Abstract
Nonself recognition in filamentous fungi is conferred by genetic differences at het (heterokaryon incompatibility) loci. When individuals that differ in het specificity undergo hyphal fusion, the heterokaryon undergoes a programmed cell death reaction or is highly unstable. In Neurospora crassa, three allelic specificities at the het-c locus are conferred by a highly polymorphic domain. This domain shows trans-species polymorphisms indicative of balancing selection, consistent with the role of het loci in nonself recognition. We determined that a locus closely linked to het-c, called pin-c (partner for incompatibility with het-c) was required for het-c nonself recognition and heterokaryon incompatibility (HI). The pin-c alleles in isolates that differ in het-c specificity were extremely polymorphic. Heterokaryon and transformation tests showed that nonself recognition was mediated by synergistic nonallelic interactions between het-c and pin-c, while allelic interactions at het-c increased the severity of the HI phenotype. The pin-c locus encodes a protein containing a HET domain; predicted proteins containing HET domains are frequent in filamentous ascomycete genomes. These data suggest that nonallelic interactions may be important in nonself recognition in filamentous fungi and that proteins containing a HET domain may be a key factor in these interactions.
SELF/NONSELF discrimination is a ubiquitous and essential function in both multicellular and microbial species. In vertebrate species, genes involved in self/nonself recognition include the major histocompatibility complex (MHC) (Jones et al. 1998), consisting of >100 highly polymorphic loci. In plants, algae, and fungi, highly polymorphic loci have been implicated in nonself recognition during sexual reproduction. Alleles at both the MHC and the sexual nonself recognition loci are highly polymorphic and often show trans-species polymorphisms, a feature associated with loci subject to balancing selection (Figueroa et al. 1988; Charlesworth 1995; Klein et al. 1998; Badrane and May 1999). Balancing selection maintains polymorphism at loci in populations, often through multiple speciation events, which, under the neutral theory of evolution, should be lost either by genetic drift or directional selection.
In filamentous fungi, nonself recognition is also important during vegetative growth. A filamentous fungal colony is composed of a network of multinucleate hyphae that are formed via hyphal fusion (Glass et al. 2004). Hyphal fusion also occurs between different individuals, which results in a vegetative heterokaryon containing genetically different nuclei in a common cytoplasm. Nonself recognition and rejection of heterokaryon formation is regulated by het loci (for heterokaryon incompatibility; also called vic loci) (Saupe 2000; Glass and Kaneko 2003). Heterokaryon incompatibility (HI) reduces the risk of transmission of infectious cytoplasmic elements (Debets et al. 1994; Cortesi et al. 2001) and exploitation by aggressive genotypes (Debets and Griffiths 1998). In some cases, DNA polymorphisms associated with het allele specificity show trans-species polymorphisms (Wu et al. 1998), indicating that some of these loci are subject to balancing selection, an observation consistent with their role in mediating nonself recognition.
Two types of genetic systems, allelic and nonallelic, regulate HI in filamentous fungi (Saupe 2000; Glass and Kaneko 2003). In nonallelic systems, mostly characterized in Podospora anserina, HI is triggered by an interaction between specific alleles at two different het loci. In allelic systems, such as those described for numerous filamentous ascomycete species, including Neurospora crassa, nonself recognition is triggered by alternative allelic specificities at a single het locus.
In N. crassa, 11 allelic het loci have been identified by genetic analysis (Perkins 1988). For the het-c locus, isolates from populations fall into one of three allelic specificity groups (Mylyk 1976; Howlett et al. 1993; Saupe and Glass 1997), referred to as het-cOR, het-cPA, or het-cGR (herein referred to as het-c1, het-c2, and het-c3, respectively). Transformants, heterokaryons, or partial diploids containing het-c alleles of alternate specificity are aconidial and show severe growth inhibition and hyphal compartmentation and death (Garnjobst and Wilson 1956; Perkins 1975; Mylyk 1976; Saupe and Glass 1997; Jacobson et al. 1998; Wu and Glass 2001). Allelic specificity at het-c is dependent upon an indel encoding 30–48 aa; swapping of this variable region between alleles switches het-c allelic specificity (Saupe and Glass 1997; Wu and Glass 2001). Co-immunoprecipitation experiments showed that a HET-C heterocomplex composed of alternative HET-C proteins is associated with HI, suggesting that a HET-C heterocomplex may play a role in nonself recognition (Sarkar et al. 2002).
In an effort to identify additional genes required for het-c incompatibility, we identified mutants that suppressed het-c incompatibility. In this article, we describe the isolation and characterization of a pin-c (partner for incompatibility with het-c) mutant, which displays temperature-sensitive HI. The pin-c locus is closely linked to het-c and encodes alleles that are highly polymorphic in strains of alternate het-c specificity. The pin-c locus encodes a protein predicted to contain a HET domain. Five of the six molecularly characterized het interactions in filamentous ascomycete species involve a protein containing a HET domain. Functional analysis of het-c and pin-c mutants showed that nonallelic interactions between het-c and pin-c are essential for nonself recognition and HI, while het-c allelic interactions contribute to the HI phenotype.
MATERIALS AND METHODS
Strains and culture conditions:
All strains used in this study are listed in Table 1. The three het-c allelic specificities are referred to as het-c1 (het-cOR-type), het-c2 (het-cPA-type), and het-c3 (het-cGR-type) (Saupe and Glass 1997). The corresponding pin-c alleles are referred to as pin-c1, pin-c2, and pin-c3. To construct mutants of het-c2, a 4-kbp fragment encoding the entire het-c2 allele was introduced into C9-2 (Table 1). To construct mutants in pin-c1 or pin-c2, a 1.5-kbp fragment from the 5′ region of pin-c1 or a 1.5-kbp fragment from the 5′ region of pin-c2 was introduced into RLM 57-30 or C9-2 (Table 1), respectively. All fragments were cloned into pCB1004, which confers hygromycin resistance (Carroll et al. 1994), and introduced into N. crassa strains via electroporation. Transformants were used in crosses to isolate mutants by repeat-induced point (RIP) mutation, a naturally occurring mutagenic process in N. crassa (Selker 1997). IK11-20 was a previously uncharacterized het-c1 mutant (Saupe et al. 1996). Strains were grown on Vogel's minimum medium (MM) (Vogel 1956) at 22° or 34°, with appropriate supplements added, if required.
TABLE 1.
Strains used in this study
| Strain | Genotypea | Origin |
|---|---|---|
| het-c1/pin-c1 strains | ||
| RLM 57-30 | cyh-1; pyr-4 A | Gift from R. L. Metzenberg |
| FGSC 4564 | ad-3B cyh-1 am1 | FGSC |
| IK09-15 | thr-2; pan-2 A | This study |
| IK09-51 | thr-2; arg-5 A | This study |
| KD02-10 | his-3; pyr-4; pan-2 a | This study |
| IK11-20 | het-c1m20; nic-3 a | This study |
| IR042 | pin-c1m42 pyr-4; pan-2 a | This study |
| IR0128 | pin-c1m128 pyr-4 a | This study |
| het-c2/pin-c2 strains | ||
| C9-2 | thr-2 a | Smith et al. (2000) |
| C9-15 | thr-2 A | Smith et al. (2000) |
| Xa-2 | arg-5; pan-2 a | Xiang and Glass (2002) |
| IK09-2 | thr-2; pan-2 A | This study |
| CJ44 | Δhet-c2 Δpin-c2 arg-5; pan-2 A | Wu and Glass (2001) |
| C7-47 | het-c2m47 thr-2 a | This study |
| KD06-15 | his-3; thr-2; pan-2 a | This study |
| XK5 | pin-c2m5 arg-5; pan-2 A | This study |
| XK108 | pin-c2m108 thr-2 arg-5 A | This study |
| IRP66 | pin-c2m66 thr-2 A | This study |
| IRP138 | pin-c2m138 thr-2 A | This study |
| IRP153 | pin-c2m153 thr-2; pan-2 A | This study |
| IRP163 | pin-c2m163 thr-2; pan-2 a | This study |
| IRP216 | pin-c2m216 thr-2 a | This study |
| IRP228 | pin-c2m228 thr-2; pan-2 a | This study |
| het-c3/pin-c3 strain | ||
| FGSC 1945 | Groveland WT a | FGSC |
FGSC, Fungal Genetic Stock Center.
All strains, except FGSC 1945, carry Oak Ridge alleles at all other het loci other than those designated in the table. Uppercase designation refers to mutant allele number.
Heterokaryon tests:
Heterokaryon tests were performed as described (Xiang and Glass 2002). To compare growth rates, heterokaryons were cultured in race tubes at 22° or 34°. Data were collected from three independent experiments.
Transformation:
Transformation of N. crassa conidia by electroporation was performed as described (Margolin et al. 1997). Constructs were made in pBM61 (Margolin et al. 1997) and were targeted to the his-3 locus by homologous recombination. Fifty transformants were picked for each DNA construct and growth rates and morphology were evaluated.
Hyphal compartmentation and death assays:
Conidial suspensions of strains used for forcing heterokaryons were co-inoculated onto minimal medium containing methionine (to reduce leakiness of the thr-2 marker) and incubated for 1 day to establish heterokaryotic growth. A small square of hyphae was transferred onto sterile cellophane on a MM plate containing methionine and 0.003% methylene blue, an indicator used to identify dead cells (Suzuki et al. 2000). After 1 day at 22° or 34°, the cellophane was removed from the plates, and the presence or absence of dead hyphal compartments was evaluated by microscopy. Wild-type colonies show ∼1% cell death, while het-c incompatible colonies show 20–30% dead hyphal compartments, which are often clustered within the colony (Jacobson et al. 1998; Wu and Glass 2001; Xiang and Glass 2002).
Cloning of pin-c2 and pin-c3:
The region containing pin-c2 was amplified from the het-c2 strain C9-2 (Table 1) by inverse PCR. Genomic DNA was digested with XbaI and treated with T4 DNA ligase before being used as a template. PCR was performed with the het-c2-specific primers CP1 (5′-TTAGCAGACGCAACCCTTGA-3′) and CP8 (5′-CGAACCTGGTCTGTCTTTCT-3′). The pin-c3 allele was amplified from FGSC 1945 (Table 1) by PCR using CG1 (5′-GGGACGAGCATCGGAGGTATTG-3′) and CP30 (5′-AAGTTCGTTCCGAATTGCCTCACC-3′).
Analysis of DNA sequences:
Nucleotide sequences were determined at the DNA Sequencing Facility, University of California, Berkeley, California. Amino acid sequence alignments were created by CLUSTALw1.7 with manual adjustments and shaded by MacBoxshade2.15.
RESULTS
A pin-c mutant shows temperature-dependent HI:
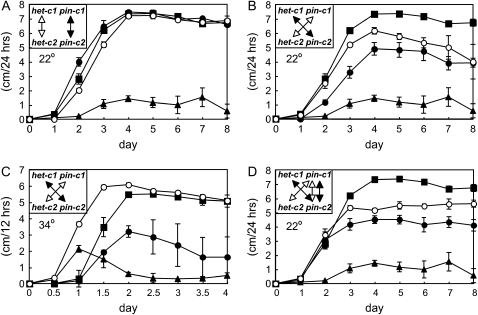
Previously, a number of mutants that suppressed het-c HI were identified (Xiang and Glass 2002, 2004; Xiang 2003). One of these mutants (XK5) showed temperature-dependent HI. A typical het-c incompatible heterokaryon (IK09-2 + FGSC 4564) is aconidial and severely inhibited in its growth and displays hyphal death at both 22° and 34° (Figure 1B). A heterokaryon between XK5 (containing a het-c2 allele) and FGSC 4564 (containing a het-c1 allele) was fully compatible when grown at 34°, but was reduced in conidiation and showed inhibited growth and hyphal death at 22° (Figure 1D, left). A heterokaryon between XK5 and a het-c2 strain (C9-15) was fully compatible at both temperatures (Figure 1C). The mutation conferring temperature-dependent het-c HI, pin-c, mapped to the het-c region.
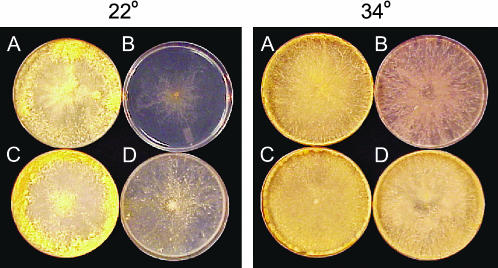
Figure 1.
Temperature-dependent het-c HI phenotype of the pin-c mutant, XK5. (A) A compatible heterokaryon IK09-51 (het-c1 pin-c1) + FGSC 4564 (het-c1 pin-c1). (B) An incompatible heterokaryon IK09-2 (het-c2 pin-c2) + FGSC 4564 (het-c1 pin-c1). (C) A XK5 (het-c2 pin-c2m) + C9-15 (het-c2 pin-c2) heterokaryon with identical het-c specificity. (D) A XK5 (het-c2 pin-c2m) + FGSC 4564 (het-c1 pin-c1) heterokaryon with alternate het-c specificity. All heterokaryons were incubated at 22° for 5 days (left) or 34° for 3 days (right).
The compatible phenotype of the (XK5 + FGSC 4564) heterokaryon at 34° suggested that the mutation in XK5 was dominant at this temperature. However, Southern blot analyses showed that the region surrounding het-c was highly polymorphic between C9-2 (the parental het-c2 strain of XK5) and RLM 57-30 (a het-c1 strain) (data not shown). C9-2 is an introgressed strain that differs in het specificity only at het-c from strains of standard laboratory genetic background (Saupe et al. 1996), such as RLM 57-30. Since pin-c mapped to the het-c region, these observations suggested that the pin-c mutation might be specific for a het-c2 strain. To assess this possibility, a triheterokaryon was forced among a het-c2 pin-cm strain (XK5), a het-c1 strain (IK09-51), and a het-c2 strain (IK09-2). Unlike the (XK5 + FGSC 4564) heterokaryon, the triheterokaryon (XK5 + IK09-51 + IK09-2) showed a typical het-c-incompatible phenotype at both 22° and 34° (Figure 2A). Thus, the inclusion of a het-c2 nucleus in a heterokaryon between XK5 and a het-c1 strain fully complemented the pin-c temperature-sensitive defect in XK5 (a het-c2 strain) and therefore the pin-c mutation in XK5 was actually recessive. These results indicated that het-c1 and het-c2 strains have strain-specific pin-c alleles.
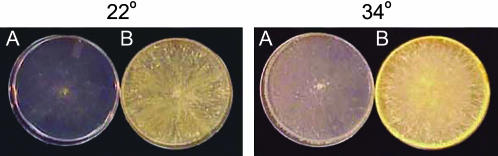
Figure 2.
Triheterokaryon tests with the pin-c mutants. (A) A XK5 (het-c2 pin-c2m) + IK09-2 (het-c2 pin-c2) + IK09-51 (het-c1 pin-c1) triheterokaryon grown at 22° or 34° shows full complementation of HI at both temperatures. (B) By contrast, a XK108 (het-c2 pin-c2m) + CJ44 (Δhet-c2 Δpin-c2) + IK09-15 (het-c1 pin-c1) triheterokaryon grown at 22° or 34° shows an identical phenotype to a heterokaryon between XK108 (het-c2 pin-c2m) and IK09-15 (het-c1 pin-c1), i.e., attenuated heterokaryon incompatibility at 22°, but full compatibility at 34°. Plates were incubated at 22° for 5 days (left) or 34° for 3 days (right).
pin-c encodes a predicted protein containing a HET domain:
Previously, a mutant (CJ44) that contained a deletion that spanned the het-c2 locus was identified (Wu and Glass 2001; Xiang and Glass 2004). The exact size of the deletion in CJ44 could not be determined because of polymorphisms in the region surrounding het-c and an unknown rearrangement at the breakpoint (Xiang and Glass 2004). When CJ44 (Δhet-c2) was used in the triheterokaryon assay (CJ44 + XK108 + IK09-15), the temperature-dependent HI phenotype of a pin-c mutant (XK108) was not complemented (Figure 2B). These results indicated that the deleted region in CJ44 included the pin-c locus. Analyses using DNA fragments surrounding the het-c1 locus as probes showed that the deleted region in CJ44 included a locus centromere distal to het-c. An ORF (NCU03494) adjacent to het-c1 is predicted to encode a protein with a HET domain (PFAM06985) (Figure 3) (http://www.broad.mit.edu/annotation/fungi/neurospora/). The HET domain is composed of three blocks of high amino acid similarity within an ∼200-aa region and is conserved among proteins involved in HI in both N. crassa and P. anserina (Espagne et al. 2002).
Figure 3.
Amino acid sequence alignment of PIN-C1, PIN-C2, and PIN-C3. The three conserved regions of the HET domain identified by alignment of 78 HET domain proteins (PFAM06985); regions that define the HET domain (I, II, and III) are underlined. The amino acid substitution Leu410Pro in the pin-c2m allele in XK5 is indicated with lowercase “p.” Solid background indicates amino acid identity, shaded boxes indicate conserved aa substitutions, and dots indicate deletion events.
NCU03494 was recovered from the het-c2 strain C9-2 by inverse PCR. To test whether NCU03494 from a het-c2 strain could complement the temperature-sensitive HI phenotype of XK5, we introduced NCU03494 from het-c1 and het-c2 strains into the pin-c mutant XK5 via transformation. The introduction of NCU03494 from a het-c1 strain (FGSC 2489) into XK5 failed to restore HI at 34°. By contrast, when NCU03494 from a het-c2 strain (C9-2) was introduced into XK5, most transformants showed the restoration of HI at 34° (data not shown), indicating that NCU03494 encodes pin-c. These results showed that pin-c from a het-c1 strain is functionally different from pin-c from a het-c2 strain. The alleles of NCU03494 in het-c1 and het-c2 strains were therefore designated as pin-c1 and pin-c2, respectively.
The predicted pin-c1 and pin-c2 polypeptides are rich in tryptophan residues (2.99–3.73%) (Tompa 2002), but otherwise have no defining features, other than the presence of a HET domain. Sequence comparison of pin-c2 from XK5 and C9-2 showed a single-nucleotide exchange, T to C, which changed a Leu 410 to a Pro codon within the predicted HET domain region (Figure 3).
The pin-c locus encodes highly polymorphic alleles:
Three functionally alternate alleles have been characterized at het-c, het-c1, het-c2, and het-c3 (Saupe and Glass 1997; Wu and Glass 2001). We therefore isolated the pin-c allele from a het-c3 strain (FGSC 1945 Table 1) by using primers to conserved regions outside of pin-c. The pin-c1, pin-c2, and pin-c3 alleles were highly polymorphic; pin-c1/pin-c2 showed 67% nt identity, pin-c1/pin-c3 showed 62% nt identity, and pin-c2/pin-c3 showed 76% nt identity. In addition to nucleotide diversity at pin-c, a total of 46 indels ranging from 1 to 86 bp in length occurred between the pin-c alleles (Figure 4). Some indels resulted in frameshift mutations, which were compensated by downstream indels to restore the pin-c ORF. All three pin-c alleles contained a single intron with splice sites at an identical location, although the length of the intron was variable: 217, 192, and 110 bp in pin-c1, pin-c2, and pin-c3, respectively. Transcripts of pin-c1 and pin-c2 were detected by RT–PCR and cDNA sequence and RFLP analysis confirmed the presence and splice sites of the predicted intron.
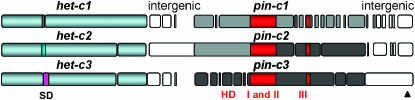
Figure 4.
Representation of het-c1/pin-c1, het-c2/pin-c2, and het-c3/pin-c3 haplotypes. The specificity domain (SD) of het-c (Saupe and Glass 1997) is represented by different colors. Outside the SD, the conserved het-c sequence is shown in blue. A comparison of nucleotide sequences of the three alternate pin-c alleles show numerous indels, as represented by open spaces. The HET domain (HD, in red) is highly conserved. The gray or black shading indicates polymorphisms in the pin-c sequence outside of the HD. The pin-c2 allele shows similarity to pin-c1 in the 5′ region (gray region) and similarity to pin-c3 in the 3′ region (black region). A high level of nucleotide identity among the three het-c haplotypes is restored in the intergenic region 3′ of pin-c (arrowhead).
The predicted protein sequences of pin-c1, pin-c2, and pin-c3 are 884, 989, and 938 aa, respectively; pin-c1/pin-c2 shows 56% aa identity, pin-c1/pin-c3 shows 47% aa identity, and pin-c2/pin-c3 shows 67% aa identity (Figure 3). The three regions of the HET domain were highly conserved (pin-c1/pin-c2: 81% aa identity; pin-c1/pin-c3: 81% aa identity; pin-c2/pin-c3: 98% aa identity; Figure 4). An AG-rich insertion in the predicted N-terminal region of pin-c2 results in 10 repeats of G(K/E)XV (Figure 3). Pairwise comparison between the pin-c alleles showed that pin-c2 was more similar to pin-c3 in the HET domain and C-terminal region, but was more similar to pin-c1 in the N-terminal region. These data suggested that the pin-c2 allele was generated by recombination within the conserved HET domain between pin-c1 and pin-c3 (Figure 4).
pin-c2 loss-of-function mutations confer temperature-dependent het-c HI, but pin-c1 mutations do not:
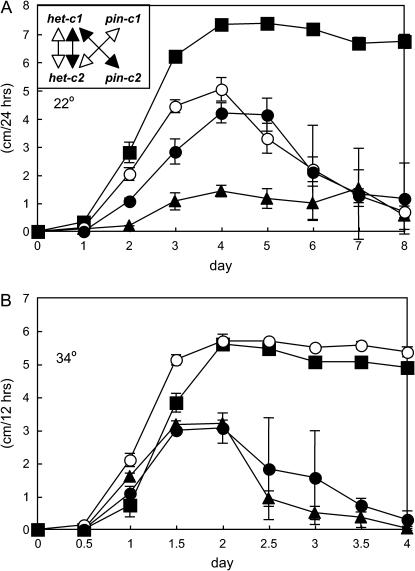
Strains containing pin-c1 and pin-c2 loss-of-function mutations were generated by RIP mutation (Selker 1997). Isogenic het-c3 pin-c3 strains were not available for heterokaryon tests and so the function of pin-c3 was evaluated by transformation (see below). Two independent pin-c1 mutants that carried stop codons at aa positions Q56 and W280 (IRO42 and IRO128, respectively; Table 1) were identified. Three independent pin-c2 mutants were recovered that carried stop codons at aa position W32 (IRP138, IRP216, IRP228; Table 1), plus three additional mutants with a stop codon at W316 (IRP66), at Q151 (IRP163), or at W291 (IRP153; Table 1). These pin-c1m and pin-c2m strains were indistinguishable in vegetative growth and sexual reproduction from wild type. However, similar to the XK5 mutant, all six pin-c2m mutants showed temperature-dependent het-c incompatibility when paired in a heterokaryon with a het-c1 strain. These (het-c1 pin-c1 + het-c2 pin-c2m) heterokaryons showed attenuated HI at 22°, but were fully compatible at 34° (11 cm/day) (Figure 5, A and B, open circles; Table 2). These results show that the amino acid substitution (L410P) in pin-c in the XK5 mutant was a loss-of-function or hypomorphic mutation and indicate that the conserved HET domain region in PIN-C2 is essential for function. By contrast, the phenotype of a (het-c1 pin-c1m + het-c2 pin-c2) heterokaryon was similar in phenotype to a wild-type incompatible heterokaryon at 34° (Figure 5, A and B, solid circles), although it showed attenuated HI at 22° (Table 2).
Figure 5.
HI phenotype of pin-c1 and pin-c2 mutants at 22° (A) or 34° (B). (A) A RLM 57-30 (het-c1 pin-c1) + FGSC 4564 (het-c1 pin-c1) heterokaryon (▪) is fully compatible (∼7 cm/day). A FGSC 4564 (het-c1 pin-c1) + C9-2 (het-c2 pin-c2) heterokaryon (▴) is incompatible (∼1 cm/day). A FGSC 4564 (het-c1 pin-c1) + IRP228 (het-c2 pin-c2m) heterokaryon (○) initially grew at ∼5 cm/day, followed by growth arrest (∼1 cm/day). An IRO42 (het-c1 pin-c1m) and C9-2 (het-c2 pin-c2) heterokaryon (•) initially grew at ∼4 cm/day, followed by growth arrest. (B) The (RLM 57-30 + FGSC 4564) (▪) heterokaryon grew at ∼11 cm/day, while the (FGSC 4564 + C9-2) heterokaryon (▴) grew at ∼6 cm/day and underwent growth arrest. The (FGSC 4564 + IRP228) heterokaryon (○) was fully compatible (∼11 cm/day), while the (IRO42 + C9-2) heterokaryon (•) showed a phenotype similar to an incompatible control. Predicted het-c1/het-c2 allelic and het-c1/pin-c2 or het-c2/pin-c1 nonallelic interactions in the heterokaryons are indicated in the inset in A.
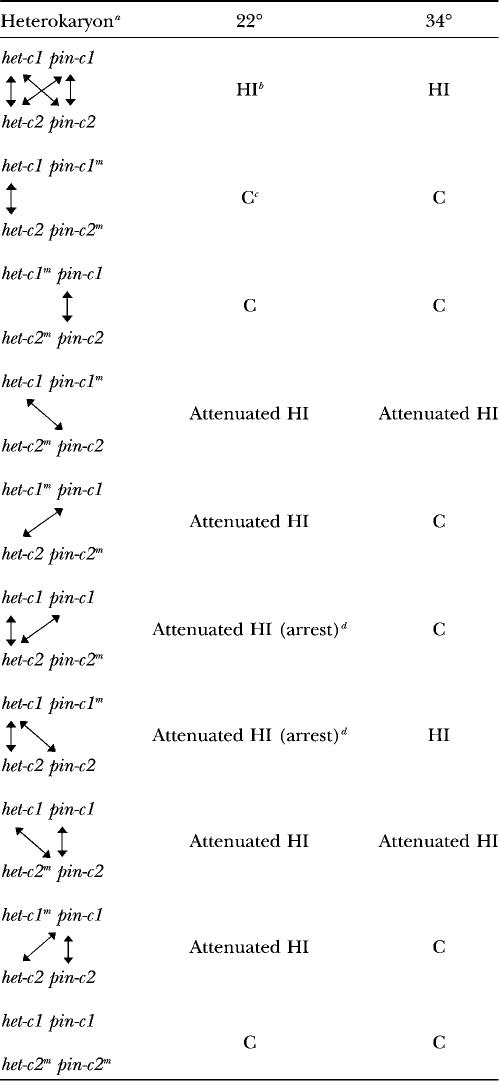
TABLE 2.
Genotype and phenotype of heterokaryons
aArrows indicate functional interactions.
bHeterokaryons showed typical HI.
cHeterokaryons showed a fully compatible phenotype (C).
dHeterokaryons showed attenuated HI, but subsequently underwent growth arrest.
Allelic interactions at het-c or pin-c do not confer HI:
The heterokaryon results presented above suggested that either pin-c functions as an independent het locus or nonallelic interactions between het-c and pin-c are required for nonself recognition and HI. To differentiate these two possibilities, we constructed strains that contained mutations at het-c and/or pin-c and assessed their phenotype in pairwise combinations via heterokaryon tests.
To test whether het-c allelic interactions were sufficient for HI, we assessed the phenotype of heterokaryons carrying mutations at pin-c. Surprisingly, a (het-c1 pin-c1m + het-c2 pin-c2m) heterokaryon was fully compatible and lacked hyphal death at both 22° (Figure 6A, open circles) and 34° (Table 2). These data indicate that allelic interactions between alternative het-c alleles were not sufficient to confer nonself recognition and HI. Thus, the het-c locus is not an allelic het locus in N. crassa.
Figure 6.
HI phenotype of heterokaryons having het-c or pin-c allelic or het-c/pin-c nonallelic interactions at 22° (A, B, and D) or 34° (C). A compatible heterokaryon (RLM 57-30 + FGSC 4564) (▪) and incompatible heterokaryon (FGSC 4564 + C9-2) (▴) were used as controls. (A) A (IRO42 + IRP216) heterokaryon (○) with a het-c allelic interaction only (het-c1 pin-c1m + het-c2 pin-c2m) was fully compatible. A IK11-20 + C7-47 heterokaryon (•) with a pin-c allelic interaction only (het-c1m pin-c1 + het-c2m pin-c2) was fully compatible. (B) A (IRO42 + C7-47) heterokaryon (•) with a het-c1/pin-c2 nonallelic interaction (het-c1 pin-c1m + het-c2m pin-c2) grew at ∼5 cm/day. A (IK11-20 + IRP228) heterokaryon (○) with a het-c2/pin-c1 nonallelic interaction (het-c1m pin-c1 + het-c2 pin-c2m) grew at ∼5.5 cm/day. (C) A (IRO42 + C7-47) heterokaryon (•) with a het-c1/pin-c2 nonallelic interaction grew at ∼5 cm/day. The (IK11-20 + IRP228) heterokaryon (○) with a het-c2/pin-c1 nonallelic interaction (het-c1m pin-c1 + het-c2 pin-c2m) was fully compatible (∼11.5 cm/day). (D) A (FGSC 4564 + C7-47) heterokaryon (•) containing het-c1/pin-c2 and pin-c1/pin-c2 interactions was identical in phenotype to a heterokaryon containing only a het-c1/pin-c2 interaction (IRO42 + C7-47) (B). A (IK11-20 + Xa-2) heterokaryon (○) with het-c2/pin-c1 and pin-c1/pin-c2 interactions was identical in phenotype to (IK11-20 + IRP228) (B). Predicted het-c or pin-c allelic or het-c/pin-c nonallelic interactions are shown in the insets as solid and open arrows, which correspond to solid or open circles in A–D.
To test whether pin-c functions as an independent het locus, a (het-c1m pin-c1 + het-c2m pin-c2) heterokaryon was constructed. Similar to the pin-cm heterokaryon above, the het-cm heterokaryon showed full compatibility and lacked hyphal death at both temperatures (Figure 6A, solid circles; Table 2). These data indicate that pin-c also does not function as an independent allelic het locus in N. crassa.
Nonallelic interactions between het-c and pin-c are essential for het-c HI:
The results presented above indicate that nonallelic interactions between het-c and pin-c must be required for nonself recognition and HI in N. crassa. This hypothesis was evaluated using the following heterokaryons: (het-c1 pin-c1m + het-c2m pin-c2) for het-c1/pin-c2 nonallelic interaction and (het-c1m pin-c1 + het-c2 pin-c2m) for het-c2/pin-c1 nonallelic interaction. Both of these heterokaryons showed hyphal death and growth rate reduction to ∼5 cm/day at 22° (Figure 6B, solid and open circles, respectively). Unlike a het-c-incompatible heterokaryon (Figure 6B, triangles), severe growth inhibition and growth arrest did not occur in heterokaryons with only one het-c/pin-c nonallelic interaction (Table 2).
As expected, a (het-c1m pin-c1 + het-c2 pin-c2m) heterokaryon was fully compatible at 34° and displayed a temperature-dependent HI phenotype identical to the original XK5 mutant (Figure 6C, open circles). These data indicate that the genetic interaction between het-c2 and pin-c1 is thermosensitive (Table 2). By contrast, the (het-c1 pin-c1m + het-c2m pin-c2) heterokaryon showed attenuated HI with a reduction in growth rate to ∼5 cm/day at 34° (Figure 6C, solid circles).
The above heterokaryon results showed that het-c/pin-c nonallelic interactions are essential for HI in N. crassa. However, the phenotype of the incompatible heterokaryons carrying only one nonallelic het-c/pin-c combination was much less severe than that of a typical wild-type het-c-incompatible heterokaryon (Figure 6, B and C). These data suggest that het-c or pin-c allelic interactions contribute to the phenotype of het-c HI when nonallelic interactions are present or that the het-c/pin-c interactions act synergistically or both.
het-c, but not pin-c, allelic interactions contribute to het-c HI when het-c/pin-c nonallelic interactions are functional:
To evaluate the possibility that pin-c allelic interactions may be important for HI when het-c/pin-c nonallelic interactions were functional, we compared the phenotype of the following heterokaryons: (het-c1 pin-c1m + het-c2m pin-c2) for het-c1/pin-c2 nonallelic interaction only (Figure 6B, solid circles) and (het-c1 pin-c1 + het-c2m pin-c2) for het-c1/pin-c2 nonallelic interaction plus pin-c allelic interaction (Figure 6D, solid circles). Both heterokaryons were identical in phenotype. Similarly, a (het-c1m pin-c1 + het-c2 pin-c2m) heterokaryon (Figure 6B, open circles) was identical in phenotype to a (het-c1m pin-c1 + het-c2 pin-c2) heterokaryon (Figure 6D, open circles). These data indicate that pin-c allelic interactions do not contribute to HI when het-c/pin-c nonallelic interactions are functional.
To determine whether het-c allelic interactions contribute to HI when het-c/pin-c nonallelic interactions are functional, we compared the phenotype of the following heterokaryons: (het-c1 pin-c1m + het-c2m pin-c2) for het-c1/pin-c2 interaction only (Figure 6B, solid circles) to (het-c1 pin-c1m + het-c2 pin-c2) for het-c1/pin-c2 and het-c allelic interactions (Figure 5A, solid circles). Heterokaryons carrying both het-c allelic and het-c1/pin-c2 nonallelic interactions were initially similar in phenotype to heterokaryons carrying only a het-c1/pin-c2 nonallelic interaction (growth rate of ∼4 cm/day). However, the (het-c1 pin-c1m + het-c2 pin-c2) heterokaryon, which carried both het-c/pin-c nonallelic and het-c allelic interactions, subsequently underwent growth rate reduction (to ∼1 cm/day) and growth arrest (Figure 5A, solid circles). Similarly, a heterokaryon with both het-c allelic and het-c2/pin-c1 nonallelic interactions (het-c1 pin-c1 + het-c2 pin-c2m) (Figure 5A, open circles) underwent growth rate reduction to ∼1 cm/day and growth arrest, unlike a heterokaryon containing only het-c2/pin-c1 non-allelic interaction (het-c1m pin-c1 + het-c2 pin-c2m) (Figure 6B, open circles). These data indicate that both het-c allelic and het-c/pin-c nonallelic interactions contribute to the het-c HI phenotype (Table 2) and that their interactions are synergistic, rather than additive.
Alternate pin-c alleles function to induce nonself recognition and heterokaryon incompatibility:
The above heterokaryon tests indicated that nonself recognition and HI are mediated by nonallelic interactions between het-c and pin-c. To assess the function of pin-c3, we performed transformation experiments in het-c1 pin-c1 and het-c2 pin-c2 strains.
The three alternate pin-c alleles were targeted to the his-3 locus via transformation into KD02-10 (a het-c1 pin-c1 strain; Table 1). The KD02-10 (pin-c1) transformants all showed a compatible phenotype (∼7.5 cm/day). By contrast, a significant portion of the KD02-10 (pin-c2) and KD02-10 (pin-c3) transformants (∼20%) showed variable conidiation and a growth rate reduction to ∼5 cm/day (at 22°). Similarly, ∼16% of KD06-15 (het-c2 pin-c2) transformants carrying pin-c3 showed reduced growth (∼3.5 cm/day) with variable conidiation. These results indicated that pin-c3 was functional in inducing HI and were consistent with the phenotype of heterokaryons carrying only one het-c/pin-c nonallelic interaction (see Figure 6, B and D). By contrast, a more typical, severe het-c incompatible phenotype was observed when het-c1 was introduced into KD06-15 (∼1 cm/day) or when het-c2 was introduced into KD02-10 (∼3 cm/day). Such transformants have both het-c/pin-c nonallelic and het-c allelic interactions contributing to the HI phenotype. These transformation data were consistent with het-c allelic interactions increasing the severity of the incompatibility phenotype when het-c/pin-c nonallelic interactions are functional.
DISCUSSION
In this article, we demonstrate that het-c nonself recognition and HI require nonallelic interactions between het-c and the closely linked locus, pin-c, providing the first definitive molecular and genetic proof that an allelic het locus functions in a nonallelic manner. Each of the three het-c specificities is represented by a specific haplotype with a particular genetic constitution at both het-c and pin-c. Nonallelic interactions at het-c have not been reported, primarily due to the temperature-sensitive nature of the het-c2/pin-c1 interaction and decreased severity in HI in strains with only one functional het-c/pin-c nonallelic interaction. Our analyses showed that a typical het-c HI phenotype requires three synergistic interactions: two nonallelic interactions between het-c and pin-c, which are essential for nonself recognition and HI, and an allelic interaction at het-c, which increases the severity of the HI phenotype.
The data presented in this article indicate that nonself recognition and HI at the het-c locus are mediated by nonallelic interactions between het-c and pin-c. Molecular characterization of two other allelic het loci in N. crassa (mat and het-6) indicates that nonallelic interactions may be the norm for nonself recognition and HI. The genes required for mat incompatibility, mat A-1 and mat a-1, encode evolutionarily unrelated transcription factors (Glass et al. 1990; Staben and Yanofsky 1990). The het-6 haplotype is composed of un-24 and het-6; allele-specific polymorphisms at un-24 and het-6 show severe linkage disequilibrium, suggesting that nonallelic interactions between un-24 and het-6 play a role in het-6 incompatibility (Mir-Rashed et al. 2000). These observations suggest that, similar to mat, het-c, and het-6, interactions at other allelic het loci in N. crassa may also involve nonallelic interactions between closely linked genes.
The pin-c locus encodes a HET domain protein. Remarkably, five of the six molecularly characterized het interactions in filamentous ascomycete species involve genes encoding predicted proteins with HET domains. These include mat incompatibility in N. crassa, which requires the HET domain protein TOL (Shiu and Glass 1999) and het-6 incompatibility; the het-6 locus encodes a HET domain protein (Smith et al. 2000). In P. anserina, het-e and het-d, involved in nonallelic interactions with het-c, encode HET domain proteins (Espagne et al. 2002). The only molecularly characterized het interaction that may not involve a HET domain protein is the het-s locus, which encodes a prion (Coustou et al. 1997). We predict that most, if not all, het interactions in filamentous ascomycete species require a HET domain gene and that nonself recognition requires nonallelic interactions between a HET domain gene and a second gene, whose nature can vary. For example, in N. crassa, the un-24 locus encodes a ribonucleotide reductase (Smith et al. 2000), the mat locus encodes transcription factors (Glass et al. 1990; Staben and Yanofsky 1990), and the het-c locus encodes a plasma membrane protein (Sarkar et al. 2002), while in P. anserina, the het-c locus encodes a glycolipid transfer protein (Mattjus et al. 2003).
Predicted HET domain genes are common in the genomes of filamentous ascomycete species, with ∼50 predicted HET domain proteins in N. crassa, >150 in Stagonospora nodorum, 38 in Magnaporthe grisea, but only 9 in Aspergillus nidulans. Genes encoding HET domain proteins are apparently absent from the genomes of the ascomycete yeast species Saccharomyces cerevisiae and Schizosaccharomyces pombe and the basidiomycete species Ustilago maydis, Cryptococcus neoformans, and Coprinus cinereus. Predicted HET domain proteins are otherwise dissimilar outside of the HET domain and may contain other functional regions, such as a NACHT, acetyltransferase, or protein kinase domains. The NACHT domain (PFAM: PF05729) is common in apoptosis proteins and includes an ∼350-aa nucleoside triphosphatase (NTPase) domain. Preliminary DNA sequence analysis indicates that some of the predicted N. crassa HET domain genes show a level of polymorphism similar to that of pin-c, suggesting that some of these HET domain genes may also function in nonself recognition and HI in N. crassa.
Nonallelic interactions between linked genes are a common theme among nonself recognition systems. In the MHC, the class II loci DQA1 and DQB1 are multiallelic and code for polypeptides that form a heterodimer (Kwok et al. 1993). These two loci are ∼20 kbp apart, but behave as a single genetic unit (Cullen et al. 1997). In the Brassicaceae, self incompatibility relies on nonallelic interactions between the S-locus receptor kinase (SRK) and its ligand S-locus cysteine-rich protein (SCR) (Nasrallah 2002). In the basidiomycete U. maydis, formation of the infectious dikaryon requires an interaction between two closely linked loci, bE and bW (Kahmann et al. 1999). Similar to other nonself recognition systems (Cullen et al. 1997; Awadalla and Charlesworth 1999), a het-c haplotype may be maintained either by the suppression of recombination or by lethality of recombinant progeny. In the Brassicaceae, to maintain the linkage between alleles within a given S-haplotype, recombination is suppressed in the S-locus region, although recombination has occurred in the evolutionary history of these alleles (Awadalla and Charlesworth 1999). A comparison of pin-c1, pin-c2, and pin-c3 alleles suggests that the pin-c2 allele arose due to recombination between pin-c1 and pin-c3. Further analysis of het-c/pin-c interactions will provide an exceptional model to explore the role of recombination vs. mutation in the evolution of matched allelic polymorphisms.
Another feature that het-c HI shares with other nonself recognition systems is that of extreme polymorphism. Similarity between the highly polymorphic SCR alleles in Brassica campestris rarely exceeds 30% (Watanabe et al. 2000; Fobis-Loisy et al. 2004), while in the Solanaceae, alleles at the S-locus are ∼40% identical at the amino acid level (Ioerger et al. 1990). In U. maydis, the N-terminal domain of the multiallelic bE and bW polypeptides is highly variable (Kämper et al. 1995). The pin-c alleles are extremely polymorphic and contain numerous indels and amino acid substitutions. Polymorphisms at nonself recognition loci are maintained by balancing selection, which often leads to trans-species polymorphisms (Ioerger et al. 1990; Klein et al. 1998; May et al. 1999). Allelic polymorphisms that predate speciation occur at het-c (Wu et al. 1998; Jacobson et al. 2004) in the genus Neurospora and its close relatives, Sordaria and Gelasinospora. We predict that polymorphisms regulating allelic specificity at het-c and pin-c will be in severe linkage disequilibrium in N. crassa populations, that polymorphisms at pin-c will be maintained in other Neurospora species, and that the evolutionary history of het-c and pin-c polymorphisms will show congruence.
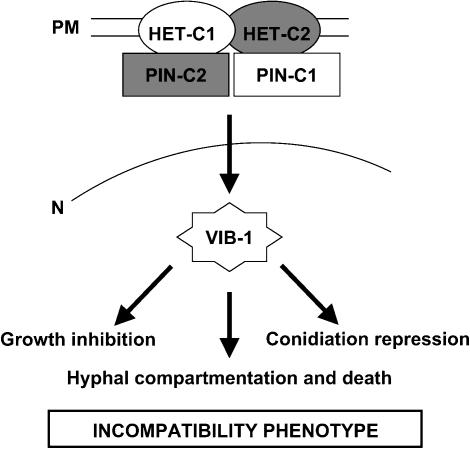
Nonself recognition is often mediated by protein-protein interactions. For example, nonself recognition in U. maydis is mediated by formation of a bE and bW heterocomplex, which occurs only if they are derived from different haplotypes (Kämper et al. 1995). Previously, we proposed that a HET-C heterocomplex is required for nonself recognition and HI (Sarkar et al. 2002). Our data show that genetic differences at pin-c are required for nonself recognition, suggesting that PIN-C may also physically interact with HET-C (Figure 7). The temperature-sensitive nature of the het-c2/pin-c1 interaction supports this hypothesis. Nonself recognition mediated by an interaction between PIN-C and the HET-C heterocomplex might trigger entry into the programmed cell death pathway (Figure 7). Further characterization of HET-C/PIN-C interaction in N. crassa provides an excellent model to unravel molecular mechanisms associated with nonself recognition and programmed cell death in filamentous fungi, the evolution of nonself recognition systems, and the function of HET domain proteins.
Figure 7.
Model for heterokaryon incompatibility mediated by genetic differences at het-c/pin-c. When a hyphal compartment is heterozygous for het-c and pin-c (for example, during a hyphal fusion event or in a partial diploid constructed via a cross or by transformation), nonself recognition is mediated by interactions between HET-C and PIN-C encoded by alternative haplotypes. The recognition event is predicted to occur at the plasma membrane because HET-C is a plasma membrane protein (Sarkar et al. 2002) and PIN-C is predicted to reside in the cytoplasm. The nonself recognition signal is amplified by HET-C1/PIN-C2, HET-C2/PIN-C1, and HET-C1/HET-C2 interactions. The signal is transduced to VIB-1, which is predicted to encode a transcription factor (Xiang and Glass 2002). VIB-1 is required for the repression of conidiation, growth inhibition, and hyphal compartmentation and death. Other factors are also important, because mutations at vib-1 do not completely suppress HI.
Acknowledgments
We thank Maggie Barton for help with DNA sequencing; Dave Jacobson for providing strains used in mapping studies and help with pedigree analysis; Kim Lichtenecker for help with cell death assays; and the Glass Laboratory for critical reading of the manuscript. This work was supported by a RO1 grant (GM60468) from the National Institutes of Health to N.L.G.
References
- Awadalla, P., and D. Charlesworth, 1999. Recombination and selection at Brassica self-incompatibility loci. Genetics 152: 413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badrane, H., and G. May, 1999. The divergence-homogenization duality in the evolution of the b1 mating type gene of Coprinus cinereus. Mol. Biol. Evol. 16: 975–986. [DOI] [PubMed] [Google Scholar]
- Carroll, A. M., J. A. Sweigard and B. Valent, 1994. Improved vectors for selecting resistance to hygromycin. Fungal Genet. Newsl. 41: 22. [Google Scholar]
- Charlesworth, D., 1995. Multi-allelic self-incompatibility polymorphisms in plants. BioEssays 17: 31–38. [Google Scholar]
- Cortesi, P., C. E. McCulloch, H. Y. Song, H. Q. Lin and M. G. Milgroom, 2001. Genetic control of horizontal virus transmission in the chestnut blight fungus, Cryphonectria parasitica. Genetics 159: 107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coustou, V., C. Deleu, S. Saupe and J. Bégueret, 1997. The protein product of the het-s heterokaryon incompatibility gene of the fungus Podospora anserina behaves as a prion analog. Proc. Natl. Acad. Sci. USA 94: 9773–9778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen, M., J. Noble, H. Erlich, K. Thorpe, S. Beck et al., 1997. Characterization of recombination in the HLA class II region. Am. J. Hum. Genet. 60: 397–407. [PMC free article] [PubMed] [Google Scholar]
- Debets, A. J. M., and A. J. F. Griffiths, 1998. Polymorphism of het-genes prevents resource plundering in Neurospora crassa. Mycol. Res. 102: 1343–1349. [Google Scholar]
- Debets, F., X. Yang and A. J. F. Griffiths, 1994. Vegetative incompatibility in Neurospora: its effect on horizontal transfer of mitochondrial plasmids and senescence in natural populations. Curr. Genet. 26: 113–119. [DOI] [PubMed] [Google Scholar]
- Espagne, E., P. Balhadere, M.-L. Penin, C. Barreau and B. Turcq, 2002. HET-E and HET-D belong to a new subfamily of WD40 proteins involved in vegetative incompatibility specificity in the fungus Podospora anserina. Genetics 161: 71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa, F., E. Gunther and J. Klein, 1988. MHC polymorphisms pre-dating speciation. Nature 335: 265–271. [DOI] [PubMed] [Google Scholar]
- Fobis-Loisy, I., C. Miege and T. Gaude, 2004. Molecular evolution of the S locus controlling mating in the Brassicaceae. Plant Biol. 6: 109–118. [DOI] [PubMed] [Google Scholar]
- Garnjobst, L., and J. F. Wilson, 1956. Heterocaryosis and protoplasmic incompatibility in Neurospora crassa. Proc. Natl. Acad. Sci. USA 42: 613–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass, N. L., and I. Kaneko, 2003. Fatal attraction: nonself recognition and heterokaryon incompatibility in filamentous fungi. Eukaryot. Cell 2: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass, N. L., J. Grotelueschen and R. L. Metzenberg, 1990. Neurospora crassa A mating-type region. Proc. Natl. Acad. Sci. USA 87: 4912–4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass, N. L., C. Rasmussen, M. G. Roca and N. D. Read, 2004. Hyphal homing, fusion and mycelial interconnectedness. Trends Microbiol. 12: 135–141. [DOI] [PubMed] [Google Scholar]
- Howlett, B., J. F. Leslie and D. D. Perkins, 1993. Putative multiple alleles at the vegetative (heterokaryon) incompatibility loci het-c and het-8 in Neurospora crassa. Fungal Genet. Newsl. 40: 40–42. [Google Scholar]
- Ioerger, T. R., A. G. Clark and T. H. Kao, 1990. Polymorphism at the self-incompatibility locus in the Solanaceae predates speciation. Proc. Natl. Acad. Sci. USA 87: 9732–9735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson, D. J., K. Beurkens and K. L. Klomparens, 1998. Microscopic and ultrastructural examination of vegetative incompatibility in partial diploids heterozygous at het loci in Neurospora crassa. Fungal Genet. Biol. 23: 45–56. [DOI] [PubMed] [Google Scholar]
- Jacobson, D. J., A. J. Powell, J. R. Dettman, G. S. Saenz, M. M. Barton et al., 2004. Neurospora in temperate forests of western North America. Mycologia 96: 66–74. [PubMed] [Google Scholar]
- Jones, E. Y., J. Tormo, S. W. Reid and D. I. Stuart, 1998. Recognition surfaces of MHC class I. Immunol. Rev. 163: 121–128. [DOI] [PubMed] [Google Scholar]
- Kahmann, R., C. Basse and M. Feldbrügge, 1999. Fungal-plant signalling in the Ustilago maydis-maize pathosystem. Curr. Opin. Microbiol. 2: 647–650. [DOI] [PubMed] [Google Scholar]
- Kämper, J., M. Reichmann, T. Romeis, M. Bölker and R. Kahmann, 1995. Multiallelic recognition: nonself-dependent dimerization of the bE and bW homeodomain proteins in Ustilago maydis. Cell 81: 73–83. [DOI] [PubMed] [Google Scholar]
- Klein, J., A. Sato, S. Nagl and C. O'hUigin, 1998. Molecular trans-species polymorphism. Annu. Rev. Ecol. Syst. 29: 1–21. [Google Scholar]
- Kwok, W. W., S. Kovats, P. Thurtle and G. T. Nepom, 1993. HLA-DQ allelic polymorphisms constrain patterns of class II heterodimer formation. J. Immunol. 150: 2263–2272. [PubMed] [Google Scholar]
- Margolin, B. S., M. Freitag and E. U. Selker, 1997. Improved plasmids for gene targeting at the his-3 locus of Neurospora crassa by electroporation. Fungal Genet. Newsl. 44: 34–36. [Google Scholar]
- Mattjus, P., B. Turcq, H. M. Pike, J. G. Molotkovsky and R. E. Brown, 2003. Glycolipid intermembrane transfer is accelerated by HET-C2, a filamentous fungus gene product involved in cell-cell incompatibility response. Biochemistry 42: 535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May, G., F. Shaw, H. Badrane and X. Vekemans, 1999. The signature of balancing selection: fungal mating compatibility gene evolution. Proc. Natl. Acad. Sci. USA 96: 9172–9177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir-Rashed, N., D. J. Jacobson, M. R. Dehghany, O. C. Micali and M. L. Smith, 2000. Molecular and functional analyses of incompatibility genes at het-6 in a population of Neurospora crassa. Fungal Genet. Biol. 30: 197–205. [DOI] [PubMed] [Google Scholar]
- Mylyk, O. M., 1976. Heteromorphism for heterokaryon incompatibility genes in natural populations of Neurospora crassa. Genetics 83: 275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrallah, J. B., 2002. Recognition and rejection of self in plant reproduction. Science 296: 305–308. [DOI] [PubMed] [Google Scholar]
- Perkins, D. D., 1975. The use of duplication-generating rearrangements for studying heterokaryon incompatibility genes in Neurospora. Genetics 80: 87–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins, D. D., 1988. Main features of vegetative incompatibility in Neurospora crassa. Fungal Genet. Newsl. 35: 44–46. [Google Scholar]
- Sarkar, S., G. Iyer, J. Wu and N. L. Glass, 2002. Nonself recognition is mediated by HET-C heterocomplex formation during vegetative incompatibility. EMBO J. 21: 4841–4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saupe, S. J., 2000. Molecular genetics of heterokaryon incompatibility in filamentous ascomycetes. Microbiol. Mol. Biol. Rev. 64: 489–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saupe, S. J., and N. L. Glass, 1997. Allelic specificity at the het-c heterokaryon incompatibility locus of Neurospora crassa is determined by a highly variable domain. Genetics 146: 1299–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saupe, S. J., G. A. Kuldau, M. L. Smith and N. L. Glass, 1996. The product of the het-C heterokaryon incompatibility gene of Neurospora crassa has characteristics of a glycine-rich cell wall protein. Genetics 143: 1589–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selker, E. U., 1997. Epigenetic phenomena in filamentous fungi: Useful paradigms or repeat-induced confusion? Trends Genet. 13: 296–301. [DOI] [PubMed] [Google Scholar]
- Shiu, P. K., and N. L. Glass, 1999. Molecular characterization of tol, a mediator of mating-type-associated vegetative incompatibility in Neurospora crassa. Genetics 151: 545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, M. L., O. C. Micali, S. P. Hubbard, N. Mir-Rashed, D. J. Jacobson et al., 2000. Vegetative incompatibility in the het-6 region of Neurospora crassa is mediated by two linked genes. Genetics 155: 1095–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staben, C., and C. Yanofsky, 1990. Neurospora crassa a mating-type region. Proc. Natl. Acad. Sci. USA 87: 4917–4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, C., M. Kawano, T. Kashiwagi, Y. Arata, T. Kawasumi et al., 2000. Lethal effect of the expression of a killer gene SMK1 in Saccharomyces cerevisiae. Protein Eng. 13: 73–76. [DOI] [PubMed] [Google Scholar]
- Tompa, P., 2002. Intrinsically unstructured proteins. Trends Biochem. Sci. 27: 527. [DOI] [PubMed] [Google Scholar]
- Vogel, H. J., 1956. A convenient growth medium. Microbiol. Genet. Bull. 13: 42–46. [Google Scholar]
- Watanabe, M., A. Ito, Y. Takada, C. Ninomiya, T. Kakizaki et al., 2000. Highly divergent sequences of the pollen self-incompatibility (S) gene in class-I S haplotypes of Brassica campestris (syn. rapa) L. FEBS Lett. 473: 139–144. [DOI] [PubMed] [Google Scholar]
- Wu, J., and N. L. Glass, 2001. Identification of specificity determinants and generation of alleles with novel specificity at the het-c heterokaryon incompatibility locus of Neurospora crassa. Mol. Cell. Biol. 21: 1045–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, J., S. J. Saupe and N. L. Glass, 1998. Evidence for balancing selection operating at the het-c heterokaryon incompatibility locus in a group of filamentous fungi. Proc. Natl. Acad. Sci. USA 95: 12398–12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang, Q., 2003. Genetic analysis of hyphal fusion and heterokaryon incompatibility in Neurospora crassa. Ph.D. Thesis, University of California, Berkeley, CA.
- Xiang, Q., and N. L. Glass, 2002. Identification of vib-1, a locus involved in vegetative incompatibility mediated by het-c in Neurospora crassa. Genetics 162: 89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang, Q., and N. L. Glass, 2004. Chromosome rearrangements in isolates that escape from het-c heterokaryon incompatibility in Neurospora crassa. Curr. Genetics 44: 329–338. [DOI] [PubMed] [Google Scholar]