Abstract
Cyclooxygenase-2 (COX2) is a primary inflammatory mediator that converts arachidonic acid into precursors of vasoactive prostaglandins, producing reactive oxygen species in the process. Under normal conditions COX2 is not detectable, except at low abundance in the brain. This study demonstrates a distinctive pattern of COX2 increases in the brain over time following traumatic brain injury (TBI). Quantitative lysate ribonuclease protection assays indicate acute and sustained increases in COX2 mRNA in two rat models of TBI. In the lateral fluid percussion model, COX2 mRNA is significantly elevated (>twofold, p < 0.05, Dunnett) at 1 day postinjury in the injured cortex and bilaterally in the hippocampus, compared to sham-injured controls. In the lateral cortical impact model (LCI), COX2 mRNA peaks around 6 h postinjury in the ipsilateral cerebral cortex (fivefold induction, p < 0.05, Dunnett) and in the ipsilateral and contralateral hippocampus (two- and sixfold induction, respectively, p < 0.05, Dunnett). Increases are sustained out to 3 days postinjury in the injured cortex in both models. Further analyses use the LCI model to evaluate COX2 induction. Immunoblot analyses confirm increased levels of COX2 protein in the cortex and hippocampus. Profound increases in COX2 protein are observed in the cortex at 1–3 days, that return to sham levels by 7 days postinjury (p < 0.05, Dunnett). The cellular pattern of COX2 induction following TBI has been characterized using immunohistochemistry. COX2-immunoreactivity (-ir) rises acutely (cell numbers and intensity) and remains elevated for several days following TBI. Increases in COX2-ir colocalize with neurons (MAP2-ir) and glia (GFAP-ir). Increases in COX2-ir are observed in cerebral cortex and hippocampus, ipsilateral and contralateral to injury as early as 2 h postinjury. Neurons in the ipsilateral parietal, perirhinal and piriform cortex become intensely COX2-ir from 2 h to at least 3 days postinjury. In agreement with the mRNA and immunoblot results, COX2-ir appears greatest in the contralateral hippocampus. Hippocampal COX2-ir progresses from the pyramidal cell layer of the CA1 and CA2 region at 2 h, to the CA3 pyramidal cells and dentate polymorphic and granule cell layers by 24 h postinjury. These increases are distinct from those observed following inflammatory challenge, and correspond to brain areas previously identified with the neurological and cognitive deficits associated with TBI. While COX2 induction following TBI may result in selective beneficial responses, chronic COX2 production may contribute to free radical mediated cellular damage, vascular dysfunction, and alterations in cellular metabolism. These may cause secondary injuries to the brain that promote neuropathology and worsen behavioral outcome.
Keywords: neural gene expression, quantitative mRNA measurements, hippocampus, parietal cortex, perirhinal cortex, piriform cortex
INTRODUCTION
Following traumatic brain injury (TBI), arachidonic acid is released from cell membranes (Sevanian and Kim, 1985; Shohami et al., 1989; Dhillon et al., 1994; Prasad et al., 1994; Lewén et al., 1996; Marklund et al., 1996) and converted to prostaglandins by cyclooxygenases. In animal models of TBI, brain prostaglandin levels have been shown to rise rapidly postinjury (Ellis et al., 1989; Dewitt et al., 1988; Shohami et al., 1987). Prostaglandin changes in these studies seemed to be associated with brain injury itself; changes in cerebral blood flow following TBI rarely declined to levels associated with ischemia-induced prostaglandin increases (Obrist et al., 1984; Povlishock and Kontos, 1985; Kempski et al., 1987; Ellis et al., 1988; Yamakami and McIntosh, 1989, 1991; Bouma and Muizelaar, 1992; Muir et al., 1992; Kochanek et al., 1995; von Stück et al., 1996).
Prostaglandins affect cerebrovascular tone (Ellis et al., 1979; Kontos et al., 1980a,b; Wei et al., 1980), regulate platelet aggregation, and act as chemokines (Cao et al., 1995). In addition, prostaglandins mediate a number of processes in the central nervous system, for example, temperature regulation (Saper and Breder, 1992), glutamate release (Nishihara et al., 1995), and hippocampal long-term potentiation (Wolf et al., 1995; Nishizaki et al., 1999).
Cyclooxygenase-2 (COX2) is a primary inflammatory mediator that converts arachidonic acid from damaged membranes into vasoactive prostaglandins, producing reactive oxygen species in the process (Kontos et al., 1980a; Ellis et al., 1989). Following TBI, these free radicals damage neural membranes, white matter, and the tight junctions that form the blood-brain barrier. Peroxidative reactions may also be implicated in the progressive vascular damage that endangers autoregulation and leads to arteriolar spasm and thrombosis. Thus, the products of COX2 likely play a role in secondary responses that may result in increased intracranial pressure, vasospasm, and ischemia, resulting in worsened outcomes.
While COX2 is normally rare or absent in most organs of the body, low levels of COX2 mRNA are expressed in mammalian brain (Feng et al., 1993; Seibert et al., 1994). COX2 has also been found in postmortem human brain (reviewed by O'Banion, 1999). COX2 protein is normally found in neuronal cell bodies and dendrites (Tsubokura et al., 1991; Breder et al., 1992, 1995; Kaufmann et al., 1996). Basal levels of COX2 are found in the neurons of the cortex and hippocampus, but not in glia or endothelial cells. COX2 mRNA has been readily observed in rat brain in hippocampal pyramidal and granule cells, cerebellar granule cells, the pyramidal cells of piriform cortex, cellular layers of cerebral cortex, central nucleus of the amygdala, and several hypothalamic nuclei (Li et al., 1993; Yamagata et al., 1993; Breder et al., 1995). Lower levels are detectable in many other neurons throughout the brain.
The first indication of an inducible, inflammatory cyclooxygenase was from Needleman's group, who observed increased COX protein levels in human fibroblasts treated with interleukin-1 (IL-1) or lipopolysaccha-ride (LPS; Raz et al., 1988; Fu et al., 1990). Levels of COX1, prostacyclin synthetase and thromboxane synthase are thought to remain relatively constant during inflammatory challenge, whereas COX2 expression is inducible.
COX2 expression increases in the CNS in response to numerous insults. While basal COX2 appears to be regulated by normal glutamatergic synaptic activity in the adult brain, COX2 can be transiently induced by pathological exposure to glutamate agonists or blocked by NMDA receptor mediated inhibition (Yamagata et al., 1993; Chen et al., 1995; Kaufmann et al., 1996; Adams et al., 1996; Marcheselli and Bazan, 1996). Neuronal induction of COX2 occurs after traumatic brain injury (Graham et al., 1996a; Strauss et al., 1997, 1998), spinal cord injury (Resnick et al., 1998), cerebral ischemia (Collaco-Moraes et al., 1996; Graham et al., 1996b; Nogawa et al., 1997; Kong et al., 1997; Sanz et al., 1997; Walton et al., 1997), spreading depression (Caggiano et al., 1996), and seizures (Yamagata et al., 1993; Adams et al., 1996; Marcheselli and Bazan, 1996; Wallace et al., 1998). COX2 expression is also regulated by inflammatory cytokines both in the periphery and in the CNS (Seibert et al., 1994; Moore et al., 1997; Ott et al., 1994; Young and Ott, 1996). COX2 appears in cerebral endothelial cells, leptomeninges, and choroid plexus after systemic LPS, IL-1β (but not IL-6), and TNFα (Cao et al., 1997; Busija et al., 1996; van Dam et al., 1996; Lacroix and Rivest, 1998).
Finally, abnormal brain COX2 expression has been observed clinically in Alzheimer's disease (Lukiw and Bazan, 1997; Pasinetti and Aisen, 1998), spinal cord injury, and cerebral infarction (Sairanen et al., 1998). The discrete localization and distinct regulation of COX2 gene expression in the brain provide a rational basis to suspect that perturbation of prostanoid metabolism may play a significant role in the neuropathology of TBI. However, no systematic studies have been reported on the regional neuroanatomical changes in COX2 after TBI. The cerebral cortex and hippocampus are clearly associated with the major neurological deficits observed in TBI. The current studies examine the distribution of COX2 induction in these brain regions following a lateral cortical contusion model of TBI in the rat.
METHODS AND MATERIALS
Traumatic Brain Injury
All animal protocols used were approved by Temple University IACUC. For the lateral cortical impact studies, Sprague-Dawley rats (male, 300–400 g, Harlan) were preanesthetized with isoflurane (Abbott Labs, North Chicago, IL) and maintained at a surgical level of anesthesia with a facemask using oxygen and isoflurane (∼0.75%, Verni-trol vaporizing system, Ohio Medical Products, Madison, WI). The modified valve assembly from a Baby Blue Infant Resuscitator (Vital Signs Inc., Totowa, NJ) was used to prevent the rebreathing of exhaled gas. After immobilization in a stereotaxic device, a 6-mm craniectomy was performed between the left lateral ridge and the sagittal suture, midway between bregma and lambda, leaving the dura mater intact. Animals were randomly assigned to sham or moderate injury groups. Traumatic brain injury was induced via a cortical contusion using a pneumatic piston (5-mm diameter, 4 m/sec, 250 msec) to a depth of 3 mm. The injury site encompassed much of the left somatosensory and visual areas, as well as parietal association cortex. The skin was approximated using nylon sutures without replacing the bone flap and anesthesia was terminated. Postinjury exclusion criteria used were latency of pinna and corneal reflexes (5 min), as well as righting response (10 min). Sham-operated controls were surgically prepared but were not injured. The lateral fluid percussion injuries were performed as described (McIntosh et al., 1989) using male Sprague-Dawley rats of the same size and vendor. An injury of moderate severity (2.3–2.5 atm) was induced.
Tissue Preparation
All reagents were from Fisher Scientific, unless otherwise noted. For the lateral cortical impact (LCI) mRNA studies, conscious animals were decapitated in a sharpened guillotine. Brains were rapidly dissected, frozen on powdered dry ice and stored at −80°C. Frozen brains were coronally sectioned, alternating 20-μm and 300-μm sections in a cryostat microtome (IEC) at −8°C. Pretreated slides (cleaned with 9% nitric acid and twice coated with 1.5% gelatin in 5 mM chromium potassium sulfate) were used and the thin sections were stored with a desiccant at −80°C. Thick sections were rapidly thaw mounted on slides, refrozen on powdered dry ice, and stored at −80°C. Fresh frozen thin sections were used for in situ hybridization (autoradiographs), micropunches were removed from thick sections for RNA and protein analyses (described below). For the lateral fluid percussion TBI mRNA study, fresh brain regions (ipsi- and contralateral to injury) were rapidly dissected (on PBS saturated filter paper on ice), sonicated in 6M GE (∼250 mg/mL), frozen on dry ice, and stored at −80°C. Cortex (inferolateral to the craniectomy site), hippocampus (the entire vital structure), and whole cerebellum were studied. In contrast, for the lateral cortical impact studies, micropunches were dissected (at −10°C using a 1,000-μm cannula) from 300-μm frozen sections (Palkovits, 1973). Specific brain regions were immediately transferred to and sonicated in 40 μl of 6M guanidine thiocyanate, 0.13M EDTA (6M GE) and stored at −80°C (Strauss and Jacobowitz, 1993). For micropunch immunoblots, brain regions of interest were dissected (as above), immediately sonicated in ice cold buffer H (10 mM potassium phosphate, 10 mM EDTA, 1 mM dithiothreitol, pH 7.4) and clarified (20 min at 15k ×g, 0°C). An aliquot of supernatant was taken for total protein analysis (Bradford microassay, Biorad) before the specimen was mixed with 0.25 volumes of 5× Laemmli buffer, boiled 5 min, and frozen at −80°C.
For histochemistry, LCI animals were deeply anesthetized with isoflurane and perfused transcardially with phosphate buffered saline (PBS, ∼125 mL) and then PBS containing 4% formaldehyde (PBSF, ∼200 mL). Brains were removed and stored in PBSF for 1 h at 4°C. Cryoprotection was performed by incubation for 3 days in 20% sucrose (in PBS) at 4°C, after which brains were frozen on powdered dry ice and stored at −80°C. Cryostat microtome sectioning (20 μm) was performed at −20°C. Sections were positioned on pretreated slides and stored with a desiccant at −80°C. These were used for in situ hybridization (emulsion dipping) and immunohistochemistry.
Immunohistochemistry
Before immunohistochemistry, sections were post-fixed 10 min with PBSF. For horseradish peroxidase (HRP) staining, sections were quenched for endogenous peroxidase followed by 4% Carnation dry milk (BLOTTO) for 20 min. These sections were incubated (3 days at 4°C) with monoclonal anti-human COX2 (1:2,000, Cayman). After washing in BLOTTO and incubation with anti-mouse IgG-HRP conjugated secondary antibodies (1:200, Jackson ImmunoResearch), the signal was visualized by standard immunohistochemical methods using diaminobenzidine. Colocalization studies were achieved by fluorescence immunohistochemistry as described (Isaacs et al., 1995), using rabbit anti-COX2 (1:2,000, Cayman), and cell-type specific antigens: monoclonal anti-MAP2 (1:1,000, kind gift of Dr. Itzhak Fisher) for neurons and monoclonal anti-GFAP IgG (1:2,000, Zymed Laboratories) for activated astrocytes. Appropriate secondary antibodies, conjugated to either Cy3 or fluorescein (Jackson ImmunoResearch), were applied as above. Negative controls, performed by omitting primary antibodies, showed no significant staining in these studies. Sections were visualized using an Olympus fluorescence microscope. Results were based on blinded observations from multiple animals (n = 3 per time point) using single and double labeling procedures.
Molecular Probes
The COX2 cDNA was cloned using a high-fidelity reverse transcriptase-polymerase chain reaction system (Clontech) from total rat brain RNA, using the primer sequences of DuBois et al. (1994). It consisted of the 724–base pair (bp) fragment (position 377–1,101 with respect to the initiation codon) subcloned into pGEM-5Zf+ (Promega). The cyclophilin cDNA (CYC, a “housekeeping” gene, has a constant level of expression under most circumstances) was subcloned into the same vector using a 309-bp fragment (−8 to 300 [Danielson et al., 1988]). Clones were confirmed by restriction enzyme analyses. RNA probes were synthesized from linearized, purified cDNA templates, using T7 RNA polymerase in the presence of 32P-labeled ribonucleoside triphosphates (specific activity 5 × 105 dpm/ng). Full-length antisense transcripts were purified by acrylamide-urea gel electrophoresis, autoradiography (10 sec), excision from the gel and elution in 0.5 M ammonium acetate (pH 6.3), 1 mM EDTA, 0.2% sodium dodecylsulfate (SDS).
In Situ Hybridization
Several thin sections were taken from each level of interest for in situ hybridization histochemistry as described (Strauss and Jacobowitz, 1993), except that 35S-labeled RNA probes (specific activity 7.5 × 105 dpm/ng, produced as above) were hybridized at 42°C and washed at 50°C. Fixed sections were used for emulsion dipping experiments. Negative controls included application of sense probe and pretreatment of selected sections with ribonuclease (RNase) before postfixing. Neither of these control conditions exhibited any signal. Results were based on observations from multiple animals (n = 3 per time point).
mRNA Quantitation
Moles of mRNA can be determined using lysate RNase Protection Assays (RPAs; Strauss and Jacobowitz, 1993). Briefly, an excess of 32P-labeled syngeneic antisense RNA probe was hybridized directly with the target mRNA in the tissue lysate (40 μL) overnight at 37°C. RNase (24 μg/mL) was added and the double-stranded RNA hybrids (protected from RNase degradation) were purified away from background contaminants by organic extractions, ethanol precipitation, and native polyacrylamide gel electrophoresis. Each gel was air dried between two sheets of cellophane (EazyBreeze, Hoefer) and autoradiographed overnight at −80°C. Gel pieces containing full-sized hybrids were excised from the gel, using the autoradiograph as a guide. The radioactive decay in each band, measured by scintillation counting, was converted to moles (via the specific activity) and to grams of mRNA (via the ratio of probe to message length). Values presented are normalized by total protein (mRNA fg/μg protein); normalization using an internal control mRNA, cyclophilin (COX2/CYC, mole%) gave the same results.
Micropunch Immunoblot Analysis
Proteins (25 μg) from specific brain regions were separated on denaturing polyacrylamide gels (7%) and electrophoretically transferred to nitrocellulose. Multiple blots were processed in parallel (back to back) throughout the process for optimal comparisons. Membranes were blocked (4% bovine serum albumin, 0.5% Tween 20) and incubated (2 h shaking at room temperature) with primary antiserum: rabbit anti-mouse COX2 (1:2,000, Cayman Chemical), rabbit anti-mouse COX1 (1:2,000, Cayman Chemical), or rabbit anti-mouse prostacyclin synthase (1:2,000, kind gift of Dr. Stuart Myers). Membranes were washed in PBS, 0.1% Tween 20, incubated with donkey anti-rabbit IgG conjugated to horseradish peroxidase (1:5,000, preadsorbed with rat serum, Pierce Chemical), washed again and incubated 5 min in chemiluminescence reagent (Supersignal, Pierce Chemical). Membranes were wrapped in plastic and exposed to x-ray film (0.5–10 min). Autoradiographs were analyzed by densitometry, using NIH Image to quantify the 72-kDa COX2 band. Membranes were stripped (shaking 30 min at 50°C in 2% SDS, 100 mM tris (pH 8), 100 mM 2-mercaptoethanol) and re-exposed to film for 1 h before reuse (up to three times).
Data Analysis
Results are presented as mean ± SE. Statistical analyses were carried out using STATVIEW software (Abacus Concepts, Inc.). Analysis of variance was performed followed by post-hoc comparisons comparing experimental groups to sham controls (Dunnett's test) or to other experimental groups (Scheffé's test). A p value of < 0.05 was required to reject the null hypothesis that the group means were equivalent.
RESULTS
These studies were conducted using a (3 × 2 × t) design, assessing COX2 (1) mRNA levels, (2) protein levels, and (3) cell type–specific distribution. These results were obtained for COX2 expression ipsilateral and contralateral to injury at several time points. Our LCI studies focused on a neuroanatomical slice (bregma −2.8 to −4.3 mm [Paxinos and Watson, 1986]) that included the anterior part of the injury site, dorsal hippocampus, and other regions that normally express COX2 (Breder et al., 1995). In each section, results for cerebral cortex are presented first, followed by hippocampus.
Localization of COX2 mRNA Increases Following Lateral Cortical Impact Traumatic Brain Injury
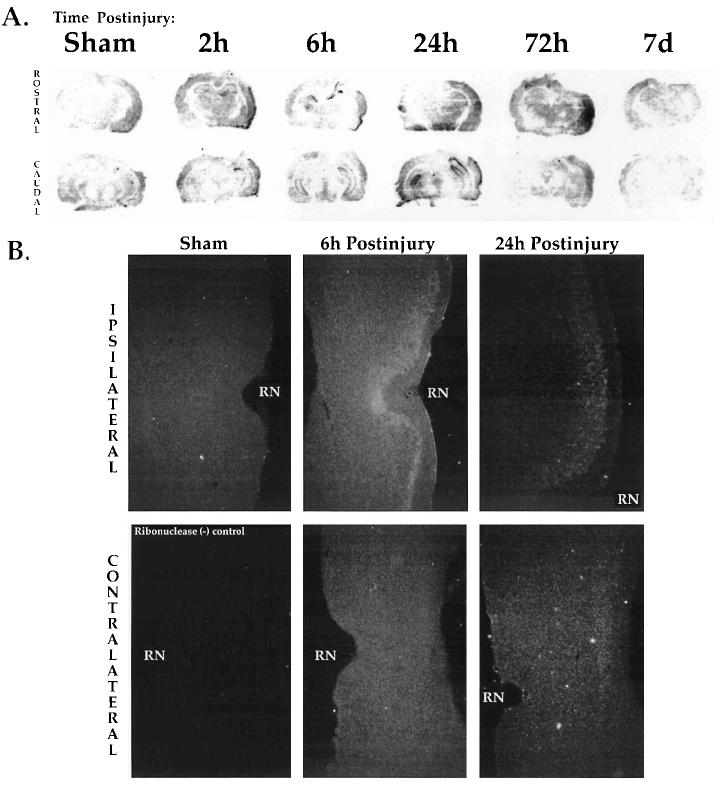
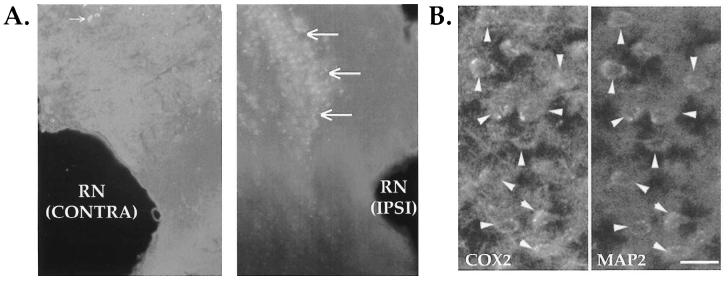
In situ hybridization histochemistry (ISHH) was utilized to qualitatively localize neuroanatomical and temporal fluctuations in COX2 mRNA following lateral cortical impact TBI. Fresh frozen sections (Fig. 1A) were from the same brains used for micropunch analyses (Figs. 2 and 3). Sham-injured and naive brains showed a low level of COX2 mRNA dispersed throughout the cortex (Fig. 1A, left) that was not present in the ribonuclease negative controls. Shams also showed slight increases in the ipsilateral cortex at the earliest time points, but this was the maximum extent of sham signal observed. Within 2 h postinjury, COX2 signal in the cortex appeared to increase in the superficial and middle layers, proximal to the site of injury (Fig. 1A, at 2 h). By 6 h, increased signal was detected in the ipsilateral piriform and amygdaloid regions (Fig. 1A, at 6 h). COX2 mRNA also increased in the ipsilateral perirhinal cortex, adjacent to the rhinal notch (Fig. 1B, formalin-fixed tissue). At 24 h postinjury, COX2 mRNA in the ipsilateral cortex appeared greater than sham levels, whereas most of the piriform signal had dissipated (Fig. 1A,B, at 24 h). After 72 h, COX2 mRNA in the injured cortex remained elevated, with apparently increased signal in the mid-cortical layers, particularly in the perirhinal region (Fig. 1A, at 72 h). Only slight changes were observed in the contralateral parietal cortex.
FIG. 1.
Localization of COX2 mRNA in rat brain after lateral cortical impact TBI. (A) Representative autoradiographic images of COX2 in situ hybridization histochemistry (ISHH) in sham brains, and at 2 h, 6 h, 24 h, 72 h, and 7 days after LCI TBI (ipsilateral side to the right). The rostral (top row) and caudal (bottom row) extent of the sections studied are shown. Sham brains (left) were from 6 h (top) or 24 h (bottom). (B) COX2 mRNA induction in the ipsilateral (top row) but not in the contralateral (bottom row) perirhinal cortex at 6 and 24 h postinjury. Emulsion ISHH showed low level COX2 signal in the shams and contralateral to injury. Postinjury COX2 induction was maximal in perirhinal cortex at 6 h. Sections (20-μm) were from formalin perfused, fixed, and cryoprotected brains. The lower left section was a negative control, pretreated with ribonuclease. A dark-field condenser was used to obtain these images (× 40). RN, rhinal notch.
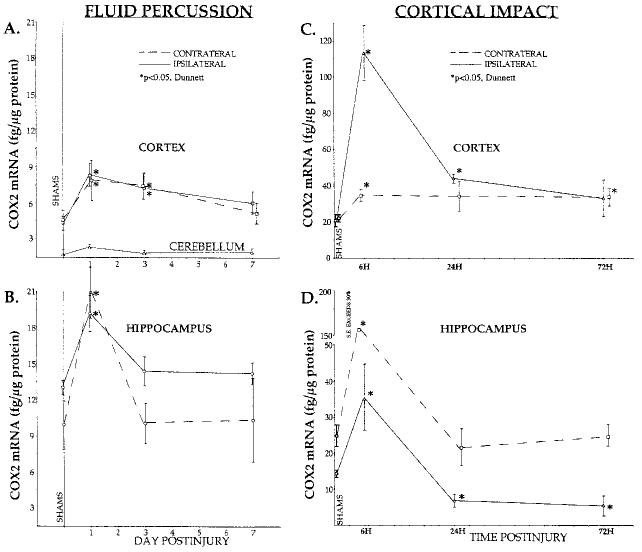
FIG. 2.
Quantitation of COX2 mRNA induction in two rat models of traumatic brain injury. (A,B) Lateral fluid percussio n TBI: COX2 mRNA levels increased bilaterally at 1 day in cortex and hippocampus. COX2 remained elevated in the cortex at 3 days, and a trend toward increase was observed at 7 days postinjury (p = 0.08). Lysates from parietal cortex (inferolateral to the craniectomy, site of most tissue damage; A) and whole hippocampus (B) were analyzed (n = 6 rats per each point). (C,D) Lateral cortical impact TBI: Bilateral elevations in COX2 mRNA were observed at 6 h in cortex and hippocampus. COX2 remained elevated in injured cortex for 24–72 h postinjury. Note the decreased levels of COX2 in the ipsilateral hippocampus at 24 and 72 h. Micropunch lysates of parietal cortex (4 × 1,000 μm micropunches, anterolateral to the injury site, bregma −2.8 to −3.8 mm [Paxinos and Watson, 1986; C), and dorsal hippocampus (4 × 1,000 μm, at the same level; D) were analyzed (n = 6 rats per each point). Micropunches were taken from thick sections from the same brains used to generate ISHH data (Fig. 1A). Changes in COX2 mRNA were measured using a lysate RNase protection assay. Shams (n = 2 at each time point) exhibited similar basal levels of COX2 mRNA and were grouped at 0 days postinjury. The values presented are normalized by total protein (mRNA fg/μg protein); normalization using cyclophilin (mole%) gave the same results. *p < 0.05, Dunnett.
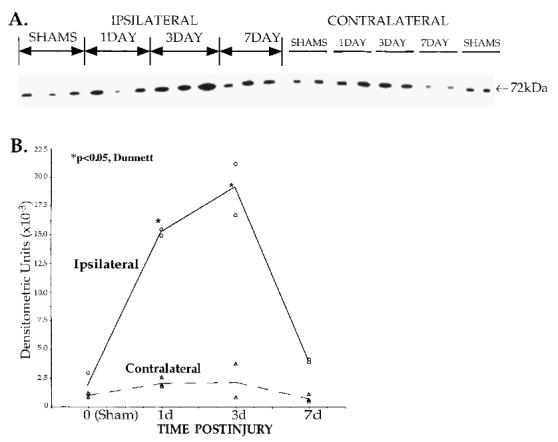
FIG. 3.
COX2 protein increases in the parietal cortex following lateral cortical impact TBI. (A) Immunoblot analysis of COX2 protein in parietal cortex micropunches (4 × 1,000 μm, from same brains used in RPAs and ISHH, n = 3 per time point). Each lane was loaded with 25 μg of total protein. Electrophoresis, transfer, and detection of multiple blots were performed concurrently to improve reliability. Densitometry was used to evaluate the 72-kilodalton COX2 band (ø = specimen deleted from analysis due to loading problem). (B) Densitometry indicated a marked elevation of COX2 protein in injured cortex at 1–3 days postinjury (*p < 0.05, Dunnett), returning toward sham levels by 7 days. Basal levels of COX2 were present in the sham animals, sacrificed at 1, 3, and 7 days postsurgery and grouped at time 0.
Within 2–6 h postinjury ISHH also showed COX2 mRNA induction in the hippocampus (Fig. 1A, at 2 and 6 h). Strongly increased signal was observed primarily in the ipsilateral granule cell layers of CA2, CA3, and dentate gyrus, as well as in the contralateral hippocampus. Between 6 and 24 h, COX2 mRNA was induced bilaterally in the dentate gyrus (Fig. 1A, at 6 and 24 h). There appeared to be much variability in the ipsilateral CA1 to CA3 regions at the 24-h time point. In several cases, the ipsilateral CA1–2 exhibited strong signal while the CA3 signal was attenuated. By 72 h postinjury, COX2 mRNA signal in the hippocampal region had dissipated. At 7 days, COX2 signal in both the cortex and hippocampus had returned toward sham levels (Fig. 1A at 7 days).
Quantitation of COX2 mRNA in Parietal Cortex and Hippocampus
In order to quantify COX2 mRNA induction following TBI, lysate RPAs were performed. Brain regions from two TBI models, the lateral cortical impact (LCI) and lateral fluid percussion (LFP) models, were analyzed. Uninjured (sham) rat brains revealed baseline levels of COX2 mRNA bilaterally in the parietal cortex and hippocampus (Fig. 2, SHAMS). An order of magnitude lower level was detected in the cerebellum (LFP, Fig. 2A), and no COX2 mRNA was detected in the dorsal thalamic nuclei (LCI, data not shown).
Both acute and prolonged elevations of COX2 mRNA were observed after TBI in the LFP (Fig. 2A,B) and LCI (Fig. 2C,D) models. Levels of COX2 mRNA in injured cortex (Fig. 2, top) were induced two- to fivefold at 6–24 h, and remained elevated at 72 h postinjury, as compared to shams (p < 0.05, Dunnett). Contralateral cortex showed corresponding statistically significant increases in both models, though the changes observed after LCI were less dramatic. At 7 days, the cortex specimens from the LFP rats showed a trend toward increased COX2 mRNA (Fig. 2A, p = 0.08).
Hippocampus (Fig. 2, bottom) also exhibited early bilateral two- to fivefold COX2 mRNA increases in both injury models. The contralateral hippocampus expressed higher levels of COX2 mRNA than ipsilateral hippocampus at 6 h after LCI (p < 0.05, Scheffé, Fig. 2D; note the discontinuous ordinate and large error at this time).
No alterations in cyclophilin mRNA levels (measured concurrently in each specimen) or total sample protein were observed. Finally, using the same LFP specimens, no significant changes were observed in COX1 and prostacyclin synthase mRNA levels (data not shown).
Increases in COX2 Protein Following Lateral Cortical Impact Traumatic Brain Injury
Immunoblot analysis (Fig. 3A, using micropunches from the same LCI thick sections as Fig. 2C) showed a significant increase of COX2 protein in the ipsilateral parietal cortex starting at 24 h postinjury (p < 0.05, Dunnett; Fig. 3B and Table 1). Earlier time points (2 and 6 h), though elevated above sham levels, yielded high variability and were eliminated from this analysis. In a separate experiment, hippocampal COX2 protein was also measured using micropunch immunoblot analysis (Table 1). The peak level of hippocampal COX2 appeared to occur at 24 h postinjury. This was followed by an apparent return to sham levels ipsilaterally by 3 days and contralaterally between 3 to 6 days postinjury. Due to high variability, these changes did not reach statistical significance using parametric analyses. As mentioned above, this degree of variability was attributed to interanimal variation in the rate and extent of de novo COX2 transcription and translation following injury.
Table 1.
COX2 Levels in Cortex and Hippocampus: Immunoblot Analysisa
| Brain region |
||
|---|---|---|
| Time postinjury | Ipsilateral | Contralateral |
| Cerebral cortex (n = 3) | ||
| 0 (sham) | 100 ± 65% | 100 ± 25% |
| 1 day | 745 ± 3%* | 223 ± 16% |
| 3 days | 928 ± 2%* | 228 ± 88% |
| 7 days | 197 ± 4% | 56 ± 45% |
| Hippocampus (n = 4) | ||
| 0 (sham) | 100 ± 31% | 100 ± 38% |
| 2 h | 99 ± 26% | 233 ± 47% |
| 1 day | 229 ± 47% | 256 ± 50% |
| 3 days | 26 ± 68% | 238 ± 66% |
| 6 days | 12 ± 93% | 26 ± 51% |
Mean values presented are %SHAM ± coefficient of variation, corrected for the mean sham value for each tissue. Statistics were performed on the raw densitometric units obtained upon scanning and peak area analysis using NIH Image software. Hippocampus was a separate experiment, performed as described in the legend of Figure 3.
p < 0.05, Dunnett.
Patterns of COX2 Induction in the Rat Brain Following Lateral Cortical Impact Traumatic Brain Injury
Patterns of neuroanatomical and temporal changes, as well as cell type–specific expression of COX2-immunoreactivity (-ir) are based on peroxidase and dual fluorescence immunohistochemistry studies. Both revealed similar patterns of COX2-ir in these studies. Sham control rat brains exhibited a uniform, bilateral distribution of lightly staining COX2-ir neurons (large cells) in the superficial and middle layers of dorsal and ventral cortex, in the pyramidal and granule cell layers of hippocampus, and in the amygdaloid region. A few faintly staining smaller COX2-ir cells were observed in the middle layers of cortex and in the pia mater. Choroid plexus, thalamus, hypothalamus were usually negative, but the habenula consistently showed one or two faintly COX2-ir neurons. There were a few large and small COX2-ir cells in the sham hippocampus (less than in the cortex). COX2-ir appeared to localize to the cytoplasm and in cellular processes; no nuclear staining was observed.
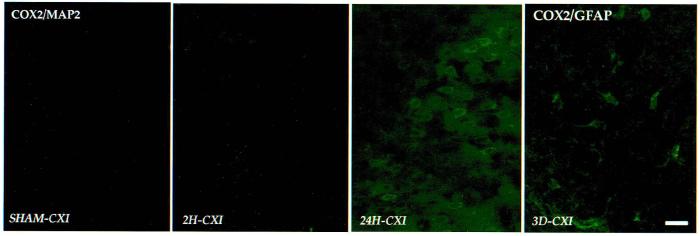
Following lateral cortical impact TBI, acute and prolonged increases of COX2-ir (cell number and intensity) were observed in the cerebral cortex (ipsilaterally) and the hippocampus (bilaterally), compared to sham controls (Figs. 4-7). COX2 was induced in both neurons (MAP2-ir, Fig. 4A,C) and astrocytes (GFAP-ir, Fig. 4B,D). Increased neuronal COX2-ir appeared in the injured and adjacent cortex at 2 h, 24 h, and 3 days postinjury (Fig. 5). The intensity of cortical COX2 staining at 24 h was markedly increased (Fig. 5, third panel), but returned to sham levels by 7 days. Many COX2-ir pyknotic neurons were seen within the site of injury, as well (not shown). Contralateral parietal cortex showed no increases in cell number or intensity of COX2 immunoreactivity.
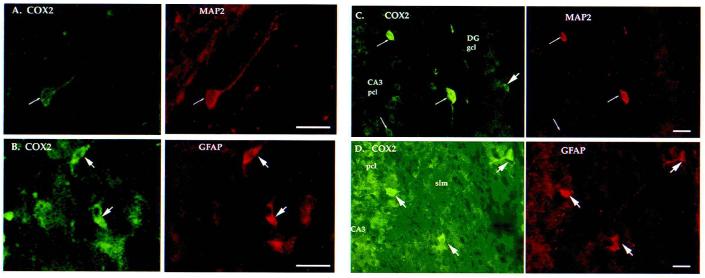
FIG. 4.
COX2 was expressed in both neurons and astrocytes after lateral cortical impact of TBI. Immunofluorescence colocalization was used to detect cell type?specific expression of COX2. (A) COX2 (left) and MAP2 (right) immunoreactive neuron in the ipsilateral parietal cortex at 24 h postinjury. (B) COX2 (left) and GFAP (right) immunoreactive cortical astrocytes, adjacent section to (A). (C) COX2 (left) and MAP2 (right) immunoreactive neurons in the contralateral hippocampus at 6 h postinjury. (D) Highly aborized COX2 (left) and GFAP (right) immunoreactive astrocytes appeared in the contralateral hippocampus, CA3 region, at 6 h postinjury. Dual labeling was performed as described in Methods. All histochemical sections shown in Figures 4-7 were rostral to the injury site (bregma −2.3 to −4.5 mm [Paxinos and Watson, 1986]). pcl, pyramidal cell layer; gcl, granule cell layer; slm, stratum lacunosum-moleculare. Thick arrows point to the astrocytes, thin arrows to neurons. Bar = 50 μm.
FIG. 7.
Intense COX2 immunoreactiv ity in the perirhinal cortex after lateral cortical impact TBI. (A) A patch of intensely labeled neurons (COX2/MAP2, arrows) appeared adjacent to the ipsilateral (IPSI) but not contralateral (CONTRA) rhinal notch at 3 days postinjury. Small arrow points to a few COX2 neurons contralateral to injury (× 6). RN, rhinal notch. (B) Contralatera l perirhinal cortex, 7 days postinjury. The patch of intensely COX2-ir neurons (arrowheads) appeared bilaterally, adjacent to the rhinal notch at 2h (not shown) and 7d postinjury. Bar = 50μm.
FIG. 5.
Temporal induction of COX2 immunoreactivity in the injured parietal cortex. COX2-ir cells showed a pattern of COX2 induction that complemented the mRNA and protein studies. COX2/MAP2 labeling (first three panels): Sham parietal cortex (left) contained many low intensity COX2-ir neurons. Between 2 h (second panel) and 24 h postinjury (third panel), increased numbers and intensity of COX2-ir neurons appeared in ipsilateral parietal cortex. COX2/GFAP labeling (far right): At 3 days postinjury, mostly COX2-ir astrocytes were observed near the site of injury, and the intensity of COX2-ir was decreased at this time. Dual labeling was performed as described in Methods; only COX2-ir is shown. CXI, ipsilateral parietal cortex. Bar = 50 μm.
Increased COX2-ir was also observed in GFAP-ir and other nonneuronal cells in injured cortex. Astrocytic COX2 expression (GFAP-ir, COX2-ir) was observed in the superficial and middle layers of injured and adjacent cortex, as early as 2 h after injury, but peaked at 3 days (Fig. 5, far right), returning to sham levels by 7 days. A locus of GFAP-ir, COX2-ir cells developed within the injury site starting at 24 h (Fig. 6A), encapsulating the cavity that developed by 3–7 days postinjury (Fig. 6B). Diffuse COX2 staining was observed in the internal capsule and corpus callosum in many injured animals, as well. Occasionally, COX2-ir cells were observed in the pia mater, in endothelial cells of larger blood vessels, and in choroid plexus.
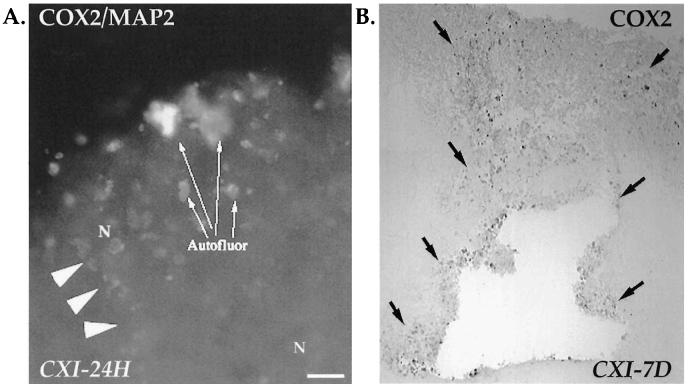
FIG. 6.
Encapsulation of the cortical injury site by nonneuronal COX2-ir cells. (A) Injured cortex showed the initiation of a COX2-ir capsule at 24 h postinjury. Dual labeling (COX2/MAP2) indicated that these cells were predominantly nonneuronal, COX2-ir astrocytes, and other cell types (arrowheads). N, neuron; Autofluor, autofluorescence was detected in the injured cortex starting at 24 h (this could have been due to phagocytosis of damaged neural components by inflammatory cells that may have been COX2-ir). Bar = 50 μm. (B) COX2 immunoreactivity (peroxidase staining) in the injured cortex at 7 days postinjury revealed the encapsulation of the injury site by nonneuronal COX2-ir cells (black arrows; × 14). Negligible staining was seen in the contralateral parietal cortex (not shown).
In the hippocampus, COX2 staining was modestly increased by 2 h postinjury, increasing bilaterally in the CA1–2 pyramidal neurons, with many astrocytes distributed in the contralateral CA2 (stratum lacunosum-moleculare, slm). By 6 h, neuronal COX2-ir was substantially increased bilaterally, with greater cell numbers and intensity contralateral to injury (CA2, CA3, and dentate gyrus; Fig. 4C,D). COX2-ir in the CA1–CA2 regions remained elevated (compared to shams) but not as much as at 2 h. At 24 h postinjury, the intensity of neuronal COX2-ir remained elevated in the contralateral regions only. COX2-ir astrocytes were present mainly in the molecular layer bilaterally. Several highly arborized astrocytes were observed from 6 to 24 h in the contralateral CA2–CA3 (slm; Fig. 4D). By 3 days postinjury, COX2 expression was limited to several intensely stained pyramidal and granule neurons in contralateral CA1–CA3 and the dentate hilus, respectively, with few remaining COX2-ir astrocytes. At 7 days, hippocampal COX2-ir had returned to sham levels.
A patch of intensely COX2-ir neurons appeared in the perirhinal cortex, adjacent to the rhinal notch (Fig. 7A, IPSI). Dual immunofluorescence confirmed only neurons (Fig. 7B). Interestingly, at 2 h and 7 days postinjury these cells appeared bilaterally; however, between 6 h and 3 days, the patch was either obscured by a general increase in COX2-ir (ipsilaterally) or had disappeared (contralaterally). Outside the site of injury, a diffuse increase in ipsilateral cortical COX2-ir was observed at 24 h and 3 days postinjury, but returned to sham levels by 7 days. In addition, a slim band of intensely COX2-ir neurons appeared ipsilaterally, paralleling the piriform cortex, but extending to the inferomedial border of temporal cortex (amygdaloid region). This intense band was present at 6 h but vanished between 24 h and 3 days postinjury.
DISCUSSION
The pattern emerging from these studies was that of early COX2 gene induction in neurons and glia of the injured cortex and hippocampus (bilaterally) following TBI. At 2–6 h postinjury, COX2 was highly induced in the ipsilateral piriform cortex, as well as some ventromedial areas. By 72 h postinjury, COX2 expression in the ipsilateral cortex remained elevated (intensified signal in the middle layers of the parietal cortex, the perirhinal cortex, and piriform cortex). COX2-ir neurons in the perirhinal and piriform cortex, extending into the amygdaloid region have been previously reported (Yamagata et al., 1993; Li et al., 1993; Breder et al., 1995). However, at early (2 h) and late (7 days) times following LCI TBI, a patch of intensely COX2-ir neurons appeared bilaterally in the perirhinal cortex, adjacent to the rhinal notch. A prolonged (1–7 days) induction of COX2 was also observed in many nonneuronal cells encapsulating the injury site and in a small number of hippocampal astrocytes (bilaterally). The appearance of COX2-ir pyknotic cells and autofluorescent (inflammatory) cells in the injured cortex commenced at about 1 day postinjury and continued throughout the entire period of observation.
There was a transient bilateral increase in the COX2-ir (cell number and intensity) in the hippocampus following TBI. Hippocampal pyramidal neurons exhibited increased COX2-ir bilaterally in the CA1–2 regions at 2 h postinjury. This was accompanied by a brief but intense induction of astrocytic COX2-ir in the contralateral hippocampal CA2–3 regions. There appeared to be a temporal “wave” in the COX2 expression from CA1–2 neurons (2–6 h) to CA3 and dentate gyrus (6–24 h). At 24 h postinjury, COX2-ir was still modestly elevated in the CA3 (contralateral) and dentate gyrus (bilateral). By 3 days postinjury, hippocampal CA1–CA3 regions exhibited sham levels of COX2-ir, with the exception of a few astrocytes in the contralateral CA3 and dentate gyrus. By 7 days, hippocampal COX2 expression had completely returned to sham levels. Interestingly, Nakayama et al. (1998) reported the induction of COX2 mRNA moving in the opposite direction (dentate gyrus → CA3 → CA2-1) between 2 and 24 h following transient global ischemia. Whether this reflects on the observations that mainly CA1 neurons are vulnerable to apoptosis after ischemic insult, while mostly CA3 neurons are lost after TBI, remains to be determined.
Quantitative lysate RPAs showed two- to sixfold increases in hippocampal and cortical levels of COX2 mRNA in LFP and LCI rat models of TBI. Highly variable peak COX2 mRNA levels at the 2–6-h time points were probably due to interanimal variation in the rate of COX2 induction. While slight neuroanatomical differences might be suspected, repeat determinations showed consistent cortical and hippocampal COX2 mRNA levels in coronal sections rostral and caudal to the sections micropunched for these studies. Differences in COX2 levels between the two models might be attributable to the method of collection. The micropunch dissections (LCI samples) were performed using the ISHH observations as a guide, whereas the homogenization of gross brain regions (LFP specimens) would tend to dilute the signal amidst less affected tissue. Decreased ipsilateral hippocampal COX2 mRNA in the LCI specimens at 1–3 days (Fig. 2D) could reflect neuronal loss. This was not seen with LFP specimens, also possibly due to the method of collection. In a novel immunoblot procedure using micropunches, COX2 protein levels in the ipsilateral parietal cortex were shown to peak around 24 h and remain elevated for at least 3 days following LCI TBI.
The paucity of ipsilateral CA3 hippocampal signal after 24 h was conspicuous (Figs. 1A, 2D). This was probably due to ipsilateral apoptotic cell loss, characteristic of lateral cortical brain injuries (Conti et al., 1998; Kaya et al., 1999). The mRNA results matched the histochemistry and immunoblot results, except at 7 days postinjury. At this time, the bilateral appearance of COX2-ir neurons in the perirhinal cortex was not reflected in the mRNA or the immunoblot analyses. This might be due to differences in the sampling techniques (micropunch analyses were performed on parietal cortex dorsal to the rhinal notch).
Our observations of COX2 mRNA, protein, and histology establish distinct temporal and neuroanatomical patterns of gene expression following traumatic brain injury. COX2 is expressed early after TBI and remains elevated for an extended period of at least 3–7 days in brain regions proximal and distal to the site of injury. Many studies describe the brief induction of COX2 after various transient cerebral insults. In other models of brain injury, COX2 expression has been shown to increase from 1 to 24 h, or in the case of transient global ischemia for up to 48 h, but then return to normal (Adams et al., 1996; Kong et al., 1997; Nakayama et al., 1996). A more prolonged pattern emerges after moderate TBI in the rat. In addition, recent studies of global ischemia (Nakayama et al., 1998; Sairanen et al., 1998) demonstrated a 72-h (or longer) induction of COX2. These findings support the hypotheses that the duration of COX2 elevation may reflect the severity of the injury, and/or the repression of COX2 induction may become compromised in more severely affected brain areas. A long period of COX2 overexpression could contribute to progressive loss of neural tissue via oxidative damage, prostaglandin receptor–mediated events, or a combination of these that effect changes at the level of gene expression. These may result in secondary injuries to the brain, promote neuropathology, and worsen behavioral outcome. Recently, Pasinetti and Aisen (1998) have suggested that COX2 may be an etiological factor in Alzheimer's disease. The connection between COX2, traumatic brain injury and cognitive decline has recently become a topic of investigation (Luukinen et al., 1999).
There is an apparent distinction between the induction of COX2 following TBI and that in response to inflammatory stimuli. Cortical impact TBI stimulates cortical and subcortical COX2 expression in neurons and glia, whereas COX2 induced systematically or centrally by LPS (Cao et al., 1999) appears in cerebral endothelial cells, leptomeninges, and choroid plexus. This may represent a fundamental difference in the mechanism of COX2 function between these two types of injury. Endothelial COX2 is most likely associated with the inflammatory response, temperature regulation and cerebral autoregulation. Neural-derived COX2 may be regulated by glutamatergic activity under normal pathological conditions (Yamagata et al., 1993; Chen et al., 1995; Kaufmann et al., 1996; Adams et al., 1996; Marcheselli and Bazan, 1996), and might therefore be associated with cell survival (Ho et al., 1998).
The acute and chronic increases in brain COX2 that follow moderate TBI are maximal in areas known to be specifically associated with the neurological and cognitive deficits that accompany virtually all clinically relevant head trauma. COX2 induction in the hippocampus and perirhinal cortex bilaterally, and in the piriform cortex could be functionally associated with these deficits. LCI is thought to be a model of focal brain contusion. Some contralateral injury may occur, however, this is not supported by morphological evidence and would not account for the dramatic COX2 induction in the contralateral hippocampus. A possible pathway for this phenomenon is proposed as follows. Starting with the early neuroanatomists, the commisural connections in the hippocampus have been thoroughly studied (Ramón y Cajal, 1995). The CA1 provides the major cortical output of the hippocampus, and has widespread projections to the contralateral CA1, subicular zones, as well as entorhinal cortex (van Groen and Wyss, 1990). Strong homotopic communication exists both in rodents and primates, particularly in the dentate gyrus and subicular zones (West et al., 1979; Amaral et al., 1984). In the primate, the presubiculum gives rise to the major contralateral projections of the hippocampus, mostly to the contralateral entorhinal cortex. Homotopic connections from the entorhinal cortex to the dentate gyrus (molecular layer), CA1, and CA2–3 (slm) may be projected contralaterally through the CA1 (slm) and subicular zones to the contralateral medial entorhinal cortex (Ino et al., 1998; van Groen and Wyss, 1990). COX2 expression is stimulated postsynaptically by glutamatergic neurotransmission in the developing and adult brain (Kaufmann et al., 1996). COX2 mRNA can be induced in cultured cerebellar granule cells by excitotoxic concentrations of glutamate (K. Strauss and A. Marini, manuscript in preparation). A potential mechanism, then, for the induction of COX2 in the contralateral hippocampus would be an increase in glutamatergic neurotransmission. This might be caused by a direct release of excitatory amino acids due to the injury (Faden et al., 1989; Katayama et al., 1990; Palmer et al., 1993), stimulation of ipsilateral hippocampal excitatory crossover neurons, or a loss of inhibitory inputs across the comimssures. The literature does not support an increase in extracellular excitatory amino acids contralateral to injury. However, exclusively excitatory (possibly mossy cell) fibers have been found to cross from the prosubiculum to the contralateral medial entorhinal cortex (van Haeften et al., 1998). Reports of crossing inhibitory (γ-amino butyric acid, GABA) fibers have long been available (Leranth and Frotscher, 1987; Babb et al., 1988). Lowenstein et al. (1992) reported that the loss of hilar neurons caused a hyperexcitability in dentate granule cells within 4 h after TBI in the rat. In addition, some of the contralaterally projecting GABAergic neurons are postsynaptic to acetylcholine inputs (Leranth and Frotscher, 1987). Recently, Dixon (1996, 1997) and others have demonstrated decreased cholinergic function in the hippocampus following TBI. This might lead to decreased GABAergic input in the contralateral hippocampus and result in increased COX2 expression via disinhibition of excitatory (possibly mossy) neurons in the dentate gyrus. Of course, the picture may be much more complex; the loss of excitatory crossing fibers could reduce the inhibition of contralateral GABA interneurons, resulting in increased contralateral glutamate release, etc.
Finally, an initial measurement of total prostaglandin levels in parietal cortex at 24 h postinjury showed approximately a fourfold increase ipsilateral to injury (data not shown). This increase corresponds directly to the observed mRNA and protein increases. The changes could be much greater in specific brain regions that were COX2-ir by immunohistochemistry (e.g., perirhinal or piriform cortex, dentate gyrus). To obtain a more complete picture of the physiological role of COX2 following TBI, the prostaglandin and free radical products formed in these brain regions will have to be measured. This could be accomplished either by in vivo stereotactic microdialysis or via an immunoassay sensitive enough to detect individual prostaglandin levels in the extracts of micropunches from frozen brain sections.
We demonstrated that COX2 in the brain was elevated for more than 72 h after TBI. Interestingly, the peak of COX2 expression corresponded temporally and neuroanatomically to the induction of apoptosis in these TBI models. However, the role of COX2 in the pathophysiology of TBI is uncertain. Treatments that prevent COX2 induction (glucocorticoids) or inhibit enzyme activity (indomethacin) have had, at best, equivocal success in the clinical treatment of TBI (Benedek et al., 1987; Biestro et al., 1995; Dahl et al., 1996). If, contrary to current opinion, COX2 induction were beneficial, then inhibition might result in a worsened outcome. In the laboratory, milder insults (transient focal ischemia, kainic acid injections, etc.) result in a transient induction of COX2 (2–24 h). In contrast, after more substantial challenges (moderate TBI, global ischemia, Alzheimer's disease?), COX2 induction occurs early, but remains elevated for prolonged periods. We propose that the initial response of COX2 induction is adaptive, while the prolonged expression observed following injury is pathological. Two inferences may be drawn from this hypothesis. First, that an active process is necessary to turn down the gain on COX2 expression following certain types of induction. Second, if the initial phase of COX2 expression is beneficial, then delayed pharmacological treatment with steroids or COX2-specific inhibitors could result in better outcomes in animal models of TBI, and may lead to new clinical treatment paradigms, as well.
ACKNOWLEDGMENTS
Russell Cole, Jeffery Sodl, and Tamika Weerasingha for excellent technical assistance; Dr. Ed Gruberg for assistance with the dark field microscopy, Dr. Ady Kendler for providing the GFAP antisera, and Dr. Itzhak Fisher for the MAP2 antisera. Special thanks to Dr. Tracy McIntosh for many valuable discussions. Thanks also to the Fels Institute of Cancer Research and Molecular Biology for the use of the fluorescence microscope, video camera, and imaging software. This study was supported in part by funds from NINDS RO1 NS38654, Rubin Family Award, Sam & Bertha Brochstein Fund, Newell DeValpine Foundation.
REFERENCES
- ADAMS J, COLLACO-MORAES Y, DEBELLEROCHE JS. Cyclooxygenase-2 induction in cerebral cortex: an intracellular response to synaptic excitation. J. Neurochem. 1996;66:6–13. doi: 10.1046/j.1471-4159.1996.66010006.x. [DOI] [PubMed] [Google Scholar]
- AMARAL DG, INSAUSTI R, COWAN WM. The commissural connections of the monkey hippocampal formation. J. Comp. Neurol. 1984;224:307–336. doi: 10.1002/cne.902240302. [DOI] [PubMed] [Google Scholar]
- BABB TL, PRETORIUS JK, KUPFER WR, BROWN WJ. Distribution of glutamate-decar boxylase-immunoreactive neurons and synapses in the rat and monkey hippocampus: light and electron microscopy. J. Comp. Neurol. 1988;278:121–138. doi: 10.1002/cne.902780108. [DOI] [PubMed] [Google Scholar]
- BENEDEK G, TOTH-DARU P, JANAKY J, HORTOBAGYI A, OBAL F., Jr. Indomethacin is effective against neurogenic hyperthermia following cranial trauma or brain surgery. Can. J. Neurol. Sci. 1987;14:145–148. doi: 10.1017/s0317167100026275. [DOI] [PubMed] [Google Scholar]
- BIESTRO AA, ALBERTI RA, SOCA AE, CANCELA M, PUPPO CB, BOROVICH B. Use of indomethacin in brain-injured patients with cerebral perfusion pressure impairment: preliminary report. J. Neurosurg. 1995;83:627–630. doi: 10.3171/jns.1995.83.4.0627. [DOI] [PubMed] [Google Scholar]
- BOUMA GJ, MUIZELAAR JP. Cerebral blood flow, cerebral blood volume, and cerebrovascular reactivity after severe head injury. J. Neurotrauma. 1992;9:S333–S348. [PubMed] [Google Scholar]
- BREDER CD, SMITH WL, RAZ A, et al. Distribution and characterization of cyclooxygenase immunoreactivity in the ovine brain. J. Comp. Neurol. 1992;322:409–438. doi: 10.1002/cne.903220309. [DOI] [PubMed] [Google Scholar]
- BREDER CD, DEWITT D, KRAIG RP. Characterization of inducible cyclooxygenase in rat brain. J. Comp. Neurol. 1995;355:296–315. doi: 10.1002/cne.903550208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUSIJA DW, THORE C, BEASLEY T, BARI F. Induction of cyclooxygenase-2 following anoxic stress in piglet cerebral arteries. Microcirculation. 1996;3:379–386. doi: 10.3109/10739689609148310. [DOI] [PubMed] [Google Scholar]
- CAGGIANO AO, BREDER CD, KRAIG RP. Long-term elevation of cyclooxygenase- 2, but not lipoxygenase, in regions synaptically distant from spreading depression. J. Comp. Neurol. 1996;376:447–462. doi: 10.1002/(SICI)1096-9861(19961216)376:3<447::AID-CNE7>3.0.CO;2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAO C, MATSUMURA K, YAMAGATA K, WATANABE Y. Induction by lipopolysaccharide of cyclooxygenase- 2 mRNA in rat brain; its possible role in the febrile responses. Brain Res. 1995;697:187–196. doi: 10.1016/0006-8993(95)00839-i. [DOI] [PubMed] [Google Scholar]
- CAO C, MATSUMURA K, WATANABE Y. Induction of cyclooxygenase-2 in the brain by cytokines. Ann. N.Y. Acad. Sci. 1997;813:307–309. doi: 10.1111/j.1749-6632.1997.tb51710.x. [DOI] [PubMed] [Google Scholar]
- CAO C, MATSUMURA K, OZAKI M, WATANABE Y. Lipopolysaccharide injected into the cerebral ventricle evokes fever through induction of cyclooxygenase-2 in brain endothelial cells. J. Neurosci. 1999;19:716–725. doi: 10.1523/JNEUROSCI.19-02-00716.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN J, MARSH T, ZHANG JS, GRAHAM SH. Expression of cyclo-oxygenase 2 in rat brain following kainate treatment. Neuroreport. 1995;6:245–248. [PubMed] [Google Scholar]
- COLLACO-MORAES Y, ASPEY B, HARRISON M, DE BELLEROCHE J. Cyclo-oxygenase-2 messenger RNA induction in focal cerebral ischemia. J. Cereb. Blood Flow Metab. 1996;16:1366–1372. doi: 10.1097/00004647-199611000-00035. [DOI] [PubMed] [Google Scholar]
- CONTI AC, RAGHUPATHI R, TROJANOWSKI JQ, McINTOSH TK. Experimental brain injury induces regionally distinct apoptosis during the acute and delayed post-traumatic period. J. Neurosci. 1998;18:5663–5672. doi: 10.1523/JNEUROSCI.18-15-05663.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAHL B, BERGHOLT B, COLD GE, et al. CO2 and indomethacin vasoreactivity in patients with head injury. Acta Neurochir. (Wien) 1996;138:265–273. doi: 10.1007/BF01411736. [DOI] [PubMed] [Google Scholar]
- DANIELSON PE, FORSS-PETTER S, BROW MA, et al. p1B15: a cDNA clone of the rat mRNA encoding cyclophilin. DNA. 1988;7:261–267. doi: 10.1089/dna.1988.7.261. [DOI] [PubMed] [Google Scholar]
- DEWITT DS, KONG DL, LYETH BG, et al. Experimental traumatic brain injury elevates brain prostaglandin E2 and thromboxane B2 levels in rats. J. Neurotrauma. 1988;5:303–313. doi: 10.1089/neu.1988.5.303. [DOI] [PubMed] [Google Scholar]
- DHILLON HS, DONALDSON D, DEMPSEY RJ, PRASAD MR. Regional levels of free fatty acids and Evans blue extravasation after experimental brain injury. J. Neurotrauma. 1994;11:405–415. doi: 10.1089/neu.1994.11.405. [DOI] [PubMed] [Google Scholar]
- DIXON CE, BAO J, LONG DA, HAYES RL. Reduced evoked release of acetylcholine in the rodent hippocampus following traumatic brain injury. Pharmacol. Biochem. Behav. 1996;53:679–686. doi: 10.1016/0091-3057(95)02069-1. [DOI] [PubMed] [Google Scholar]
- DIXON CE, MA X, MARION DW. Effects of CDP-choline treatment on neurobehavioral deficits after TBI and on hippocampal and neocortical acetylcholine release. J. Neurotrauma. 1997;14:161–169. doi: 10.1089/neu.1997.14.161. [DOI] [PubMed] [Google Scholar]
- DuBOIS RN, TSUJII M, BISHOP P, AWAD JA, MAKITA K, LANAHAN A. Cloning and characterization of a growth factor-inducible cyclooxygenase gene from rat intestinal epithelial cells. Am. J. Physiol. 1994;266:G822–G827. doi: 10.1152/ajpgi.1994.266.5.G822. [DOI] [PubMed] [Google Scholar]
- ELLIS EF, WEI EP, KONTOS HA. Vasodilation of cat cerebral arterioles by prostaglandins D2, E2, G2, and I2. Am. J. Physiol. 1979;237:H381–H385. doi: 10.1152/ajpheart.1979.237.3.H381. [DOI] [PubMed] [Google Scholar]
- ELLIS EF, HOLT SA, WEI EP, KONTOS HA. Kinins induce abnormal vascular reactivity. Am. J. Physiol. 1988;255:H397–H400. doi: 10.1152/ajpheart.1988.255.2.H397. [DOI] [PubMed] [Google Scholar]
- ELLIS EF, POLICE RJ, RICE LY, GRABEEL M, HOLT S. Increased plasma PGE2, 6-keto-PGF1 alpha, and 12-HETE levels following experimental concussive brain injury. J. Neurotrauma. 1989;6:31–37. doi: 10.1089/neu.1989.6.31. [DOI] [PubMed] [Google Scholar]
- FADEN AI, DEMEDIUK P, PANTER SS, VINK R. The role of excitatory amino acids and NMDA receptors in traumatic brain injury. Science. 1989;244:798–800. doi: 10.1126/science.2567056. [DOI] [PubMed] [Google Scholar]
- FENG L, SUN W, XIA Y, et al. Cloning two isoforms of rat cyclooxygenase: differential regulation of their expression. Arch. Biochem. Biophys. 1993;307:361–368. doi: 10.1006/abbi.1993.1601. [DOI] [PubMed] [Google Scholar]
- FU JY, MASFERRER JL, SEIBERT K, RAZ A, NEEDLEMAN P. The induction and suppression of prostaglandin H2 synthase (cyclooxygenase ) in human monocytes. J. Biol. Chem. 1990;265:16737–16740. [PubMed] [Google Scholar]
- GRAHAM SH, CLARK RSB, STETLER RA, et al. Increased expression of the immediate early gene cyclooxygenase-2 after controlled cortical impact in rat hippocampus and cortex. J. Neurotrauma. 1996a;13:A620. [Google Scholar]
- GRAHAM SH, NAKAYAMA M, ZHU R, CHEN J. Cyclooxygenase 2 and the pathogenesis of delayed neuronal death after global ischemia. Neurology. 1996b;46S:A406. [Google Scholar]
- HO L, OSAKA H, AISEN PS, PASINETTI GM. Induction of cyclooxygenase (COX)–2 but not COX-1 gene expression in apoptotic cell death. J. Neuroimmunol. 1998;89:142–149. doi: 10.1016/s0165-5728(98)00132-5. [DOI] [PubMed] [Google Scholar]
- INO T, KANEKO T, MIZUNO N. Direct projections from the entorhinal cortical layers to the dentate gyrus, hippocampus, and subicular complex in the cat. Neurosci. Res. 1998;32:241–265. doi: 10.1016/s0168-0102(98)00093-5. [DOI] [PubMed] [Google Scholar]
- ISAACS KR, WINSKY L, STRAUSS KI, JACOBOWITZ DM. Quadruple colocalization of calretinin, calcitonin gene-related peptide, vasoactive intestinal peptide, and substance P in fibers within the villi of the rat intestine. Cell Tissue Res. 1995;280:639–651. doi: 10.1007/BF00318366. [DOI] [PubMed] [Google Scholar]
- KATAYAMA Y, BECKER DP, TAMURA T, HOVDA DA. Massive increases in extracellular potassium and the indiscriminate release of glutamate following concussive brain injury. J. Neurosurg. 1990;73:889–900. doi: 10.3171/jns.1990.73.6.0889. [DOI] [PubMed] [Google Scholar]
- KAUFMANN WE, WORLEY PF, PEGG J, BREMER M, ISAKSON P. COX-2, a synaptically induced enzyme, is expressed by excitatory neurons at postsynaptic sites in rat cerebral cortex. Proc. Natl. Acad. Sci. U.S.A. 1996;93:2317–2321. doi: 10.1073/pnas.93.6.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAYA SS, MAHMOOD A, LI Y, YAVUZ E, GOKSEL M, CHOPP M. Apoptosis and expression of p53 response proteins and cyclin D1 after cortical impact in rat brain. Brain Res. 1999;818:23–33. doi: 10.1016/s0006-8993(98)01204-9. [DOI] [PubMed] [Google Scholar]
- KEMPSKI O, SHOHAMI E, VON LUBITZ D, HALLENBECK JM, FEUERSTEIN G. Postischemic production of eicosanoids in gerbil brain. Stroke. 1987;18:111–119. doi: 10.1161/01.str.18.1.111. [DOI] [PubMed] [Google Scholar]
- KOCHANEK PM, MARION DW, ZHANG WG, et al. Severe controlled cortical impact in rats: assessment of cerebral edema, blood flow, and contusion volume. J. Neurotrauma. 1995;12:1015–1025. doi: 10.1089/neu.1995.12.1015. [DOI] [PubMed] [Google Scholar]
- KONG DL, HARA K, WEINSTEIN PR, SHARP FR. Cyclooxygenase-2 mRNA and protein expression in a mouse model of cerebral artery occlusion (MCAO) Soc. Neurosci. Abst. 1997;23:2181. [Google Scholar]
- KONTOS HA, WEI EP, POVLISHOCK JT, DIETRICH WD, MAGIERA CJ, ELLIS EF. Cerebral arteriolar damage by arachidonic acid and prostaglandin G2. Science. 1980a;209:1242–1245. doi: 10.1126/science.7403881. [DOI] [PubMed] [Google Scholar]
- KONTOS HA, DIETRICH WD, WEI EP, ELLIS EF, POVLISHOCK JT. Abnormalities of the cerebral microcirculation after traumatic injury: the relationship of hypertension and prostaglandins. Adv. Exp. Med. Biol. 1980b;131:243–256. doi: 10.1007/978-1-4684-3752-2_19. [DOI] [PubMed] [Google Scholar]
- LACROIX S, RIVEST S. Effect of acute systemic inflammatory response and cytokines on the transcription of the genes encoding cyclooxygenase enzymes (COX-1 and COX-2) in the rat brain. J. Neurochem. 1998;70:452–466. doi: 10.1046/j.1471-4159.1998.70020452.x. [DOI] [PubMed] [Google Scholar]
- LERANTH C, FROTSCHER M. Cholinergic innervation of hippocampal GAD- and somatostatin-immunoreactive commissural neurons. J. Comp. Neurol. 1987;261:33–47. doi: 10.1002/cne.902610104. [DOI] [PubMed] [Google Scholar]
- LEWÉN A, SALCI K, MARKLUND N, HILLERED L. The spin trap PBN reduces extracellular glycerol and lactate levels following traumatic brain injury in rats. J. Neurotrauma. 1996;13:A600. [Google Scholar]
- LI DY, VARMA DR, CHATTERJEE TK, FERNANDEZ H, ABRAN D, CHEMTOB S. Fewer PGE2 and PGF2 alpha receptors in brain synaptosomes of newborn than of adult pigs. J. Pharmacol. Exp. Ther. 1993;267:1292–1297. [PubMed] [Google Scholar]
- LOWENSTEIN DH, THOMAS MJ, SMITH DH, McINTOSH TK. Selective vulnerability of dentate hilar neurons following traumatic brain injury: a potential mechanistic link between head trauma and disorders of the hippocampus. J. Neurosci. 1992;12:4846–4853. doi: 10.1523/JNEUROSCI.12-12-04846.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUKIW WJ, BAZAN NG. Cyclooxygenase 2 RNA message abundance, stability, and hypervariability in sporadic alzheimer neocortex. J. Neurosci. Res. 1997;50:937–945. doi: 10.1002/(SICI)1097-4547(19971215)50:6<937::AID-JNR4>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- LUUKINEN H, VIRAMO P, KOSKI K, LAIPPALA P, KIVELÄ S-L. Head injuries and cognitive decline among older adults. Neurology. 1999;52:557–562. doi: 10.1212/wnl.52.3.557. [DOI] [PubMed] [Google Scholar]
- MARCHESELLI VL, BAZAN NG. Sustained induction of prostaglandin endoperoxide synthase-2 by seizures in hippocampus. Inhibition by a platelet-activatin g factor antagonist. J. Biol. Chem. 1996;271:24794–24799. doi: 10.1074/jbc.271.40.24794. [DOI] [PubMed] [Google Scholar]
- MARKLUND N, SALCI K, LEWÉN A, HILLERED L. Extracellular glycerol as a marker for membrane phospholipid degradation in traumatic brain injury. J. Neurotrauma. 1996;13:A600. doi: 10.1097/00001756-199704140-00026. [DOI] [PubMed] [Google Scholar]
- McINTOSH TK, VINK R, NOBLE L, et al. Traumatic brain injury in the rat: characterization of a lateral fluid-percussion model. Neuroscience. 1989;28:233–244. doi: 10.1016/0306-4522(89)90247-9. [DOI] [PubMed] [Google Scholar]
- MOORE SA, YODER EJ, RICH F. Cerebrovascular cyclooxygenase- 2 expression is regulated by pro- and anti-inflammatory cytokines. Soc. Neurosci. Abst. 1997;23:1166. [Google Scholar]
- MUIR JK, BOERSCHEL M, ELLIS EF. Continuous monitoring of posttraumatic cerebral blood flow using laser-Doppler flowmetry. J. Neurotrauma. 1992;9:355–362. doi: 10.1089/neu.1992.9.355. [DOI] [PubMed] [Google Scholar]
- NAKAYAMA M, UCHIMURA K, ZHU L, et al. Cyclooxygenas e-2 gene expression in neurons following focal cerebral ischemia. Soc. Neurosci. Abst. 1996;22:1670. [Google Scholar]
- NAKAYAMA M, UCHIMURA K, ZHU RL, et al. Cyclooxygenas e-2 inhibition prevents delayed death of CA1 hippocampal neurons following global ischemia. Proc. Natl. Acad. Sci. U.S.A. 1998;95:10954–10959. doi: 10.1073/pnas.95.18.10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISHIHARA I, MINAMI T, WATANABE Y, ITO S, HAYAISHI O. Prostaglandin E2 stimulates glutamate release from synaptosomes of rat spinal cord. Neurosci. Lett. 1995;196:57–60. doi: 10.1016/0304-3940(95)11839-o. [DOI] [PubMed] [Google Scholar]
- NISHIZAKI T, NOMURA T, MATSUOKA T, TSU-JISHITA Y. Arachidonic acid as a messenger for the expression of long-term potentiation. Biochem. Biophys. Res. Commun. 1999;254:446–449. doi: 10.1006/bbrc.1998.9961. [DOI] [PubMed] [Google Scholar]
- NOGAWA S, FANGYI Z, ROSS ME, IADECOLA C. Cyclo-oxygenas e-2 gene expression in neurons contributes to ischemic brain damage. J. Neurosci. 1997;17:2746–2755. doi: 10.1523/JNEUROSCI.17-08-02746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'BANION MK. Cyclooxygenas e-2: molecular biology, pharmacology, and neurobiology. Crit. Rev. Neurobiol. 1999;13:45–82. doi: 10.1615/critrevneurobiol.v13.i1.30. [DOI] [PubMed] [Google Scholar]
- OBRIST WD, LANGFITT TW, JAGGI JL, CRUZ J, GENNARELLI TA. Cerebral blood flow and metabolism in comatose patients with acute head injury. Relationship to intracranial hypertension. J. Neurosurg. 1984;61:241–253. doi: 10.3171/jns.1984.61.2.0241. [DOI] [PubMed] [Google Scholar]
- OTT L, McCLAIN CJ, GILLESPIE M, YOUNG B. Cytokines and metabolic dysfunction after severe head injury. J. Neurotrauma. 1994;11:447–472. doi: 10.1089/neu.1994.11.447. [DOI] [PubMed] [Google Scholar]
- PALKOVITS M. Isolated removal of hypothalamic or other brain nuclei of the rat. Brain Res. 1973;59:449–450. doi: 10.1016/0006-8993(73)90290-4. [DOI] [PubMed] [Google Scholar]
- PALMER AM, MARION DW, BOTSCHELLER ML, SWEDLOW PE, STYREN SD, DEKOSKY ST. Traumatic brain injury–induced excitotoxicity assessed in a controlled cortical impact model. J. Neurochem. 1993;61:2015–2024. doi: 10.1111/j.1471-4159.1993.tb07437.x. [DOI] [PubMed] [Google Scholar]
- PASINETTI GM, AISEN PS. Cyclooxygenase-2 expression is increased in frontal cortex of Alzheimer's disease brain. Neuroscience. 1998;87:319–324. doi: 10.1016/s0306-4522(98)00218-8. [DOI] [PubMed] [Google Scholar]
- PAXINOS G, WATSON C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1986. [Google Scholar]
- POVLISHOCK JT, KONTOS HA. Continuing axonal and vascular change following experimental brain trauma. CNS Trauma. 1985;2:285–298. doi: 10.1089/cns.1985.2.285. [DOI] [PubMed] [Google Scholar]
- PRASAD MR, DHILLON HS, CARBARY T, DEMPSEY RJ, SCHEFF SW. Enhanced phosphodiestric breakdown of phophotidylinosi tol bisphosphate after experimental brain injury. J. Neurochem. 1994;63:773–776. doi: 10.1046/j.1471-4159.1994.63020773.x. [DOI] [PubMed] [Google Scholar]
- RAMÓN Y CAJAL S. In: Histology of the Nervous System of Man and Vertebrates. Swanson N, Swanson LW, editors. Oxford University Press; New York: 1995. pp. 638–640. [Google Scholar]
- RAZ A, WYCHE A, SIEGEL N, NEEDLEMAN P. Regulation of fibroblast cyclooxygenase synthesis by interleukin-1. J. Biol. Chem. 1988;263:3022–3028. [PubMed] [Google Scholar]
- RESNICK DK, GRAHAM SH, DIXON CE, MARION DW. Role of cyclooxygenase 2 in acute spinal cord injury. J. Neurotrauma. 1998;15:1005–1013. doi: 10.1089/neu.1998.15.1005. [DOI] [PubMed] [Google Scholar]
- SAIRANEN T, RISTIMÄKI A, KARJALAINEN-LINDSBERG ML, PAETAU A, KASTE M, LINDSBERG PJ. Cyclooxygenase-2 is induced globally in infarcted human brain. Ann. Neurol. 1998;43:738–747. doi: 10.1002/ana.410430608. [DOI] [PubMed] [Google Scholar]
- SANZ O, ESTRADA A, FERRER I, PLANAS AM. Differential cellular distribution and dynamics of HSP70, cyclooxygenase-2 , and c-Fos in the rat brain after transient focal ischemia or kainic acid. Neuroscience. 1997;80:221–232. doi: 10.1016/s0306-4522(97)00089-4. [DOI] [PubMed] [Google Scholar]
- SAPER CB, BREDER CD. Endogenous pyrogens in the CNS: role in the febrile response. Prog. Brain Res. 1992;93:419–429. doi: 10.1016/s0079-6123(08)64587-2. [DOI] [PubMed] [Google Scholar]
- SEIBERT K, ZHANG Y, LEAHY K, et al. Pharmacological and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proc. Natl. Acad. Sci. U.S.A. 1994;91:12013–12017. doi: 10.1073/pnas.91.25.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEVANIAN A, KIM E. Phospholipase A2–dependent release of fatty acids from peroxidized membranes. J. Free Radic. Biol. Med. 1985;1:263–271. doi: 10.1016/0748-5514(85)90130-8. [DOI] [PubMed] [Google Scholar]
- SHOHAMI E, SHAPIRA Y, SIDI A, COTEV S. Head injury induces increased prostaglandin synthesis in rat brain. J. Cereb. Blood Flow Metab. 1987;7:58–63. doi: 10.1038/jcbfm.1987.8. [DOI] [PubMed] [Google Scholar]
- SHOHAMI E, SHAPIRA Y, YADID G, REISFELD N, YEDGAR S. Brain phospholipase A2 is activated after experimental closed head injury in the rat. J. Neurochem. 1989;53:1541–1546. doi: 10.1111/j.1471-4159.1989.tb08550.x. [DOI] [PubMed] [Google Scholar]
- STRAUSS KI, COLE RW, MEHTA S, NARAYAN RK. A dehydroepiandros terone analog improves behavioral outcome and alters cyclooxygenase-2 gene expression after traumatic brain injury in the rat. J. Neurotrauma. 1998;15:A898. [Google Scholar]
- STRAUSS KI, JACOBOWITZ DM. Quantitative measurement of calretinin and β-actin mRNA in rat brain micropunches without prior isolation of RNA. Brain Res. Mol. Brain Res. 1993;20:229–239. doi: 10.1016/0169-328x(93)90045-q. [DOI] [PubMed] [Google Scholar]
- STRAUSS KI, RAGHUPATHI R, McINTOSH TK, NARAYAN RK. Eicosanoid-related genes are modulated in a rat model of traumatic brain injury. J. Neurotrauma. 1997;14:A794. [Google Scholar]
- TSUBOKURA S, WATANABE Y, EHARA H, et al. Localization of prostaglandin endoperoxide synthase in neurons and glia in monkey brain. Brain Res. 1991;543:15–24. doi: 10.1016/0006-8993(91)91043-z. [DOI] [PubMed] [Google Scholar]
- VAN DAM AM, DEVRIES HE, KUIPER J, et al. Interleukin-1 receptors on rat brain endothelial cells: a role in neuroim mune interaction. FASEB J. 1996;10:351–356. doi: 10.1096/fasebj.10.2.8641570. [DOI] [PubMed] [Google Scholar]
- VAN GROEN T, WYSS JM. Extrinsic projections from area CA1 of the rat hippocampus: olfactory, cortical, subcortical, and bilateral hippocampal formation projections. J. Comp. Neurol. 1990;302:515–528. doi: 10.1002/cne.903020308. [DOI] [PubMed] [Google Scholar]
- VON STÜCK SL, LEE SM, HOVDA DA, BECKER DP. l-Arginine increases cerebral blood flow following cortical contusion injury: implications regarding vulnerability. J. Neurotrauma. 1996;13:A618. [Google Scholar]
- VAN HAEFTEN T, WOUTERLOOD FG, JORRITSMA-BYHAM B, WITTER MP. GABAergic presubicular projections to the medial entorhinal cortex of the rat. J. Neurosci. 1997;17:862–874. doi: 10.1523/JNEUROSCI.17-02-00862.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALLACE CS, LYFORD GL, WORLEY PF, STEWARD O. Differential intracellular sorting of immediate early gene mRNAs depends on signals in the mRNA sequence. J. Neurosci. 1998;18:26–35. doi: 10.1523/JNEUROSCI.18-01-00026.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALTON M, SIRIMANNE E, WILLIAMS C, et al. Prostaglandin H synthase-2 and cytosolic phospholipase A2 in the hypoxic-ischemi c brain: role in neuronal death or survival? Mol. Brain Res. 1997;50:165–170. doi: 10.1016/s0169-328x(97)00181-2. [DOI] [PubMed] [Google Scholar]
- WEI EP, ELLIS EF, KONTOS HA. Role of prostaglandins in pial arteriolar response to CO2 and hypoxia. Am. J. Physiol. 1980;238:H226–H230. doi: 10.1152/ajpheart.1980.238.2.H226. [DOI] [PubMed] [Google Scholar]
- WEST JR, NORNES HO, BARNES CL, BRON-FENBRENNER M. The cells of origin of the commissural afferents to the area dentata in the mouse. Brain Res. 1979;160:203–215. doi: 10.1016/0006-8993(79)90419-0. [DOI] [PubMed] [Google Scholar]
- WOLF MJ, IZUMI Y, ZORUMSKI CF, GROSS RW. Long-term potentiation requires activation of calcium-independent phospholipase A2. FEBS Lett. 1995;377:358–362. doi: 10.1016/0014-5793(95)01371-7. [DOI] [PubMed] [Google Scholar]
- YAMAGATA K, ANDREASSON KI, KAUFMANN WE, BARNES CA, WORLEY PF. Expression of a mitogen-inducible cyclooxygenase in brain neurons: regulation by synaptic activity and glucocorticoids. Neuron. 1993;11:371–386. doi: 10.1016/0896-6273(93)90192-t. [DOI] [PubMed] [Google Scholar]
- YAMAKAMI I, McINTOSH TK. Effects of traumatic brain injury on regional cerebral blood flow in rats as measured with radiolabeled microspheres. J. Cereb. Blood Flow Metab. 1989;9:117–124. doi: 10.1038/jcbfm.1989.16. [DOI] [PubMed] [Google Scholar]
- YAMAKAMI I, McINTOSH TK. Alterations in regional cerebral blood flow following brain injury in the rat. J. Cereb. Blood Flow Metab. 1991;11:655–660. doi: 10.1038/jcbfm.1991.117. [DOI] [PubMed] [Google Scholar]
- YOUNG B, OTT L. Nutritional and metabolic management of the head-injured patients. In: Narayan RK, Wilberger JF, Povlishock JT, editors. Neurotrauma. McGraw-Hill; New York: 1996. pp. 345–363. [Google Scholar]