Abstract
The Saccharomyces cerevisiae C-type cyclin and its cyclin-dependent kinase (Cdk8p) repress the transcription of several stress response genes. To relieve this repression, cyclin C is destroyed in cells exposed to reactive oxygen species (ROS). This report describes the requirement of cyclin C destruction for the cellular response to ROS. Compared to wild type, deleting cyclin C makes cells more resistant to ROS while its stabilization reduces viability. The Slt2p MAP kinase cascade mediates cyclin C destruction in response to ROS treatment but not heat shock. This destruction pathway is important as deleting cyclin C suppresses the hypersensitivity of slt2 mutants to oxidative damage. The ROS hypersensitivity of an slt2 mutant correlates with elevated programmed cell death as determined by TUNEL assays. Consistent with the viability studies, the elevated TUNEL signal is reversed in cyclin C mutants. Finally, two results suggest that cyclin C regulates programmed cell death independently of its function as a transcriptional repressor. First, deleting its corepressor CDK8 does not suppress the slt2 hypersensitivity phenotype. Second, the human cyclin C, which does not repress transcription in yeast, does regulate ROS sensitivity. These findings demonstrate a new role for the Slt2p MAP kinase cascade in protecting the cell from programmed cell death through cyclin C destruction.
NATURAL antioxidants such as the free radical scavengers glutathione and catalase usually exist in balance with reactive oxygen species (ROS) produced as a by-product of oxidative phosphorylation (reviewed in (Gate et al. 1999). Acute oxidative damage, commonly referred to as oxidative stress, results when this balance is perturbed by the introduction of compounds such as heavy metals or hydrogen peroxide (H2O2) that stimulates free radical production. In response to oxidative stress, genes encoding antioxidants or free radical scavengers are induced to counter the damage caused by reactive oxygen. Cells can respond to oxidative stress by arresting cell cycle progression until the damage is repaired. Alternatively, oxidative damage can elicit programmed cell death (PCD) or apoptosis during which the cell undergoes a series of events leading to nuclear fragmentation and loss of membrane integrity (reviewed in Jiang and Wang 2004). Programmed cell death appears to be a highly conserved process extending from mammals to fungi (Skulachev 2002). For example, execution of the apoptotic program requires the activation of a class of proteases termed caspases (Green and Kroemer 2004). Although several caspases have been identified in human cells, only one has been found in yeast (Madeo et al. 2002), suggesting that the system governing PCD may be less complex in lower organisms.
In budding yeast, the induction of oxidative stress responsive genes requires the activation of well-studied transcription factors, including heat shock factor (Liu and Thiele 1996), Yap1p (Stephen et al. 1995), Rlm1p (Watanabe et al. 1995), and Msn2p/Msn4p (Martinez-Pastor et al. 1996). These factors are effectors of signal transduction networks that transmit a particular signal to the nucleus (Gustin et al. 1998). For example, exposure to ROS stimulates the Slt2p/Mpk1p MAP kinase pathway (Alic et al. 2003; Vilella et al. 2005), which in turn activates Rlm1p (Dodou and Treisman 1997; Staleva et al. 2004). Similarly, the osmolarity sensing MAP kinase Hog1p targets the transcription factors Hot1p and Msn2p/Msn4p (Rep et al. 1999; Alepuz et al. 2001, 2003). However, multiple pathways can be activated by a single stress as the Fus1p mating response MAP kinase also responds to ROS (Staleva et al. 2004).
In addition to transcription factor activation, the removal of repressor function is also required for stress gene induction. Two transcriptional repressors, cyclin C (also known as UME3/SRB11) (Liao et al. 1995; Cooper et al. 1997) and the protein kinase that it activates (Cdk8, also known as UME5/SRB10) (Surosky et al. 1994), inhibit the transcription of several stress response genes (Cooper et al. 1997; Holstege et al. 1998). Unlike Rlm1p, which binds a specific promoter element, cyclin C–Cdk8 associates with the RNA polymerase II holoenzyme (Kuchin et al. 1995; Liao et al. 1995; Tassan et al. 1995; Maldonado et al. 1996; Cooper and Strich 1999). To relieve cyclin C–Cdk8 repression, the cyclin is destroyed in response to a subset of some (e.g., heat shock, oxidative stress) but not all (e.g., hyper-, hypotonic medium) stressors (Cooper et al. 1997, 1999). Heat and oxidative stress-induced destruction of cyclin C occurs via multiple pathways. For example, three separate cis-acting regions that are required for cyclin destruction in response to heat shock have been defined (Cooper et al. 1997). However, only one of these regions, defined by two single amino acid substitutions (A110V or E170K), is necessary for oxidative stress-induced destruction (Cooper et al. 1997).
ROS-induced cyclin C destruction also requires the conserved signaling molecule phospholipase C (Plc1p) (Cooper et al. 1999) and the nuclear factor Ask10p (Cohen et al. 2003). In this study, we report that cyclin C destruction is an important component of the oxidative stress response pathway. Deleting cyclin C protects the cell from ROS while a stabilized allele induces hypersensitivity. The MAP kinase Slt2p/Mpk1p mediates the ROS-induced cyclin C destruction. Mutating SLT2 protects cyclin C from degradation following H2O2 treatment while ectopic activation of this pathway triggers cyclin destruction in the absence of stress. This degradation pathway is important for viability as slt2Δ mutants display an elevated PCD response following H2O2 treatment that is suppressed by deleting cyclin C. Interestingly, genetic studies indicate that cyclin C controls ROS sensitivity independently of its role as a transcriptional repressor. These results indicate that Stl2p plays a prosurvival role by directing the destruction of cyclin C, thus protecting the cell from ROS-induced PCD.
MATERIALS AND METHODS
Strains, growth conditions, and plasmids:
Yeast strains used in these studies (Table 1) were derived from the W303 background strain RSY10 (Strich et al. 1989) unless otherwise indicated. The use of cyclin C and Cdk8p to indicate Srb11/Ume3p and Srb10/Ume5p, respectively, follows the guidelines established to unify the nomenclature for components of the RNA polymerase II mediator complex (Bourbon et al. 2004). Yeast culture conditions have been described previously (Cooper et al. 1997). Gene disruptions indicated in this article were constructed by homologous recombination (Longtine et al. 1998). pLR101 and pLR102 contain a single myc-epitope-tagged wild type or the A110V mutant allele of cyclin C, respectively, under the control of the ADH1 promoter (Cooper et al. 1997) inserted into the single-copy plasmid pRS315 (Sikorski and Hieter 1989). The BCK1-20 expression plasmid and the kinase dead mutant of SLT2 (p2119) was provided by D. Levin, Johns Hopkins University. A single amino terminal HA-epitope-tagged derivative of the human cyclin C was constructed by inserting the cDNA (a gift from S. Reed, Scripts Instruments) in frame with the HA epitope (12CA5) in pJTMCS (a gift from T. Yen, Fox Chase Cancer Center). The tagged cDNA was placed under the control of the ADH1 promoter on a single-copy plasmid to form pKC409.
TABLE 1.
Yeast strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| RSY10 | MATaade2 ade6 can1-100 his3-11,15 leu2-3,112 trp1-1 ura3-1 | Strich et al. (1989) |
| RSY391a | cycC∷LEU2 | Cooper et al. (1997) |
| RSY815a | bck1∷URA3 | This study |
| RSY921 | MATatrp1-1 ura3-1 mkk1 mkk2 | Irie et al. (1993) |
| RSY1007a | slt2∷his5+ cycC∷LEU2 | This study |
| RSY1057a | slt2∷his5+ | This study |
| RSY376a | cdk8∷TRP1 | This study |
| RSY1046a | mca1∷TRP1 | This study |
| RSY1021a | cdk8∷TRP1 slt2∷his5+ | This study |
| RSY1023a | mca1∷TRP1 slt2∷his5+ | This study |
Isogenic with RSY10.
Stress protocols and analyses:
Heat shock and oxidative stress time-course experiments were conducted and cyclin C was visualized by Western blot analysis as previously described (Cooper et al. 1997, 1999). Plate assays were conducted with freshly prepared complete minimal plates with or without H2O2 as indicated in this article. Midlog-phase cells (5 × 106 cells/ml) were lightly sonicated, counted, and then serially diluted (1:10) in water. The dilutions were then spotted onto the plates and incubated at 23° for 3 days before being photographed.
PCD assays:
The PCD assays were conducted exactly as described previously (Madeo et al. 2002). TdT-mediated dUTP nick end labeling (TUNEL) assays were conducted per the manufacturer's instructions with spheroplasted cells prepared as described (Guacci et al. 1997). The percentage of TUNEL-positive nuclei were calculated from at least 200 cells from two to three separate experiments.
Cyclin C–Cdk8 modeling:
To model the cyclin C–Cdk8p complex, the closest homologous complex of known structure was first identified as a template for model building using PSI-BLAST (Altschul et al. 1997) to search the nonredundant protein sequence database (Wheeler et al. 2003). A position-specific substitution matrix (PSSM) was constructed using both cyclin C and Cdk8p as query sequences. PSI-BLAST was run for 12 rounds and the PSSM was saved after each round. These matrices were used to search a database of sequences of known structure from the Protein Data Bank (PDB) (Berman et al. 2000). A 2.2-Å crystal structure of Cyclin A and CDK2 was identified (PDB entry 1QMZ) (Brown et al. 1999) as homologous to the cyclin C–Cdk8 complex with sequence identities of 16% (E-value 5.0 × 10−66) and 32% (E-value 1.0 × 10−78), respectively. The alignments were edited by hand upon visual examination of the known structure in light of the alignment. Some gaps were moved into loop regions of the structure, if they were located within regular secondary structure units. Side-chain conformations for the model were built from the known backbone coordinates from the crystal structure and the sequence alignments with the program SCWRL (Canutescu et al. 2003). SCWRL uses a backbone-dependent rotamer library (Dunbrack and Cohen 1997; Dunbrack 2002) to place side chains with the lowest energy with respect to the local backbone conformation and takes account of steric interactions between side chains and between side chains and the backbone. Loops with gaps in the sequence alignment were built with the program MODELLER (Sali and Blundell 1993). SCWRL was used to rebuild the side chains on the structure built by MODELLER.
RESULTS
The Pkc1p-directed MAP kinase cascade mediates ROS-induced destruction of cyclin C:
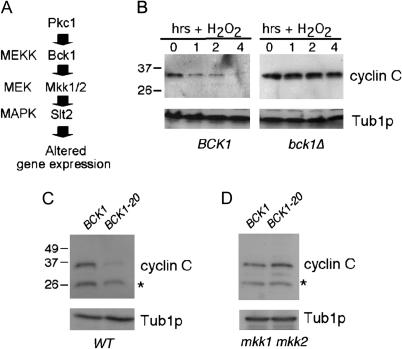
Previous studies from this laboratory found that the oxidative stress-dependent phosphorylation of Ask10p, a cyclin C interacting protein, requires the redundant Pkc1p-directed MAP kinase kinases (or MEKs) Mkk1p and Mkk2p (Cohen et al. 2003; Figure 1A). Since Ask10p is required for efficient ROS-induced destruction of cyclin C, we asked whether this signal transduction cascade is also involved. A cyclin C–myc expression construct was introduced into wild-type (BCK1) and null (bck1Δ) strains. Bck1p is the MEK kinase that activates the redundant MEKs Mkk1p and Mkk2p (Figure 1A). Both cultures were grown to midlog phase and subjected to oxidative stress (see materials and methods for details). As previously reported (Cooper et al. 1999), Western blots of immunoprecipitates revealed that cyclin C–myc levels were reduced in the wild-type strain following treatment with H2O2 (Figure 1B). However, cyclin C was stabilized in the bck1Δ strain even 4 hr following H2O2 treatment. This degree of stabilization is significantly more robust than that observed in the ask10Δ mutant (Cohen et al. 2003). These findings suggest that Ask10p is only part of the ROS cyclin C regulatory pathway while the Pkc1p MAP kinase cascade transduces the major oxidative stress destruction signal for cyclin C.
Figure 1.
Oxidative stress-induced destruction of cyclin C requires the Pkc1p MAP kinase cascade. (A) Diagram of the Pkc1p-controlled MAP kinase pathway with corresponding kinase designations on the left. (B) Wild-type BCK1 (RSY10) and bck1Δ mutant (RSY815) strains transformed with the myc–cyclin C expression plasmid were subjected to oxidative stress (0.4 mm) for the times indicated (in hours). myc–cyclin C levels were monitored by Western blots of immunoprecipitates. Tub1p served as a loading control. Molecular weight standards (in kilodaltons) are indicated on the left. (C) myc–cyclin C levels were determined in the wild-type strain RSY10 harboring either the wild-type (BCK1) or the constitutively active (BCK1-20) BCK1 allele on a plasmid. Cultures were harvested in midlog phase in the absence of stress. (D) The experiment described in C was repeated in the mkk1 mkk2 double mutant (RSY921). Tub1p served as a loading control. The asterisk indicates a nonspecific cross-reacting protein with the myc Mab.
To determine if activation of the Pkc1p MAP kinase cascade is sufficient to induce cyclin C degradation, a constitutively active allele of BCK1 (BCK1-20; Lee and Levin 1992) was employed. A plasmid harboring this allele was introduced into the wild-type strain just described and cyclin C levels were monitored in midlog cultures in the absence of stress. Cyclin C levels were markedly reduced in the strain containing BCK1-20 vs. the BCK1 wild-type allele (Figure 1C). These findings indicate that Bck1p is both necessary and sufficient to trigger cyclin C destruction. To determine whether Bck1p was functioning through the established Pkc1p MAP kinase cascade, cyclin C levels were examined in an mkk1 mkk2 double mutant expressing Bck1-20p. Mkk1p and Mkk2p normally transmit the Bck1p signal to the Slt2p MAP kinase (see Figure 1A). This experiment revealed that Mkk1p and Mkk2p are required for Bck1-20p-induced destruction of cyclin C (Figure 1D). These results indicate that the Pkc1p-directed MAP kinase pathway is required to transmit the ROS cyclin C destruction signal.
Slt2p is not required for heat-shock-induced destruction of cyclin C:
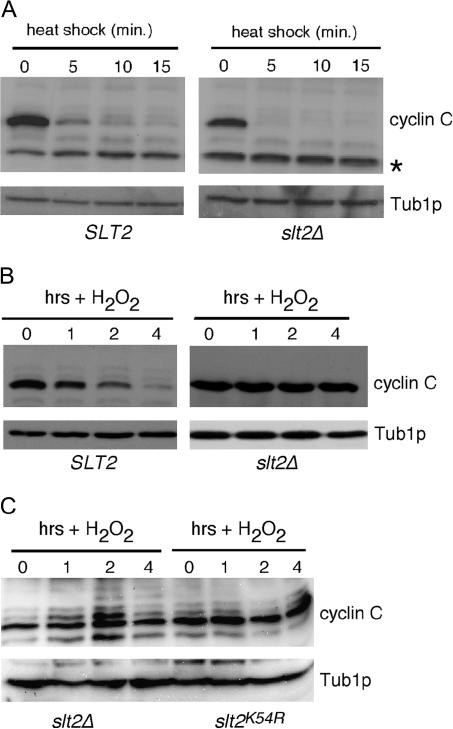
The Slt2p MAP kinase module is activated in response to heat shock (Kamada et al. 1995). Therefore, we next determined whether this pathway contributes to the previously described heat-induced destruction of cyclin C (Cooper et al. 1997). The wild-type and slt2Δ strains described above were grown to midlog phase at 30° and then subjected to heat shock (37°) (see materials and methods for details). Interestingly, Western blots of immunoprecipitates revealed that cyclin C degradation occurred with similar kinetics in the wild-type and slt2Δ strains (Figure 2A). Similar results were obtained for bck1 mutant strains (data not shown). These results indicate that Slt2p is not required for the rapid degradation of cyclin C in cells subjected to heat stress. To verify that Slt2p is required for ROS-induced cyclin C destruction, this experiment was repeated except that the cultures were exposed to H2O2 (0.4 mm). As expected, these experiments revealed that Slt2p was required for the ROS-induced destruction of cyclin C (Figure 2B). Finally, to determine if the kinase activity of Slt2p was required for oxidative stress-induced destruction of cyclin C, a kinase dead derivative of Slt2p (K54R) was introduced into the slt2Δ null strain. Similar to the vector control, expression of the kinase dead version of Slt2p failed to restore normal degradation of cyclin C following H2O2 exposure (Figure 2C). Taken together, these results may suggest that Slt2p activation by heat shock and ROS is recognized differently by the cell. Alternatively, heat shock may induce more pathways that function in parallel with Slt2p to destroy cyclin C (see discussion).
Figure 2.
Slt2p mediates only ROS-induced cyclin C destruction. (A) Wild-type SLT2 (RSY10) and isogenic null mutant slt2Δ (RSY1057) expressing myc–cyclin C were subjected to heat shock (37°) for the times indicated (in minutes). myc–Cyclin C levels were determined as in Figure 1. Tub1p serves as a loading control. The asterisk indicates a nonspecific cross-reacting protein with the myc Mab. (B) The cultures described in A were grown to midlog phase and then exposed to H2O2 treatment (0.4 mm) for the indicated times (in hours) and myc–cyclin C levels were determined as before. (C) The slt2Δ strain (RSY1057) expressing myc–cyclin C was transformed with the stl2K54R kinase dead allele of SLT2 or vector control. An oxidative stress time course was conducted and cyclin C levels were visualized as described in B.
Cyclin C destruction regulates sensitivity to oxidative stress:
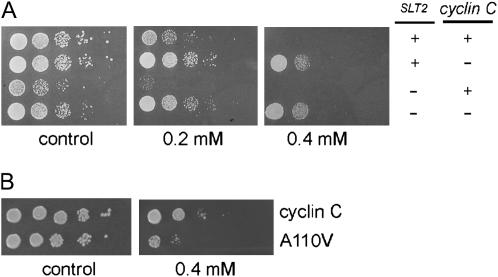
A recent study demonstrated that slt2Δ mutants were hypersensitive to an oxidized lipid (Alic et al. 2003). Since Slt2p is required for cyclin C destruction following oxidative stress, we examined the impact of altering cyclin C levels on H2O2 sensitivity with and without SLT2. Midlog-phase cultures of isogenic wild-type and slt2Δ strains were diluted serially 1:10 and spotted on complete minimal medium containing H2O2 (0.2 mm). As reported previously (Alic et al. 2003), the slt2Δ mutant was ∼50- to 100-fold more sensitive to oxidative stress compared to the wild-type control (Figure 3A). To investigate the physiological relevance of Slt2p-dependent cyclin C destruction, the sensitivity of a strain deleted for both cyclin C and SLT2 was assayed. The rationale for this experiment was that if an important function of Slt2p is triggering cyclin C destruction, then eliminating cyclin C should restore normal viability to the slt2Δ mutant. Indeed, deleting cyclin C suppressed the growth defect of slt2Δ mutants on H2O2 plates. Interestingly, the double mutant, as well as the cyclin C single mutant strain, exhibited enhanced resistance to oxidative stress compared to wild type. This effect was more evident as the H2O2 concentration increased (Figure 3A). Taken together, these findings indicate that Slt2p-mediated destruction of cyclin C is important for maintaining cell viability in response to ROS damage.
Figure 3.
Cyclin C stability regulates oxidative stress-induced cell death. (A) Log-phase cultures of the indicated genotype were serially diluted (1:10) and plated onto complete minimal medium without (control) or with (right panels) H2O2 as indicated. Strains used were (top to bottom) RSY10, RSY391, RSY1057, and RSY1007. Plates were incubated at 23° for 3 days and then photographed. (B) Wild-type strain RSY10 was transformed with either the myc–cyclin C (pLR101) or the myc–cyclin CA110V (pLR102)-expressing plasmids. The cultures were grown to midlog phase and treated as described in A except that the minimal medium used selected for plasmid maintenance.
The findings just presented are consistent with a model that Slt2p-dependent destruction of cyclin C is important for cell survival in response to oxidative stress. However, the relationship between these observations may not be direct. To further test the relationship between cyclin C destruction and cell viability following ROS treatment, we took advantage of a mutation in cyclin C described in an earlier report. This mutation consists of a single amino acid substitution (A110V) that stabilizes cyclin C in response to ROS (Cooper et al. 1999). In addition, this mutant is fully active as determined by repression assays and its ability to associate with Cdk8p in vivo (Cooper and Strich 1999). The cyclin C and A110V expression constructs were introduced into a wild-type strain. Cultures were grown to midlog phase and then serially diluted and plated onto minimal medium selecting for the plasmid either containing H2O2 (0.4 mm) or not. These experiments revealed an ∼10-fold reduction in viability in the culture expressing the stabilized allele of cyclin C (Figure 3B). No difference in plating efficiency was observed on the control plate, indicating that cyclin CA110V expression is not inherently toxic to the cell. These experiments revealed that loss of cyclin C activity protected the cell from ROS-induced cell death while preventing its destruction made strains hypersensitive. We therefore conclude that cyclin C regulation is an important component of the cellular response to oxidative stress.
The Pkc1p MAP kinase cascade targets cyclin C destruction through the element defined by the A110V mutation:
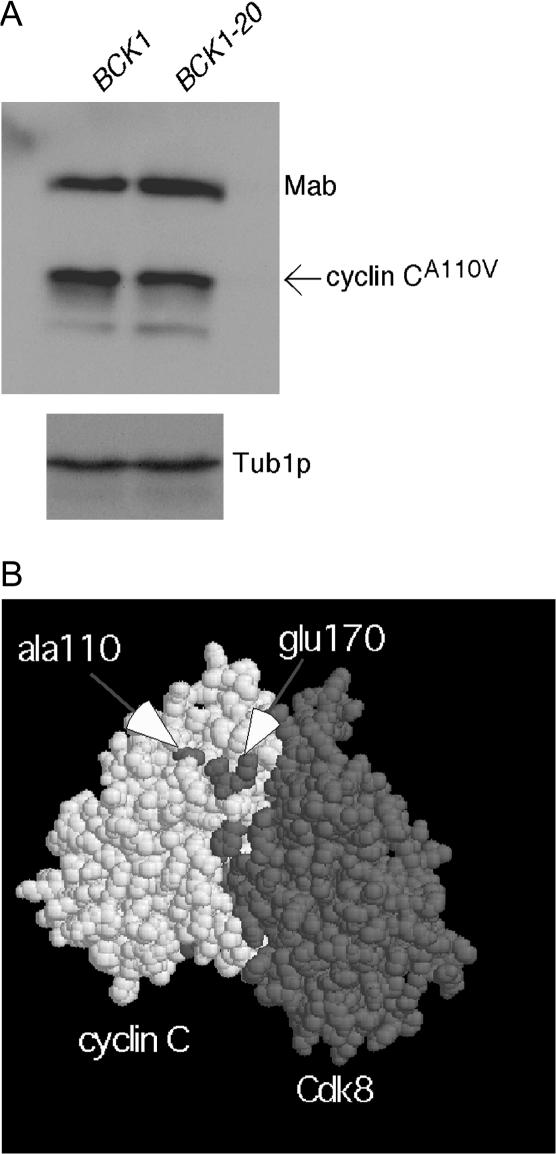
Previous studies have found that the ROS-induced degradation of cyclin C requires a cis-acting element defined by the A110V mutation. To determine if the Slt2p destruction signal operates through the putative A110 element, the BCK1-20 constitutively active allele was again employed. Cultures expressing a cyclin C derivative mutated at A110 (cyclin CA110V) and either the wild-type BCK1 gene or the BCK1-20 allele was harvested in midlog phase in the absence of stress. Extracts were prepared and cyclin CA110V levels were determined by Western blot analysis. Unlike the wild-type cyclin C (Figure 1C), cyclin CA110V steady-state levels were not altered in the presence of Bck1-20p (Figure 4A). These results indicate that Slt2p functions through the element defined by the A110 residue.
Figure 4.
Pkc1p-directed MAP kinase cascade targets cyclin C for destruction through a novel destruction element. (A) Wild-type strain RSY10 was transformed with plasmids expressing myc–cyclin CA110V and either the wild-type (BCK1) or activated (BCK1-20) allele of the MEK kinase. Cultures were harvested at midlog phase in the absence of stress. myc–cyclin CA110V levels were determined as in Figure 1. Tub1p serves as the loading control. Mab, myc monoclonal antibody used during immunoprecipitations, which is cross reactive with the secondary antibody used to develop the myc–cyclin C signal. (B) A space-filling model of cyclin C (white) and Cdk8p (shaded) is presented (see materials and methods for details). The arrows indicate the predicted locations of residues Ala110 and Glu170.
Slt2p protects the cell from H2O2-induced programmed cell death through cyclin C destruction:
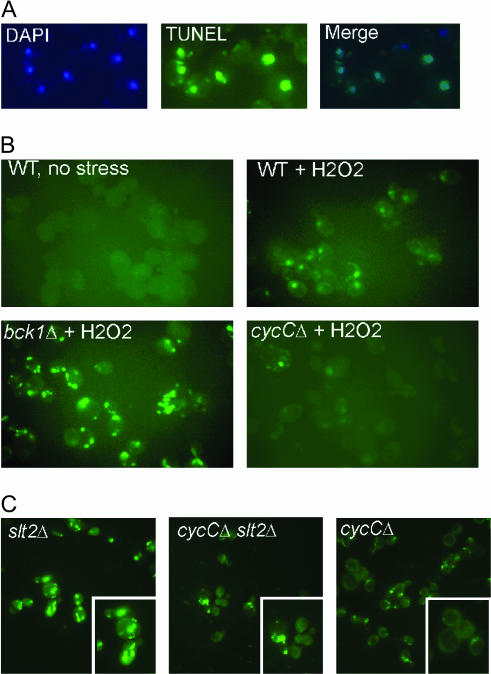
Similar to mammalian cells, exposing yeast to ROS can initiate caspase-dependent PCD characterized by nuclear fragmentation, phosphatidyl serine accessibility, and the appearance of double-strand breaks (Madeo et al. 2002). First, we repeated these experiments in our strain background and found that H2O2 exposure did induce double-strand breaks as determined by TUNEL assays (Figure 5A). Next, we determined whether the Slt2p–cyclin C pathway regulated PCD following oxidative damage. Samples were taken from midlog wild-type, bck1Δ, and cycCΔ mutant cultures both before and following treatment with H2O2 (0.4 mm; see materials and methods for details). These samples were examined for the acquisition of double-strand breaks by TUNEL assays. No TUNEL signal was observed in the cultures prior to oxidative stress (see Figure 5B, top left). Following exposure to H2O2, 35% ± 1% of wild-type nuclei were TUNEL positive (see materials and methods for details). Interestingly, bck1Δ mutants displayed an elevated percentage of TUNEL-positive cells (70 ± 6) compared to wild type. Conversely, the cyclin C mutant exhibited a reduction in H2O2-induced double-strand breaks (13% ± 2%, Figure 4B, bottom right). The appearance of TUNEL-positive cells correlated with the viability studies presented in Figure 3. As expected, an elevated TUNEL signal was also observed in the slt2Δ mutant (Figure 5C). Finally, the viability studies described in Figure 3 would predict that an slt2Δ cycCΔ double mutant would exhibit a TUNEL profile more similar to the cycCΔ single mutant. This was indeed the case as the cycCΔ slt2Δ double mutant exhibited a reduced TUNEL signal compared to the sltΔ single mutant (Figure 5C). These results indicate that Bck1p and cyclin C regulate programmed cell death but in opposite ways. Slt2p protects the cell from programmed cell death by mediating cyclin C destruction. Failure to destroy cyclin C, either in an slt2Δ mutant or through a stabilizing mutation, results in reduced ROS-induced PCD.
Figure 5.
Slt2p and cyclin C regulate programmed cell death in opposite ways. (A) A wild-type culture (RSY10) following exposure to 0.4 mm H2O2 was stained with DAPI to identify the nuclei and probed for the presence of double-strand breaks by TUNEL assays (see materials and methods for details). The merged image is shown on the right. Magnification ×400. (B) The wild-type culture RSY10 before (top, left) and following (top, right) H2O2 treatment (0.4 mm) were examined by TUNEL assays. Isogenic bck1Δ (RSY851) and cycCΔ (RSY391) mutants were also examined by TUNEL assays following H2O2 exposure. (C) Strains lacking SLT2 (RSY1057), cyclin C (RSY391), or both (RSY1007) were assayed for double-strand breaks by TUNEL. The inserts depict higher magnifications of selected cells in the same field to highlight nuclear breakdown.
Separation of Cdk8p and cyclin C functions:
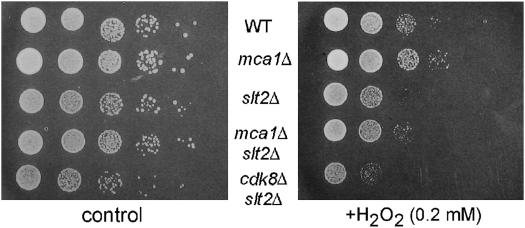
The previous section suggested a positive correlation between increased H2O2-induced PCD and hypersensitivity in slt2 mutants. To explore this relationship further, we tested whether preventing programmed cell death by deleting the MCA1 caspase (Madeo et al. 2002) would rescue the slt2 hypersensitivity phenotype. H2O2 sensitivity was tested using the plate assay described above. As observed in an earlier study (Madeo et al. 2002), the mca1Δ single mutant displayed a four- to fivefold decrease in H2O2 sensitivity compared to the wild type (Figure 6). A modest but consistent increase in viability was observed with the slt2Δ mca1Δ double mutant compared to the slt2Δ mutant alone. These results indicate that Mca1p function is required for the ROS hypersensitivity observed in slt2Δ mutants. However, this suppression did not restore wild-type viability as observed when cyclin C was deleted (Figure 4A). These results suggest that cyclin C may regulate cell survival through both Mca1-dependent and -independent pathways.
Figure 6.
Genetic interactions between Slt2p, Mca1p, and Cdk8p. Wild-type (RSY10), mca1Δ (RSY1046), slt2Δ (RSY1057), mca1Δ slt2Δ (RSY1023), and cdk8Δ slt2Δ (RSY1021) midlog cultures were harvested and tested for sensitivity to H2O2 as described in Figure 3.
One possible explanation for the ability of cyclin C mutants to completely suppress the H2O2 hypersensitivity associated with the slt2Δ allele is its role as a transcriptional repressor. Loss of cyclin C activity derepresses several stress-response genes, including catalase and several protein chaperones (Cooper et al. 1997; Holstege et al. 1998). Therefore, elevated levels of these proteins would reduce intercellular ROS concentrations or protect proteins from H2O2-induced damage. To test this possibility, the ability of a CDK8 null allele (cdk8Δ) to suppress slt2Δ H2O2 hypersensitivity was tested. Mutants lacking Cdk8p phenocopy cycCΔ strains with respect to transcriptional repression assays and meiotic phenotypes (Strich et al. 1989; Surosky et al. 1994; Cooper and Strich 2002). Surprisingly, deleting CDK8 did not restore normal growth to slt2Δ mutants growing on 0.2 mm H2O2 (Figure 6). These results indicate that cyclin C regulates H2O2 sensitivity and programmed cell death through a mechanism independent of Cdk8p activation.
The human cyclin C regulates the ROS response in yeast:
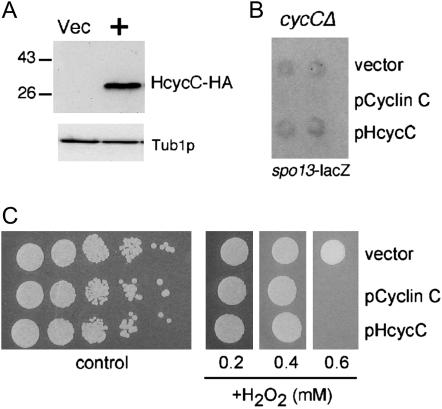
The differences between cycCΔ slt2Δ and cdk8Δ slt2Δ sensitivity to ROS could be due to an intrinsic property of cyclin C that is independent of its role in activating Cdk8p. To explore this possibility, we obtained the human cyclin C (HcycC) cDNA (a gift from S. Reed, Scripts Instruments) and fused the coding region to a single HA epitope (see materials and methods for details). This fusion gene was placed under the control of the ADH1 promoter and introduced into yeast via transformation. A signal of the appropriate molecular weight (33 kDa) was detected in the HcycC–HA transformant but not in the vector control (Figure 7A). A previous study found that HcycC cannot substitute for the yeast cyclin with respect to its transcriptional repression activity (Kuchin et al. 1995). We confirmed this result and found that HcycC is unable to complement the aberrant mitotic expression of a spo13–lacZ reporter gene in a cyclin C mutant host (Figure 7B). We next tested the H2O2 sensitivity of the cyclin C mutant strain expressing HcycC, the yeast cyclin C, or the vector control. As described in Figure 4A, the cycCΔ mutant transformed with the vector control displayed enhanced resistance to 0.6 mm H2O2 compared to the strain expressing the wild-type cyclin C (Figure 7C). Interestingly, expression of HcycC restored wild-type H2O2 sensitivity to the cycCΔ mutant strain. These results indicate that HcycC is able to complement the H2O2 resistance phenotype of cycCΔ strains but not the loss of transcriptional repression. When combined with the cdk8Δ slt2Δ double-mutant experiments, these results suggest that cyclin C controls H2O2 sensitivity by a mechanism that is independent of its transcriptional repression function. In addition, the function of cyclin C in the cellular response to ROS appears to be conserved between the yeast and human proteins.
Figure 7.
The human cyclin C regulates the ROS response in yeast. (A) HA-epitope-tagged allele of the HcycC was subjected to Western blot analysis. The vector lane (Vec) serves as a control for nonspecific cross-reactivity of the HA Mab. Tub1p serves as a loading control. Molecular weight standards (in kilodaltons) are indicated on the left. (B) The aberrant vegetative expression of the meiosis-specific spo13–lacZ reporter gene was analyzed in a cycCΔ mutant strain harboring the vector control, the yeast cyclin C expression plasmid (pLR101), or the human HcycC-expressing plasmid (pKC409). Plate assays indicating lacZ expression by cleavage of 5-bromo-4-chloro-3-indole-β-d-galactoyranosidase (X-gal) of two independent transformants are depicted. (C) H2O2 sensitivity assays were conducted as described in Figure 3 with the strains indicated in B except that the first dilution in the series was spotted onto medium containing different amounts of H2O2 as indicated. The plates were incubated for 3 days at 23° and then photographed.
DISCUSSION
In mammalian cells, MAP kinase signal transduction pathways generally have been described as either prosurvival (e.g., ERK1/2) or pro-apoptotic (e.g., JNK and p38) in overall function (reviewed in Xia et al. 1995; Weston and Davis 2002). This study describes a prosurvival role for the Slt2/Mpk1 MAP kinase pathway following oxidative stress. Specifically, ROS-induced cell death is enhanced in strains mutant for the Slt2p. The increased cell death correlates well with elevated double-strand breaks as determined by TUNEL assays and nuclear fragmentation characteristic of PCD. Our results indicate that Slt2p-mediated protection from ROS is mediated through cyclin C destruction. First, the Slt2p MAPK cascade is required for cyclin C destruction in response to H2O2 treatment while ectopic activation of this pathway triggers cyclin degradation in the absence of stress. Second, deleting cyclin C suppresses the H2O2 hypersensitivity and elevated PCD associated with slt2 mutations. Surprisingly, two results suggest that cyclin C regulates the oxidative stress response independently of its role of a transcriptional repressor. First, deleting CDK8, the kinase activated by cyclin C, does not suppress the slt2 hypersensitivity phenotype. In addition, the human cyclin C, which does not repress transcription in yeast, is still able to regulate the ROS response. These findings indicate a new role for cyclin C in the control of the oxidative stress response that may be conserved from yeast to humans.
This study found that ROS-induced destruction of cyclin C is mediated by Slt2p. However, Slt2p is not required for heat-shock-induced destruction of cyclin C even though this MAP kinase cascade is activated by elevated temperatures (Kamada et al. 1995). One possibility is that heat shock activates additional pathways that target the two other elements (destruction box, PEST) involved for heat-induced destruction (Cooper et al. 1997). The presence of parallel pathways could therefore mask the degradation defect in slt2 mutants. However, this explanation may be too simplistic. We demonstrate that Slt2p targets cyclin C for destruction through the region defined by the A110V mutation. However, mutating A110 partially stabilizes cyclin C following heat shock (Cooper et al. 1997), indicating that this domain is involved in mediating the heat-shock response. One possibility is that heat shock utilizes another MAP kinase or one in combination with Slt2p. We have tested the other stress-responsive MAP kinase Hog1p, both alone and in combination with Slt2p, for its requirement for heat-shock-induced destruction of cyclin C. Again, cyclin C destruction was not affected (our unpublished observations). These results suggest the existence of independent mechanisms that target the A110 region in response to heat shock or oxidative stress.
Two single amino acid substitutions (A110V or E170K) that exhibit similar phenotypes with respect to cyclin C regulation were identified. Both substitutions partially stabilize cyclin C following heat shock (Cooper et al. 1997) but completely protect cyclin C from ROS-induced degradation (Cooper et al. 1999). Normally, destruction elements mediating regulated proteolysis are characterized by relatively short, continuous regions (e.g., destruction box, KEN box, PEST regions). However, neither A110 nor E170 are part of a consensus destruction element motif. In addition, these residues are 60 amino acids apart on the basis of the primary sequence. This result suggested the existence of either a large destruction element or perhaps several smaller units. To gain insight into this issue, the cyclin C–Cdk8p complex was modeled against the known crystal structure of human cyclin A–Cdk2 (see materials and methods for details). The resulting model placed A110 and E170 within the cyclin box domain but not in direct contact with Cdk8p (Figure 4B). This prediction is consistent with functional studies that found that the A110V substitution did not affect either cyclin C repression activity (Cooper et al. 1997) or its ability to interact with Cdk8p (Cooper and Strich 1999). More importantly, this model predicts that E170 lies adjacent to A110 (arrows, Figure 4B) suggesting that the two residues form a new interaction surface that is a target of stress-responsive signaling pathways. In addition, previous studies have demonstrated that the A110V mutation also partially stabilizes cyclin C during meiosis (Cooper et al. 1997). Therefore, we propose that this domain, which we will refer to as a stress-meiosis activated degron (S-M degron), is at the intersection of two highly conserved signaling pathways. Additional studies are required to precisely define the boundaries of this domain. However, unlike other destruction elements, a minimal functioning element may be identified only through the use of three-dimensional models.
The association of cyclin C with the RNA polymerase II holoenzyme suggests a direct connection between the Slt2p MAP kinase cascade and the transcriptional machinery. The localization of the Hog1p MAP kinase to promoters has been well documented (Alepuz et al. 2003). In addition, a common mechanism to trigger the destruction of a protein is through phosphorylation. For example, G1 cyclins are all targeted for degradation by modification by the Cdc28p kinase (Lanker et al. 1996). The requirement of MAP kinase cascade for cyclin C destruction could suggest a similar triggering mechanism. However, the yeast cyclin C contains only one Ser–Pro motif consistent with a MAP kinase recognition site. Deleting this region does not affect cyclin destruction. Therefore, it appears more likely that the MAP kinase cascade recognizes an intermediate that in turn targets cyclin C for destruction. We previously identified a protein (Ask10p) that associates with cyclin C and is phosphorylated in response to oxidative stress (Cohen et al. 2003). However, Ask10p has only a partial role in mediating cyclin C destruction, suggesting the presence of additional regulatory pathways that connect Slt2p to the cyclin C–Cdk8p-containing RNA polymerase II holoenzyme.
This study demonstrates that deleting cyclin C suppresses the H2O2 hypersensitivity of slt2Δ mutant strains. Strains lacking cyclin C derepress many stress response genes, including chaperones and antioxidants (Cooper et al. 1997, 1999; Holstege et al. 1998). The induction of stress response genes in turn may protect the cell from the damage caused by reactive oxygen species. This type of response may be similar to the acquired stress tolerance observed when cells are subjected to mild stress prior to exposure to acute toxic conditions (Lindquist and Kim 1996). Consistent with this possibility, increased heat-shock protein expression promotes resistance to apoptosis (Samali and Cotter 1996). However, we were surprised that deleting CDK8 did not suppress the slt2Δ H2O2 hypersensitivity as the function of cyclin C and Cdk8p have always been linked. These results suggest two possibilities. First, cyclin C may be able to activate another Cdk that can substitute for Cdk8p. The human cyclin C is able to bind and activate Cdk3 in addition to Cdk8 (Ren and Rollins 2004). In yeast, the Cdk Pho85p represents the best possibility for an alternative partner for cyclin C. Similar to Cdk8p, it is not essential for growth, responds to environmental signals, and regulates transcription (Toh-e et al. 1988; Hirst et al. 1994; Kaffman et al. 1994). However, no interaction, genetic or physical, has been observed between cyclin C and Pho85p. An alternative model posits that cyclin C performs a role in the H2O2 response that is independent of its transcriptional repressor function. In support of this possibility, we found that the human cyclin C, which has not been found to control transcription in yeast, still regulates the ROS response. In addition, we have found that cyclin C is exported from the nucleus to the cytoplasm prior to its destruction (our unpublished results). Therefore, one model consistent with these data is that cyclin C controls the ROS response outside of the nucleus through a mechanism independent of transcriptional regulation.
Our findings indicate that cells lacking cyclin C are resistant to H2O2-induced PCD. This finding begs the question of why retain cyclin C throughout evolution if the cell has a selective advantage without it? A recent study found a modest growth defect in a cyclin C mutant compared to wild type (Deutschbauer et al. 2005). However, we observed one to two orders of magnitude difference in cyclin C mutant resistance to H2O2 compared to wild type. A recent study argued that altruism within a colony could explain the evolutionary advantage for PCD in single-cell organisms. In this report (Fabrizio et al. 2004), massive ROS-dependent cell death was reported in old colonies grown under laboratory or field conditions. Those few survivors within the colony live off the nutrients derived from the dying cells and undergo genetic alterations that may produce a more fit clonal population. This is consistent with the finding that PCD occurs within the center of the colony in acid-stressed cells, thus providing a nutrient pool for younger, perhaps fitter cells on the colony periphery (Vachova and Palkova 2005). Therefore, preventing cells from undergoing PCD under these adverse conditions would inhibit this type of selective process, perhaps reducing the fitness of the organism as a whole. However, resistance to ROS-induced cell death could have other manifestations. The HcycC maps to 6q21, a region that is deleted in many tumor types, including breast (Siezinger et al. 1991) and high-grade non-Hodgkin's lymphomas (Offit et al. 1993). In addition, cytogenetic loss of 6q21 is a frequent occurrence in non-small-cell lung cancer (Testa et al. 1994) and malignant mesotheliomas (Taguchi et al. 1993). Therefore, it is interesting to speculate that a similar antiapoptotic phenotype may be associated with loss of HcycC making the tumor less susceptible to chemotherapeutic agents designed to trigger programmed cell death.
Acknowledgments
We thank Edward Winter for critical reading of this manuscript and Antonios Makris and Joseph Testa for valuable discussions. We also thank Michael Gustin, David Levin, Steve Reed, Edward Winter, and Tim Yen for strains or plasmids used in this study. This work was supported by grants from the National Institutes of Health (GM57842 and CA99003) to R.S. and from the American Cancer Society (CCG106162) to K.F.C. Institutional support was also provided by the National Cancer Center (Comprehensive Cancer Center core grant CA 06927) and by an appropriation from the Commonwealth of Pennsylvania.
References
- Alepuz, P. M., A. Jovanovic, V. Reiser and G. Ammerer, 2001. Stress-induced map kinase Hog1 is part of transcription activation complexes. Mol. Cell 7: 767–777. [DOI] [PubMed] [Google Scholar]
- Alepuz, P. M., E. de Nadal, M. Zapater, G. Ammerer and F. Posas, 2003. Osmostress-induced transcription by Hot1 depends on a Hog1-mediated recruitment of the RNA Pol II. EMBO J. 22: 2433–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alic, N., V. J. Higgins, A. Pichova, M. Breitenbach and I. W. Dawes, 2003. Lipid hydroperoxides activate the mitogen-activated protein kinase Mpk1p in Saccharomyces cerevisiae. J. Biol. Chem. 278: 41849–41855. [DOI] [PubMed] [Google Scholar]
- Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang et al., 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25: 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman, H. M., T. N. Bhat, P. E. Bourne, Z. Feng, G. Gilliland et al., 2000. The Protein Data Bank and the challenge of structural genomics. Nat. Struct. Biol. 7(Suppl): 957–959. [DOI] [PubMed] [Google Scholar]
- Bourbon, H. M., A. Aguilera, A. Z. Ansari, F. J. Asturias, A. J. Berk et al., 2004. A unified nomenclature for protein subunits of mediator complexes linking transcriptional regulators to RNA polymerase II. Mol. Cell 14: 553–557. [DOI] [PubMed] [Google Scholar]
- Brown, N. R., M. E. Noble, J. A. Endicott and L. N. Johnson, 1999. The structural basis for specificity of substrate and recruitment peptides for cyclin-dependent kinases. Nat. Cell Biol. 1: 438–443. [DOI] [PubMed] [Google Scholar]
- Canutescu, A. A., A. A. Shelenkov and R. L. Dunbrack, Jr., 2003. A graph-theory algorithm for rapid protein side-chain prediction. Protein Sci. 12: 2001–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, T. J., K. Lee, L. H. Rutkowski and R. Strich, 2003. Ask10p mediates the oxidative stress-induced destruction of the Saccharomyces cerevisiae C-type cyclin Ume3p/Srb11p. Eukaryot. Cell 2: 962–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper, K. F., and R. Strich, 1999. Functional analysis of the yeast C-type cyclin Ume3p/Srb11p- RNA polymerase II holoenzyme interaction. Gene Exp. 8: 43–57. [PMC free article] [PubMed] [Google Scholar]
- Cooper, K. F., and R. Strich, 2002. Saccharomyces cerevisiae C-type cyclin UME3/SRB11 is required for efficient induction and execution of meiotic development. Eukaryot. Cell 1: 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper, K. F., M. J. Mallory, J. S. Smith and R. Strich, 1997. Stress and developmental regulation of the yeast C-type cyclin UME3 (SRB11/SSN8). EMBO J. 16: 4665–4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper, K. F., M. J. Mallory and R. Strich, 1999. Oxidative stress-induced destruction of the yeast C-type cyclin Ume3p requires the phosphatidylinositol-specific phospholipase C and the 26S proteasome. Mol. Cell. Biol. 19: 3338–3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutschbauer, A. M., D. F. Jaramillo, M. Proctor, J. Kumm, M. E. Hillenmeyer et al., 2005. Mechanisms of haploinsufficiency revealed by genome-wide profiling in yeast. Genetics 169: 1915–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodou, E., and R. Treisman, 1997. The Saccharomyces cerevisiae MADS-box transcription factor Rlm1 is a target for the Mpk1 mitogen-activated protein kinase pathway. Mol. Cell. Biol. 17: 1848–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbrack, R. L., Jr., 2002. Rotamer libraries in the 21st century. Curr. Opin. Struct. Biol. 12: 431–440. [DOI] [PubMed] [Google Scholar]
- Dunbrack, R. L., Jr., and F. E. Cohen, 1997. Bayesian statistical analysis of protein side-chain rotamer preferences. Protein Sci. 6: 1661–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrizio, P., L. Battistella, R. Vardavas, C. Gattazzo, L. L. Liou et al., 2004. Superoxide is a mediator of an altruistic aging program in Saccharomyces cerevisiae. J. Cell Biol. 166: 1055–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gate, L., J. Paul, G. N. Ba, K. D. Tew and H. Tapiero, 1999. Oxidative stress induced in pathologies: the role of antioxidants. Biomed. Pharmacother. 53: 169–180. [DOI] [PubMed] [Google Scholar]
- Green, D. R., and G. Kroemer, 2004. The pathophysiology of mitochondrial cell death. Science 305: 626–629. [DOI] [PubMed] [Google Scholar]
- Guacci, V., E. Hogan and D. Koshland, 1997. Centromere position in budding yeast: evidence for anaphase A. Mol. Biol. Cell 8: 957–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustin, M. C., J. Albertyn, M. Alexander and K. Davenport, 1998. MAP kinase pathways in the yeast Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 62: 1264–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst, K., F. Fisher, P. C. McAndrew and C. R. Godding, 1994. The transcription factor, the Cdk, its cyclin and their regulator: directing the transcriptional response to a nutritional signal. EMBO J. 13: 5410–5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstege, F. C., E. G. Jennings, J. J. Wyrick, T. I. Lee, C. J. Hengartner et al., 1998. Dissecting the regulatory circuitry of a eukaryotic genome. Cell 95: 717–728. [DOI] [PubMed] [Google Scholar]
- Irie, K., M. Takase, K. S. Lee, D. E. Levin, H. Araki et al., 1993. MKK1 and MKK2, which encode Saccharomyces cerevisiae mitogen-activated protein kinase-kinase homologs, function in the pathway mediated by protein kinase C. Mol. Cell. Biol. 13: 3076–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, X., and X. Wang, 2004. Cytochrome C-mediated apoptosis. Annu. Rev. Biochem. 73: 87–106. [DOI] [PubMed] [Google Scholar]
- Kaffman, A., I. Herskowitz, R. Tjian and E. K. O'Shea, 1994. Phosphorylation of the transcription factor PHO4 by a cyclin CDK complex, PHO80-PHO85. Science 263: 1153–1159. [DOI] [PubMed] [Google Scholar]
- Kamada, Y., U. S. Jung, J. Piotrowski and D. E. Levin, 1995. The protein kinase C-activated MAP kinase pathway of Saccharomyces cerevisiae mediates a novel aspect of the heat shock response. Genes Dev. 9: 1559–1571. [DOI] [PubMed] [Google Scholar]
- Kuchin, S., P. Yeghiayan and M. Carlson, 1995. Cyclin-dependent protein kinase and cyclin homologs SSN3 and SSN8 contribute to transcriptional control in yeast. Proc. Natl. Acad. Sci. USA 92: 4006–4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanker, S., M. H. Valdivieso and C. Wittenberg, 1996. Rapid degradation of the G1 cyclin Cln2 induced by CDK-dependent phosphorylation. Science 271: 1597–1600. [DOI] [PubMed] [Google Scholar]
- Lee, K. S., and D. E. Levin, 1992. Dominant mutations in a gene encoding a putative protein kinase (BCK1) bypass the requirement for a Saccharomyces cerevisiae protein kinase C homolog. Mol. Cell. Biol. 12: 172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao, S.-M., J. Zhang, D. A. Jeffery, A. J. Koleske, C. M. Thompson et al., 1995. A kinase-cyclin pair in the RNA polymerase II holoenzyme. Nature 374: 193–196. [DOI] [PubMed] [Google Scholar]
- Lindquist, S., and G. Kim, 1996. Heat shock protein 104 expression is sufficient for thermotolerance in yeast. Proc. Natl. Acad. Sci. USA 93: 5301–5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X.-D., and D. J. Thiele, 1996. Oxidative stress induces heat shock factor phosphorylation and HSF-dependent activation of yeast metallothionein gene transcription. Genes Dev. 10: 592–603. [DOI] [PubMed] [Google Scholar]
- Longtine, M. S., A. R. McKenzie, D. J. Demarini, N. G. Shah, A. Wach et al., 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14: 953–961. [DOI] [PubMed] [Google Scholar]
- Madeo, F., E. Herker, C. Maldener, S. Wissing, S. Lachelt et al., 2002. A caspase-related protease regulates apoptosis in yeast. Mol. Cell 9: 911–917. [DOI] [PubMed] [Google Scholar]
- Maldonado, E., R. Shiekhattar, M. Sheldon, H. Cho, R. Drapkin et al., 1996. A human RNA polymerase II complex associated with SRB and DNA-repair proteins. Nature 381: 86–89. [DOI] [PubMed] [Google Scholar]
- Martinez-Pastor, M. T., G. Marchler, C. Schuller, A. Marchler-Bauer, H. Ruis et al., 1996. The Saccharomyces cerevisiae zinc finger proteins Msn2p and Msn4p are required for transcriptional induction through the stress response element (STRE). EMBO J. 15: 2227–2235. [PMC free article] [PubMed] [Google Scholar]
- Offit, K., N. Z. Parsa, G. Gaidano, D. A. Filippa, D. Louie et al., 1993. 6q deletions define distinct clinico-pathologic subsets of non-Hodgkin's lympoma. Blood 82: 2157–2162. [PubMed] [Google Scholar]
- Ren, S., and B. J. Rollins, 2004. Cyclin C/cdk3 promotes Rb-dependent G0 exit. Cell 117: 239–251. [DOI] [PubMed] [Google Scholar]
- Rep, M., V. Reiser, U. Gartner, J. M. Thevelein, S. Hohmann et al., 1999. Osmotic stress-induced gene expression in Saccharomyces cerevisiae requires Msn1p and the novel nuclear factor Hot1p. Mol. Cell. Biol. 19: 5474–5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sali, A., and T. L. Blundell, 1993. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 234: 779–815. [DOI] [PubMed] [Google Scholar]
- Samali, A., and T. G. Cotter, 1996. Heat shock proteins increase resistance to apoptosis. Exp. Cell Res. 223: 163–170. [DOI] [PubMed] [Google Scholar]
- Siezinger, B. R., H. P. Klinger, C. Junien, Y. Nakamura, M. Le Beau et al., 1991. Report of the committee on chromosome and gene loss in human neoplasia. Cytogenet. Cell Genet. 58: 1080–1096. [Google Scholar]
- Sikorski, R. S., and P. Hieter, 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122: 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skulachev, V. P., 2002. Programmed death in yeast as adaptation? FEBS Lett. 528: 23–26. [DOI] [PubMed] [Google Scholar]
- Staleva, L., A. Hall and S. J. Orlow, 2004. Oxidative stress activates FUS1 and RLM1 transcription in the yeast Saccharomyces cerevisiae in an oxidant-dependent manner. Mol. Biol. Cell 15: 5574–5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen, D., S. Rivers and D. Jamieson, 1995. The role of the YAP1 and YAP2 genes in the regulation of the adaptive oxidative stress responses of Saccharomyces cerevisiae. Mol. Microbiol. 16: 415–423. [DOI] [PubMed] [Google Scholar]
- Strich, R., M. R. Slater and R. E. Esposito, 1989. Identification of negative regulatory genes that govern the expression of early meiotic genes in yeast. Proc. Natl. Acad. Sci. USA 86: 10018–10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surosky, R. T., R. Strich and R. E. Esposito, 1994. The yeast UME5 gene regulates the stability of meiotic mRNAs in response to glucose. Mol. Cell. Biol. 14: 3446–3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi, T., S. C. Jhanwar, J. M. Siegfried, S. M. Keller and J. R. Testa, 1993. Recurrent deletions of specific chromosomal sites in 1p, 3p, 6q, and 9p in human malignant mesothelioma. Cancer Res. 53: 4349–4355. [PubMed] [Google Scholar]
- Tassan, J.-P., M. Jaqueoud, P. Leopold, S. J. Schultz and E. A. Nigg, 1995. Identification of human cyclin-dependent kinase 8, a putative protein kinase partner for cyclin C. Proc. Natl. Acad. Sci. USA 92: 8871–8875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa, J. R., J. M. Siegfried, Z. Liu, J. D. Hunt, M. M. Feder et al., 1994. Cytogetic analysis of 63 non-small cell lung carcinomas: recurrent chromosome alterations amid frequent and widspread genomic upheaval. Genes Chromosomes Cancer 11: 178–194. [DOI] [PubMed] [Google Scholar]
- Toh-e, A., K. Tanaka, Y. Uesono and R. B. Wickner, 1988. PHO85, a negative regulator of the PHO system, is a homolog of the protein kinase gene, CDC28, of Saccharomyces cerevisiae. Mol. Gen. Genet. 214: 162–164. [DOI] [PubMed] [Google Scholar]
- Vachova, L., and Z. Palkova, 2005. Physiological regulation of yeast cell death in multicellular colonies is triggered by ammonia. J. Cell Biol. 169: 711–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilella, F., E. Herrero, J. Torres and M. A. de la Torre-Ruiz, 2005. Pkc1 and the upstream elements of the cell integrity pathway in Saccharomyces cerevisiae, Rom2 and Mtl1, are required for cellular responses to oxidative stress. J. Biol. Chem. 280: 9149–9159. [DOI] [PubMed] [Google Scholar]
- Watanabe, Y., K. Irie and K. Matsumoto, 1995. Yeast RLM1 encodes a serum response factor-like protein that may function downstream of the Mpk1 (Slt2) mitogen-activated protein kinase pathway. Mol. Cell. Biol. 15: 5740–5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston, C. R., and R. J. Davis, 2002. The JNK signal transduction pathway. Curr. Opin. Genet. Dev. 12: 14–21. [DOI] [PubMed] [Google Scholar]
- Wheeler, D. L., D. M. Church, S. Federhen, A. E. Lash, T. L. Madden et al., 2003. Database resources of the National Center for Biotechnology. Nucleic Acids Res. 31: 28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia, Z., M. Dickens, J. Raingeaud, R. J. Davis and M. E. Greenberg, 1995. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270: 1326–1331. [DOI] [PubMed] [Google Scholar]