S. R., B. I. Chu and D. P. Kiehart, 2000. Genetic analysis demonstrates a direct link between rho signaling and nonmuscle myosin function during Drosophila morphogenesis. Genetics 155: 1253–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammonds, A. S., 2002. Mutational analysis of Drososphila Stubble-stubbloid gene function in ecdysone-regulated morphogenesis. Ph.D. Thesis, University of California, Berkeley, CA.
- Hartenstein, V., and J. W. Posakony, 1989. Development of adult sensilla on the wing and notum of Drosophila melanogaster. Development 107: 389–405. [DOI] [PubMed] [Google Scholar]
- Hooper, J. D., J. A. Clements, J. P. Quigley and T. M. Antalis, 2001. Type II transmembrane serine proteases. Insights into an emerging class of cell surface proteolytic enzymes. J. Biol. Chem. 276: 857–860. [DOI] [PubMed] [Google Scholar]
- Imamura, H., K. Tanaka, T. Hihara, M. Umikawa, T. Kamei et al., 1997. Bni1p and Bnr1p: downstream targets of the Rho family small G-proteins which interact with profilin and regulate actin cytoskeleton in Saccharomyces cerevisiae. EMBO J. 16: 2745–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwakiri, K., M. Ghazizadeh, E. Jin, M. Fujiwara, T. Takemura et al., 2004. Human airway trypsin-like protease induces PAR-2-mediated IL-8 release in psoriasis vulgaris. J. Invest. Dermatol. 122: 937–944. [DOI] [PubMed] [Google Scholar]
- Jiang, H., and M. R. Kanost, 2000. The clip-domain family of serine proteinases in arthropods. Insect Biochem. Mol. Biol. 30: 95–105. [DOI] [PubMed] [Google Scholar]
- Kohno, H., K. Tanaka, A. Mino, M. Umikawa, H. Imamura et al., 1996. Bni1p implicated in cytoskeletal control is a putative target of Rho1p small GTP binding protein in Saccharomyces cerevisiae. EMBO J. 15: 6060–6068. [PMC free article] [PubMed] [Google Scholar]
- Kong, W., K. McConalogue, L. M. Khitin, M. D. Hollenberg, D. G. Payan et al., 1997. Luminal trypsin may regulate enterocytes through proteinase-activated receptor 2. Proc. Natl. Acad. Sci. USA 94: 8884–8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krem, M. M., and E. Di Cera, 2001. Molecular markers of serine protease evolution. EMBO J. 20: 3036–3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees, A. D., and L. E. R. Picken, 1945. Shape in relation to the fine structure in the bristles of Drosophila melanogaster. Proc. R. Soc. Lond. Ser. B 132: 396–423. [Google Scholar]
- Lees, A. D., and C. H. Waddington, 1942. The development of bristles in normal and some mutant types of Drosophila melanogaster. Proc. R. Soc. Lond. Ser. B 131: 87–110. [Google Scholar]
- Lewis, E. B., 1948. New mutants. Dros. Inf. Ser. 22: 72–73. [Google Scholar]
- Lin, C. Y., J. Anders, M. Johnson, Q. A. Sang and R. B. Dickson, 1999. Molecular cloning of cDNA for matriptase, a matrix-degrading serine protease with trypsin-like activity. J. Biol. Chem. 274: 18231–18236. [DOI] [PubMed] [Google Scholar]
- Lindsley, D. L., and E. H. Grell, 1968. Genetic Variations of Drosophila melanogaster. Pub. 627, Carnegie Institute, Washington, DC.
- Madison, E. L., A. Kobe, M. J. Gething, J. F. Sambrook and E. J. Goldsmith, 1993. Converting tissue plasminogen activator to a zymogen: a regulatory triad of Asp-His-Ser. Science 262: 419–421. [DOI] [PubMed] [Google Scholar]
- Moore, W. G., 1935. New mutants. Dros. Inf. Ser. 3: 27. [Google Scholar]
- Muta, T., R. Hashimoto, T. Miyata, H. Nishimura, Y. Toh et al., 1990. Proclotting enzyme from horseshoe crab hemocytes. cDNA cloning, disulfide locations, and subcellular localization. J. Biol. Chem. 265: 22426–22433. [PubMed] [Google Scholar]
- Muta, T., T. Nakamura, R. Hashimoto, T. Morita and S. Iwanaga, 1993. Limulus proclotting enzyme. Methods Enzymol. 223: 352–358. [DOI] [PubMed] [Google Scholar]
- Nelson, C. R., and P. Szauter, 1992. Cytogenetic analysis of chromosome region 89A of Drosophila melanogaster: isolation of deficiencies and mapping of Po, Aldox-1 and transposon insertions. Mol. Gen. Genet. 235: 11–21. [DOI] [PubMed] [Google Scholar]
- Netzel-Arnett, S., J. D. Hooper, R. Szabo, E. L. Madison, J. P. Quigley et al., 2003. Membrane anchored serine proteases: a rapidly expanding group of cell surface proteolytic enzymes with potential roles in cancer. Cancer Metastasis Rev. 22: 237–258. [DOI] [PubMed] [Google Scholar]
- Overton, J., 1967. The fine structure of developing bristles in wild type and mutant Drosophila melanogaster. J. Morphol. 122: 367–379. [DOI] [PubMed] [Google Scholar]
- Pena-Rangel, M. T., I. Rodriguez and J. R. Riesgo-Escovar, 2002. A misexpression study examining dorsal thorax formation in Drosophila melanogaster. Genetics 160: 1035–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pino-Heiss, S., and G. Schubiger, 1989. Extracellular protease production by Drosophila imaginal discs. Dev. Biol. 132: 282–291. [DOI] [PubMed] [Google Scholar]
- Poodry, C. A., and H. A. Schneiderman, 1971. Intercellular adhesivity and pupal morphogenesis in Drosophila melanogaster. Roux's Arch. Dev. Biol. 168: 1–9. [DOI] [PubMed] [Google Scholar]
- Pruyne, D., M. Evangelista, C. Yang, E. Bi, S. Zigmond et al., 2002. Role of formins in actin assembly: nucleation and barbed-end association. Science 297: 612–615. [DOI] [PubMed] [Google Scholar]
- Rawlings, N. D., D. P. Tolle and A. J. Barrett, 2004. MEROPS: the peptidase database. Nucleic Acids Res. 32: D160–D164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley, A. J., 2001. Rho family proteins: coordinating cell responses. Trends Cell Biol. 11: 471–477. [DOI] [PubMed] [Google Scholar]
- Rubin, G. M., and A. C. Spradling, 1982. Genetic transformation of Drosophila with transposable element vectors. Science 218: 348–353. [DOI] [PubMed] [Google Scholar]
- Sagot, I., S. K. Klee and D. Pellman, 2002. Yeast formins regulate cell polarity by controlling the assembly of actin cables. Nat. Cell Biol. 4: 42–50. [DOI] [PubMed] [Google Scholar]
- Smith, C. L., and R. DeLotto, 1992. A common domain within the proenzyme regions of the Drosophila snake and easter proteins and Tachypleus proclotting enzyme defines a new subfamily of serine proteases. Protein Sci. 1: 1225–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, C., H. Giordano and R. DeLotto, 1994. Mutational analysis of the Drosophila snake protease: an essential role for domains within the proenzyme polypeptide chain. Genetics 136: 1355–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi, T., J. L. Harris, W. Huang, K. W. Yan, S. R. Coughlin et al., 2000. Cellular localization of membrane-type serine protease 1 and identification of protease-activated receptor-2 and single-chain urokinase-type plasminogen activator as substrates. J. Biol. Chem. 275: 26333–26342. [DOI] [PubMed] [Google Scholar]
- Tan, J. L., S. Ravid and J. A. Spudich, 1992. Control of nonmuscle myosins by phosphorylation. Annu. Rev. Biochem. 61: 721–759. [DOI] [PubMed] [Google Scholar]
- Tapon, N., and A. Hall, 1997. Rho, Rac and Cdc42 GTPases regulate the organization of the actin cytoskeleton. Curr. Opin. Cell Biol. 9: 86–92. [DOI] [PubMed] [Google Scholar]
- Thummel, C. S., 1997. Dueling orphans: interacting nuclear receptors coordinate Drosophila metamorphosis. BioEssays 19: 669–672. [DOI] [PubMed] [Google Scholar]
- Thummel, C. S., 2002. Ecdysone-regulated puff genes 2000. Insect Biochem. Mol. Biol. 32: 113–120. [DOI] [PubMed] [Google Scholar]
- Thummel, C. S., and V. Pirrotta, 1992. New pCaSpeR P element vectors. Dros. Inf. Ser. 71: 150. [Google Scholar]
- Tilney, L. G., M. S. Tilney and G. M. Guild, 1995. F actin bundles in Drosophila bristles. I. Two filament cross-links are involved in bundling. J. Cell Biol. 130: 629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney, L. G., P. Connelly, S. Smith and G. M. Guild, 1996. F-actin bundles in Drosophila bristles are assembled from modules composed of short filaments. J. Cell Biol. 135: 1291–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney, L. G., P. S. Connelly, K. A. Vranich, M. K. Shaw and G. M. Guild, 1998. Why are two different cross-linkers necessary for actin bundle formation in vivo and what does each cross-link contribute? J. Cell Biol. 143: 121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney, L. G., P. S. Connelly, K. A. Vranich, M. K. Shaw and G. M. Guild, 2000. Actin filaments and microtubules play different roles during bristle elongation in Drosophila. J. Cell Sci. 113 (Pt. 7): 1255–1265. [DOI] [PubMed] [Google Scholar]
- Tilney, L. G., P. S. Connelly, L. Ruggiero, K. A. Vranich and G. M. Guild, 2003. Actin filament turnover regulated by cross-linking accounts for the size, shape, location, and number of actin bundles in Drosophila bristles. Mol. Biol. Cell 14: 3953–3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney, L. G., P. S. Connelly, L. Ruggiero, K. A. Vranich, G. M. Guild et al., 2004. The role actin filaments play in providing the characteristic curved form of Drosophila bristles. Mol. Biol. Cell 15: 5481–5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usui-Ishihara, A., P. Simpson and K. Usui, 2000. Larval multidendrite neurons survive metamorphosis and participate in the formation of imaginal sensory axonal pathways in the notum of Drosophila. Dev. Biol. 225: 357–369. [DOI] [PubMed] [Google Scholar]
- Van Aelst, L., and C. D'Souza-Schorey, 1997. Rho GTPases and signaling networks. Genes Dev. 11: 2295–2322. [DOI] [PubMed] [Google Scholar]
- von Kalm, L., D. Fristrom and J. Fristrom, 1995. The making of a fly leg: a model for epithelial morphogenesis. BioEssays 17: 693–702. [DOI] [PubMed] [Google Scholar]
- Ward, R. E., J. Evans and C. S. Thummel, 2003. Genetic modifier screens in Drosophila demonstrate a role for Rho1 signaling in ecdysone-triggered imaginal disc morphogenesis. Genetics 165: 1397–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, N., P. Madaule, T. Reid, T. Ishizaki, G. Watanabe et al., 1997. p140mDia, a mammalian homolog of Drosophila diaphanous, is a target protein for Rho small GTPase and is a ligand for profilin. EMBO J. 16: 3044–3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, S., B. Greer, J. Hooper, A. Zijlstra, B. Walker et al., 2005. The membrane-anchored serine protease, TMPRSS2, activates PAR-2 in prostate cancer cells. Biochem. J. 388: 967–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter, C. G., B. Wang, A. Ballew, A. Royou, R. Karess et al., 2001. Drosophila Rho-associated kinase (Drok) links Frizzled-mediated planar cell polarity signaling to the actin cytoskeleton. Cell 105: 81–91. [DOI] [PubMed] [Google Scholar]
- Wulfkuhle, J. D., N. S. Petersen and J. J. Otto, 1998. Changes in the F-actin cytoskeleton during neurosensory bristle development in Drosophila: the role of singed and forked proteins. Cell Motil. Cytoskeleton 40: 119–132. [DOI] [PubMed] [Google Scholar]