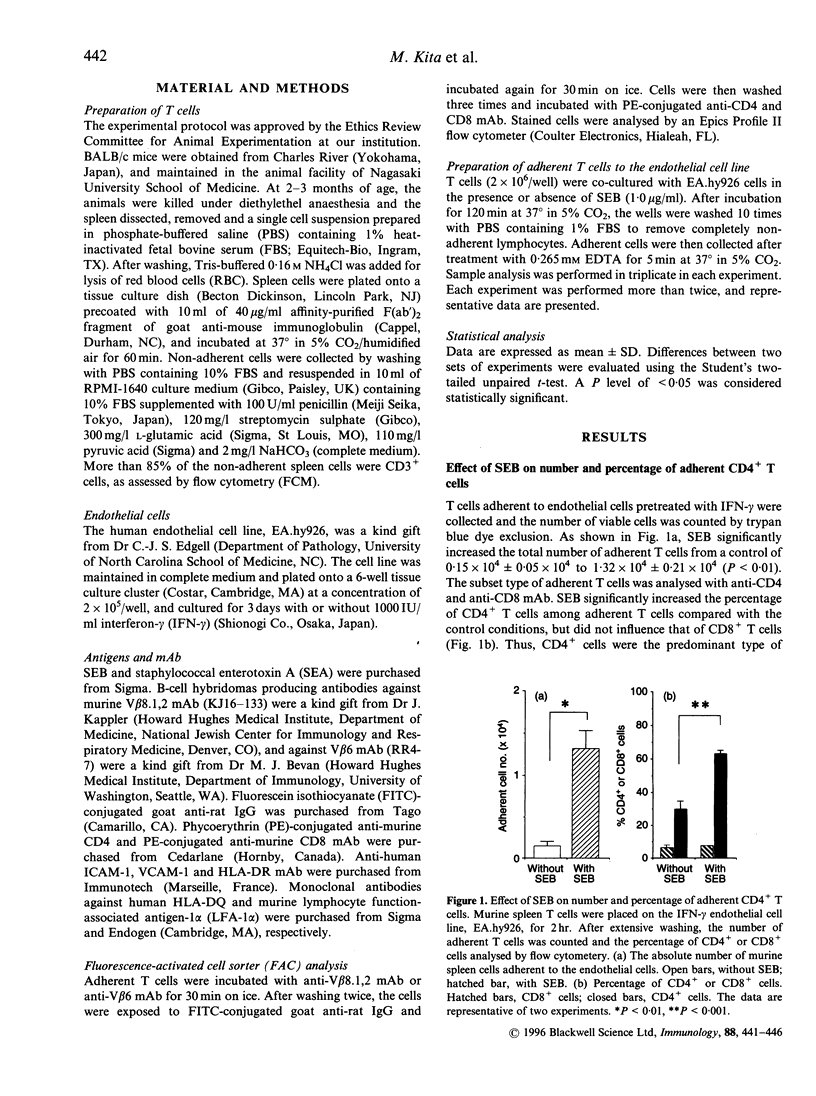
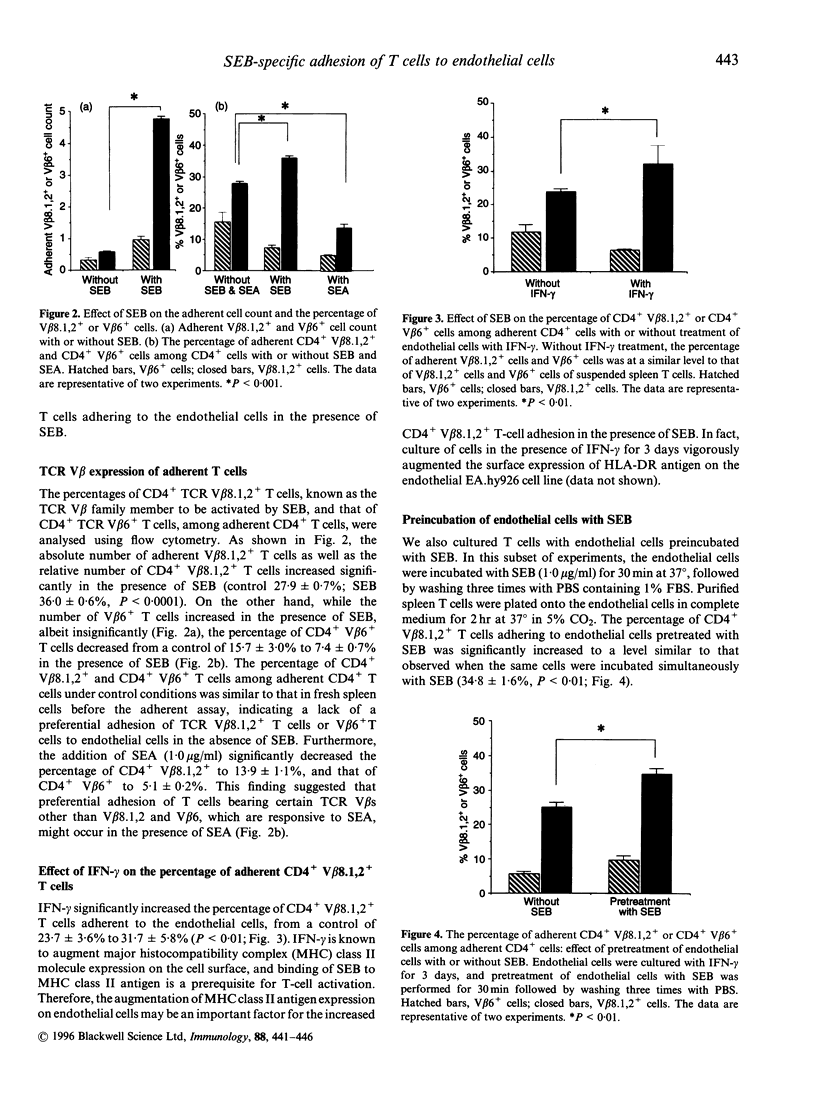
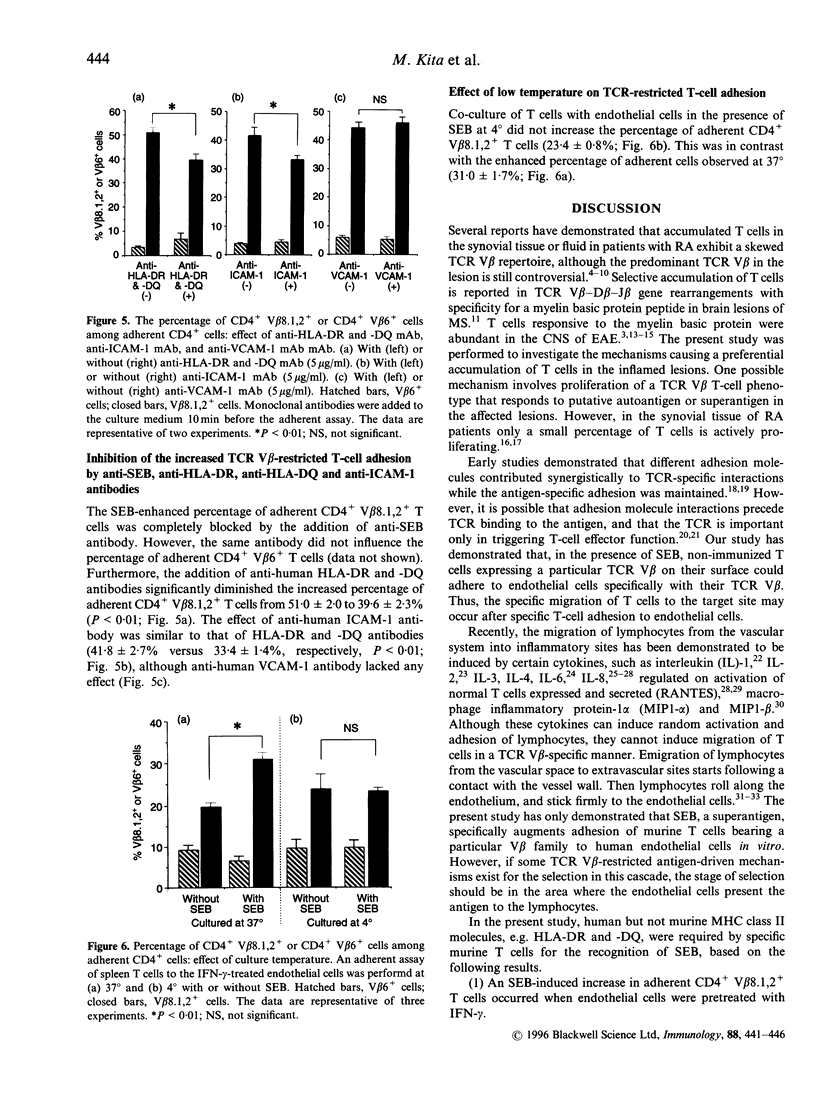
Abstract
The presence of a putative autoantigen of autoimmune disorder in a target organ may cause accumulation of specific T cells in the inflammatory region. One of the mechanisms of such accumulation involves the migration of specific-circulating T cells through the endothelial cells into the target lesion. The presence of only a few specific T cells responsive to a putative autoantigen has hampered the investigation of specific migration of circulating T cells to the target organ. We used a superantigen to investigate specific T-cell adhesion to endothelial cells, because it stimulates a large proportion of T cells with particular V beta elements and adhesion of T cells to the endothelium is a vital step in the migration process. Adhesion of murine T cells to the human endothelial cell line, EA.hy926, was specifically increased in the presence of staphylococcal enterotoxin B (SEB). The increase was interferon-gamma (IFN-gamma)-dependent, and consisted mainly of CD4+ T cells. V beta 8.1,2+ T cells preferentially adhered to endothelial cells in the presence of SEB compared with V beta 6+ T cells. Pretreatment of endothelial cells with SEB increased the adherence of V beta 8.1,2+ T cells, while anti-human leucocyte antigen (HLA)-DR and -DQ antibodies inhibited the increased adherence of V beta 8.1,2+ T cells. Our results demonstrate that increased T-cell adhesion to endothelial cells is SEB specific, and that the specificity is dependent on major histocompatibility complex (MHC) class II molecules expressed on endothelial cells and on the recognition of the SEB-MHC class II complex by V beta 8.1,2+ T cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. H., Shaw S. Leucocyte-endothelial interactions and regulation of leucocyte migration. Lancet. 1994 Apr 2;343(8901):831–836. doi: 10.1016/s0140-6736(94)92029-x. [DOI] [PubMed] [Google Scholar]
- Bacon K. B., Westwick J., Camp R. D. Potent and specific inhibition of IL-8-, IL-1 alpha- and IL-1 beta-induced in vitro human lymphocyte migration by calcium channel antagonists. Biochem Biophys Res Commun. 1989 Nov 30;165(1):349–354. doi: 10.1016/0006-291x(89)91076-0. [DOI] [PubMed] [Google Scholar]
- Bacon K., Gearing A., Camp R. Induction of in vitro human lymphocyte migration by interleukin 3, interleukin 4, and interleukin 6. Cytokine. 1990 Mar;2(2):100–105. doi: 10.1016/1043-4666(90)90003-c. [DOI] [PubMed] [Google Scholar]
- Bergroth V., Konttinen Y. T., Nykänen P., von Essen R., Koota K. Proliferating cells in the synovial fluid in rheumatic disease. An analysis with autoradiography-immunoperoxidase double staining. Scand J Immunol. 1985 Oct;22(4):383–388. doi: 10.1111/j.1365-3083.1985.tb01896.x. [DOI] [PubMed] [Google Scholar]
- Bonvoisin B., Cordier G., Revillard J. P., Lejeune E., Bouvier M. Increased DNA and/or RNA content of synovial fluid cells in rheumatoid arthritis: a flow-cytometry study. Ann Rheum Dis. 1984 Apr;43(2):222–227. doi: 10.1136/ard.43.2.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocke S., Gaur A., Piercy C., Gautam A., Gijbels K., Fathman C. G., Steinman L. Induction of relapsing paralysis in experimental autoimmune encephalomyelitis by bacterial superantigen. Nature. 1993 Oct 14;365(6447):642–644. doi: 10.1038/365642a0. [DOI] [PubMed] [Google Scholar]
- Brown K. A., Vora A., Biggerstaff J., Edgell C. J., Oikle S., Mazure G., Taub N., Meager A., Hill T., Watson C. Application of an immortalized human endothelial cell line to the leucocyte:endothelial adherence assay. J Immunol Methods. 1993 Jul 6;163(1):13–22. doi: 10.1016/0022-1759(93)90234-x. [DOI] [PubMed] [Google Scholar]
- Bucht A., Oksenberg J. R., Lindblad S., Grönberg A., Steinman L., Klareskog L. Characterization of T-cell receptor alpha beta repertoire in synovial tissue from different temporal phases of rheumatoid arthritis. Scand J Immunol. 1992 Feb;35(2):159–165. doi: 10.1111/j.1365-3083.1992.tb02846.x. [DOI] [PubMed] [Google Scholar]
- Butcher E. C. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991 Dec 20;67(6):1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- Cross A. H., Cannella B., Brosnan C. F., Raine C. S. Homing to central nervous system vasculature by antigen-specific lymphocytes. I. Localization of 14C-labeled cells during acute, chronic, and relapsing experimental allergic encephalomyelitis. Lab Invest. 1990 Aug;63(2):162–170. [PubMed] [Google Scholar]
- Doyle C., Strominger J. L. Interaction between CD4 and class II MHC molecules mediates cell adhesion. Nature. 1987 Nov 19;330(6145):256–259. doi: 10.1038/330256a0. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989 Oct 19;341(6243):619–624. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- Edgell C. J., McDonald C. C., Graham J. B. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3734–3737. doi: 10.1073/pnas.80.12.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmdahl R., Andersson M., Goldschmidt T. J., Gustafsson K., Jansson L., Mo J. A. Type II collagen autoimmunity in animals and provocations leading to arthritis. Immunol Rev. 1990 Dec;118:193–232. doi: 10.1111/j.1600-065x.1990.tb00817.x. [DOI] [PubMed] [Google Scholar]
- Howell M. D., Diveley J. P., Lundeen K. A., Esty A., Winters S. T., Carlo D. J., Brostoff S. W. Limited T-cell receptor beta-chain heterogeneity among interleukin 2 receptor-positive synovial T cells suggests a role for superantigen in rheumatoid arthritis. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10921–10925. doi: 10.1073/pnas.88.23.10921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inverardi L., Pardi R. Early events in cell-mediated recognition of vascularized xenografts: cooperative interactions between selected lymphocyte subsets and natural antibodies. Immunol Rev. 1994 Oct;141:71–93. doi: 10.1111/j.1600-065x.1994.tb00873.x. [DOI] [PubMed] [Google Scholar]
- Jenkins R. N., Nikaein A., Zimmermann A., Meek K., Lipsky P. E. T cell receptor V beta gene bias in rheumatoid arthritis. J Clin Invest. 1993 Dec;92(6):2688–2701. doi: 10.1172/JCI116886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston S. C., Dustin M. L., Hibbs M. L., Springer T. A. On the species specificity of the interaction of LFA-1 with intercellular adhesion molecules. J Immunol. 1990 Aug 15;145(4):1181–1187. [PubMed] [Google Scholar]
- Kaufmann Y., Tseng E., Springer T. A. Cloning of the murine lymphocyte function-associated molecule-1 alpha-subunit and its expression in COS cells. J Immunol. 1991 Jul 1;147(1):369–374. [PubMed] [Google Scholar]
- Kawabe Y., Ochi A. Selective anergy of V beta 8+,CD4+ T cells in Staphylococcus enterotoxin B-primed mice. J Exp Med. 1990 Oct 1;172(4):1065–1070. doi: 10.1084/jem.172.4.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld H., Berman J. S., Beer D. J., Center D. M. Induction of human T lymphocyte motility by interleukin 2. J Immunol. 1985 Jun;134(6):3887–3890. [PubMed] [Google Scholar]
- Larsen C. G., Anderson A. O., Appella E., Oppenheim J. J., Matsushima K. The neutrophil-activating protein (NAP-1) is also chemotactic for T lymphocytes. Science. 1989 Mar 17;243(4897):1464–1466. doi: 10.1126/science.2648569. [DOI] [PubMed] [Google Scholar]
- Law S. K., Gagnon J., Hildreth J. E., Wells C. E., Willis A. C., Wong A. J. The primary structure of the beta-subunit of the cell surface adhesion glycoproteins LFA-1, CR3 and p150,95 and its relationship to the fibronectin receptor. EMBO J. 1987 Apr;6(4):915–919. doi: 10.1002/j.1460-2075.1987.tb04838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard E. J., Skeel A., Yoshimura T., Noer K., Kutvirt S., Van Epps D. Leukocyte specificity and binding of human neutrophil attractant/activation protein-1. J Immunol. 1990 Feb 15;144(4):1323–1330. [PubMed] [Google Scholar]
- Ludowyk P. A., Willenborg D. O., Parish C. R. Selective localisation of neuro-specific T lymphocytes in the central nervous system. J Neuroimmunol. 1992 Apr;37(3):237–250. doi: 10.1016/0165-5728(92)90008-9. [DOI] [PubMed] [Google Scholar]
- Marrack P., Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990 May 11;248(4956):705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- Miossec P., Yu C. L., Ziff M. Lymphocyte chemotactic activity of human interleukin 1. J Immunol. 1984 Oct;133(4):2007–2011. [PubMed] [Google Scholar]
- Murphy W. J., Taub D. D., Anver M., Conlon K., Oppenheim J. J., Kelvin D. J., Longo D. L. Human RANTES induces the migration of human T lymphocytes into the peripheral tissues of mice with severe combined immune deficiency. Eur J Immunol. 1994 Aug;24(8):1823–1827. doi: 10.1002/eji.1830240815. [DOI] [PubMed] [Google Scholar]
- Oksenberg J. R., Stuart S., Begovich A. B., Bell R. B., Erlich H. A., Steinman L., Bernard C. C. Limited heterogeneity of rearranged T-cell receptor V alpha transcripts in brains of multiple sclerosis patients. Nature. 1990 May 24;345(6273):344–346. doi: 10.1038/345344a0. [DOI] [PubMed] [Google Scholar]
- Pettinelli C. B., McFarlin D. E. Adoptive transfer of experimental allergic encephalomyelitis in SJL/J mice after in vitro activation of lymph node cells by myelin basic protein: requirement for Lyt 1+ 2- T lymphocytes. J Immunol. 1981 Oct;127(4):1420–1423. [PubMed] [Google Scholar]
- Pluschke G., Ricken G., Taube H., Kroninger S., Melchers I., Peter H. H., Eichmann K., Krawinkel U. Biased T cell receptor V alpha region repertoire in the synovial fluid of rheumatoid arthritis patients. Eur J Immunol. 1991 Nov;21(11):2749–2754. doi: 10.1002/eji.1830211115. [DOI] [PubMed] [Google Scholar]
- Schall T. J., Bacon K., Camp R. D., Kaspari J. W., Goeddel D. V. Human macrophage inflammatory protein alpha (MIP-1 alpha) and MIP-1 beta chemokines attract distinct populations of lymphocytes. J Exp Med. 1993 Jun 1;177(6):1821–1826. doi: 10.1084/jem.177.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall T. J., Bacon K., Toy K. J., Goeddel D. V. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990 Oct 18;347(6294):669–671. doi: 10.1038/347669a0. [DOI] [PubMed] [Google Scholar]
- Seth A., Stern L. J., Ottenhoff T. H., Engel I., Owen M. J., Lamb J. R., Klausner R. D., Wiley D. C. Binary and ternary complexes between T-cell receptor, class II MHC and superantigen in vitro. Nature. 1994 May 26;369(6478):324–327. doi: 10.1038/369324a0. [DOI] [PubMed] [Google Scholar]
- Shaw S., Luce G. E., Quinones R., Gress R. E., Springer T. A., Sanders M. E. Two antigen-independent adhesion pathways used by human cytotoxic T-cell clones. Nature. 1986 Sep 18;323(6085):262–264. doi: 10.1038/323262a0. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., Newman W., Tanaka Y., Shaw S. Lymphocyte interactions with endothelial cells. Immunol Today. 1992 Mar;13(3):106–112. doi: 10.1016/0167-5699(92)90151-V. [DOI] [PubMed] [Google Scholar]
- Sioud M., Kjeldsen-Kragh J., Quayle A. J., Wiker H. G., Sørskaar D., Natvig J. B., Førre O. Immune responses to 18.6 and 30-kDa mycobacterial antigens in rheumatoid patients, and V beta usage by specific synovial T-cell lines and fresh T cells. Scand J Immunol. 1991 Dec;34(6):803–812. doi: 10.1111/j.1365-3083.1991.tb01605.x. [DOI] [PubMed] [Google Scholar]
- Spits H., van Schooten W., Keizer H., van Seventer G., van de Rijn M., Terhorst C., de Vries J. E. Alloantigen recognition is preceded by nonspecific adhesion of cytotoxic T cells and target cells. Science. 1986 Apr 18;232(4748):403–405. doi: 10.1126/science.3485822. [DOI] [PubMed] [Google Scholar]
- Springer T. A., Davignon D., Ho M. K., Kürzinger K., Martz E., Sanchez-Madrid F. LFA-1 and Lyt-2,3, molecules associated with T lymphocyte-mediated killing; and Mac-1, an LFA-1 homologue associated with complement receptor function. Immunol Rev. 1982;68:171–195. doi: 10.1111/j.1600-065x.1982.tb01064.x. [DOI] [PubMed] [Google Scholar]
- Thornhill M. H., Li J., Haskard D. O. Leucocyte endothelial cell adhesion: a study comparing human umbilical vein endothelial cells and the endothelial cell line EA-hy-926. Scand J Immunol. 1993 Sep;38(3):279–286. doi: 10.1111/j.1365-3083.1993.tb01726.x. [DOI] [PubMed] [Google Scholar]
- Tsuchida M., Matsumoto Y., Hirahara H., Hanawa H., Tomiyama K., Abo T. Preferential distribution of V beta 8.2-positive T cells in the central nervous system of rats with myelin basic protein-induced autoimmune encephalomyelitis. Eur J Immunol. 1993 Oct;23(10):2399–2406. doi: 10.1002/eji.1830231004. [DOI] [PubMed] [Google Scholar]
- Williams W. V., Fang Q., Demarco D., VonFeldt J., Zurier R. B., Weiner D. B. Restricted heterogeneity of T cell receptor transcripts in rheumatoid synovium. J Clin Invest. 1992 Aug;90(2):326–333. doi: 10.1172/JCI115866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi J., Baron J., Buxser S., Janeway C. A., Jr Bacterial proteins that mediate the association of a defined subset of T cell receptor:CD4 complexes with class II MHC. J Immunol. 1990 Feb 1;144(3):892–901. [PubMed] [Google Scholar]


