Abstract
In the fission yeast Schizosaccharomyces pombe the septation initiation network (SIN) is required for stabilization of the actomyosin ring in late mitosis as well as for ring constriction and septum deposition. In a genetic screen for suppressors of the SIN mutant sid2-250, we isolated a mutation, ace2-35, in the transcription factor Ace2p. Both ace2Δ and ace2-35 show defects in cell separation, and both can rescue the growth defects of some SIN mutants at low restrictive temperatures, where the SIN single mutants lyse at the time of cytokinesis. By detailed analysis of the formation and constriction of the actomyosin ring and septum in the sid2-250 mutant at low restrictive temperatures, we show that the lysis phenotype of the sid2-250 mutant is likely due to a weak cell wall and septum combined with enzymatic activity of septum-degrading enzymes. Consistent with the recent findings that Ace2p controls transcription of genes involved in cell separation, we show that disruption of some of these genes can also rescue sid2-250 mutants. Consistent with SIN mutants having defects in septum formation, many SIN mutants can be rescued at the low restrictive temperature by the osmotic stabilizer sorbitol. The small GTPase Rho1 is known to promote cell wall formation, and we find that Rho1p expressed from a multi-copy plasmid can also rescue sid2-250 at the low restrictive temperature. Together these results suggest that the SIN has a role in promoting proper cell wall formation at the division septa.
CYTOKINESIS is the final stage of the cell cycle during which the two daughter cells undergo irreversible physical separation. In animal cells, cytokinesis occurs by constriction of an actomyosin ring together with newly synthesized membrane insertion at the division site. In the fission yeast Schizosaccharomyces pombe, actomyosin ring constriction is concomitant with deposition of a multilayered division septum, which must be cleaved eventually to release the two daughter cells.
In fission yeast, a regulatory network referred to as the septation initiation network (SIN) is required to maintain the actomyosin ring as well as for actomyosin ring contraction and septum deposition. The SIN consists of several genes, including sid4+, cdc11+, spg1+, cdc7+, sid1+, cdc14+, sid2+, mob1+, and plo1+ (reviewed in McCollum and Gould 2001; Guertin et al. 2002; Simanis 2003). Disruption of any of these genes allows normal ring assembly in early mitosis, but in anaphase, the ring falls apart without constriction and the septum does not form. Although the SIN clearly has a role in maintaining the actomyosin ring in anaphase, it has been unclear whether the SIN has a direct role in septum formation. This is because the lack of septum formation in SIN mutants could be an indirect effect of actomyosin ring disassembly since the ring is required to localize septum-synthesizing enzymes (Cortes et al. 2002; Liu et al. 2002).
The division septum of S. pombe is composed of a primary septum flanked by secondary septa on either side (Johnson et al. 1973), which will constitute the new cell ends after cell separation. Deposition of the primary septum is dependent on constriction of the actomyosin ring (Liu et al. 1999, 2000) and it is laid down in a centripetal manner. After this, each daughter builds cell wall material on its own side of the primary septum, leading to the assembly of a secondary septum. The primary septum contains mainly 1,3-β-glucan and the secondary septum contains 1,3-α-glucan, 1,6-branched 1,3-β-glucan, and galactomannans (Humbel et al. 2001; Sugawara et al. 2003). Recently, several enzymes involved in synthesis of 1,3-β-glucan and 1,3-α-glucan have been identified, and all of them are large integral membrane proteins. As 1,3-β-glucan synthase subunits, Cps1p/Bgs1p and Bgs4p are required for assembly of 1,3-β-glucan and thus of the primary septum (Le Goff et al. 1999; Liu et al. 1999; Cortes et al. 2002, 2005). The enzyme complex involved in 1,3-β-glucan synthesis requires not only synthase subunits but also regulatory subunit(s). The small GTPase Rho1 was identified as a regulatory component of the 1,3-β-glucan synthase complex and cells lacking this protein undergo lysis (Arellano et al. 1996, 1997). In addition, Rho1p plays a fundamental role in many morphogenetic processes, such as polarization of the actin cytoskeleton (Cabib et al. 1998; Arellano et al. 1999; Drgonova et al. 1999).
In S. pombe, the detailed molecular mechanism that is responsible for dissolution of the septum while maintaining structural integrity remains unclear. Cell separation involves not only degradation of the primary septum but also erosion of the “septum edging” or original cell wall. It has been shown that 1,3-β-glucanase Eng1p is involved in breakdown of the primary septum (Martin-Cuadrado et al. 2003), and recent reports provide evidence that Agn1p functions as an endo-1,3-α-glucanase to hydrolyze septum-edging material (Dekker et al. 2004; Garcia et al. 2005). The expression of Eng1p and Agn1p, together with at least five other proteins required for cell separation during the last stages of the cell cycle, is transcriptionally regulated by the transcription factor Ace2p (Rustici et al. 2004; Alonso-Nunez et al. 2005). In addition, various other proteins have been directly or indirectly implicated in the process of cell separation in fission yeast, as several mutants affecting cell–cell separation have been isolated, including mutations in the forkhead transcription factor sep1+, calcineurin (ppb1+), a MAPK (pmk1+), a MAPK phosphatase (pmp1+), PP2A regulatory subunits (par1+ and par2+), septins (spn3+ and spn4+), an anillin homolog (mid2+), components of the exocyst complex (sec6+, sec8+, sec10+, and exo70+), and a Rho GTPase (rho4+) (Yoshida et al. 1994; Longtine et al. 1996; Toda et al. 1996; Ribar et al. 1997; Sugiura et al. 1998; Jiang and Hallberg 2000; Le Goff et al. 2001; Wang et al. 2002; Berlin et al. 2003; Nakano et al. 2003; Santos et al. 2003; Tasto et al. 2003; An et al. 2004; Dekker et al. 2004; Alonso-Nunez et al. 2005; Bahler 2005; Garcia et al. 2005). It is unclear how all of these proteins promote cell separation, but the exocyst complex, along with Mid2p and septins, was recently shown to be required for targeting of enzymes involved in septum cleavage to the septum (Martin-Cuadrado et al. 2005).
Through careful analysis of sid2-250 mutant phenotypes at reduced restrictive temperatures as well as of suppressing mutations, we show here that the SIN functions to regulate cell wall assembly at the septum, in addition to its role in actomyosin ring stability.
MATERIALS AND METHODS
Yeast media, strains, and genetic manipulations:
The fission yeast strains used in this study are listed in Table 1. Genetic crosses and general yeast techniques were performed as previously described (Moreno et al. 1991). S. pombe strains were grown in rich medium of yeast extract (YE) or Edinburgh minimal medium (EMM) with appropriate supplements (Moreno et al. 1991). EMM with 5 μg/ml of thiamine was used to repress expression from the nmt1 promoter. For serial dilution patch tests of growth, three serial 10-fold dilutions were made and 5 μl of each was spotted on plates with the starting cell number of 104. Cells were pregrown in liquid YE or EMM at 25° and then spotted onto YE, YE plus 1.2 m sorbitol, or EMM plates at the indicated temperatures and incubated for 3–5 days before photography. In the case of cells expressing Mid2p from plasmid pREP41-mid2+ (a gift from Kathy Gould) in wild-type or mutant cells (sid2-250, ace2Δ, and ace2Δ sid2-250), transformants were first grown in liquid EMM plus thiamine and then cells were washed to remove thiamine and diluted and dropped on EMM plates.
TABLE 1.
S. pombe strains used in this study
| Strain | Genotype |
|---|---|
| YDM105 | leu1-32 ura4-D18 ade6-210 h− |
| YDM106 | leu1-32 ura4-D18 ade6-210 h+ |
| YDM429 | sid2-250 leu1-32 ura4-D18 ade6 h+ |
| YDM1965 | ace2-35 leu1-32 ura4-D18 ade6-210 h− |
| YDM1966 | ace2-35 leu1-32 ura4-D18 ade6-210 h+ |
| YDM2025 | ace2-35 sid2-250 leu1-32 ura4-D18 ade6 h− |
| YDM2028 | ace2Δ∷kanR ura4-D18 ade6-216 leu1-32 h+ |
| YDM2027 | ace2Δ∷kanR sid2-250 ura4-D18 h− |
| YDM1264 | rlc1-GFP-ura4+ leu1-32 h− |
| YDM1374 | rlc1-GFP-ura4+ sid2-250 ura4-D18 leu1-32 h+ |
| YDM116 | sid4-A1 leu1-32 ura4-D18 ade6 h− |
| YDM2038 | ace2Δ∷kanR sid4-A1 leu1-32 ura4-D18 ade6 h− |
| YDM275 | cdc11-123 leu1-32 ura4-D18 ade6-210 h− |
| YDM3205 | ace2Δ∷kanR cdc11-123 leu1-32 ura4-D18 ade6 h? |
| YDM430 | spg1-106 leu1-32 ura4-D18 ade6-210 h+ |
| YDM2036 | ace2Δ∷kanR spg1-106 leu1-32 ura4-D18 ade6-210 h+ |
| YDM1239 | cdc7-24 h+ |
| YDM2042 | ace2Δ∷kanR cdc7-24 ura4-D18 h+ |
| YDM76 | sid1-239 leu1-32 ade6 h− |
| YDM2040 | ace2Δ∷kanR sid1-239 leu1-32 ura4-D18 ade6 h− |
| YDM445 | sid1-125 leu1-32 ura4-D18 ade6-210 h+ |
| YDM2044 | ace2Δ∷kanR sid1-125 ura4-D18 ade6 h− |
| YDM272 | cdc14-118 ura4-D18 ade6-M210 h− |
| YDM3204 | ace2Δ∷kanR cdc14-118 ura4-D18 ade6 h? |
| YDM670 | mob1-1 leu1-32 ura4-D18 ade6 his3- D1 h− |
| YDM3206 | ace2Δ∷kanR mob1-1 leu1-32 ura4-D18 ade6 h? |
| YDM1259 | mid2Δ∷ura4+ leu1-32 ura4-D18 ade6 h− |
| YDM2055 | mid2Δ∷ura4+ sid2-250 leu1-32 ura4-D18 ade6 h+ |
| YDM1948 | eng1Δ∷kanR ura4-D18 h− |
| YDM2058 | eng1Δ∷kanR sid2-250 leu1-32 ura4-D18 ade6 h+ |
| YDM2801 | agn1Δ∷ura4+ ura4-D18 leu1-32 h? |
| YDM2802 | agn1Δ∷ura4+ sid2-250 leu1-32 ura4-D18 ade6 h? |
| YDM2772 | eng1Δ∷kanR agn1Δ∷ura4+ ura4-D18 h− |
| YDM2805 | eng1Δ∷kanR agn1Δ∷ura4+ sid2-250 ura4-D18 h? |
| YDM878 | sep1 leu1-32 ura4-D18 h− |
| YDM1926 | sep1 sid2-250 leu1-32 ura4-D18 ade6 h+ |
| YDM641 | spn3Δ∷ura4+ leu1-32 ura4-D18 ade6 h+ |
| YDM1498 | spn3Δ∷ura4+ sid2-250 ura4-D18 h− |
| YDM2569 | rho3Δ:ura4+ h+ |
| YDM2613 | rho3Δ:ura4+ sid2-250 h? |
| YDM2603 | rho4Δ∷kanR leu1-32 ura4-D18 h− |
| YDM2658 | rho4Δ∷kanR sid2-250 leu1-32 ura4-D18 his3-D1 h+ |
| YDM2609 | sec8-1 h− |
| YDM2625 | sec8-1 sid2-250 leu1-32 his3-D1 h+ |
| YDM2519 | par1Δ∷ura4+ leu1-32 ura4-D18 ade6 h+ |
| YDM2510 | par1Δ∷ura4+ sid2-250 leu1-32 ura4-D18 ade6-216 h+ |
| YDM2522 | par2Δ∷Leu2+ ura4-D18 leu1-32 ade6 h+ |
| YDM2481 | par2Δ∷Leu2+ sid2-250 leu1-32 ura4-D18 ade6his3-D1 h− |
| YDM2482 | par1Δ∷his3+ par2Δ∷Leu2+ sid2-250 leu1-32 ura4-D18 ade6 his3-D1 h+ |
| YDM643 | spn4Δ∷ura4+ leu1-32 ura4-D18 ade6 h− |
| YDM2289 | spn4Δ∷ura4+ sid2-250 leu1-32 ura4-D18 ade6 h− |
Sequencing of the ace2-35 mutation:
To confirm that sup35 represented a mutation in the gene ace2+, the coding region for the ace2+ gene was amplified by PCR from the wild-type S. pombe genome and cloned into the pREP41–GFP vector (Craven et al. 1998) and expressed in sup35 cells. Oligonucleotides used for PCR amplification were 5′-GGCCGTCGACAATGTCGCTTTCATATTTATC-3′ and 5′-GGCCGGATCCGTGCTGTCTGCGATCTACGCC-3′, and the product was digested with SalI and BamHI and then subcloned into the pREP41–GFP vector containing the thiamine-repressible nmt1 promoter (Maundrell 1990).
To sequence the open reading frame (ORF) of the ace2+ gene and flanking regions in the sup35 mutant, the ace2+ gene was amplified from sup35 cells using two primers, 5′-CATCCATGATCCAGTTGTTG-3′ and 5′-GTTCATGTACGATGCTTG-3′, which are 520 bases before the start codon (ATG) and 140 bases after the stop codon (TAA), respectively. Two independent PCR reactions were sequenced, and the same mutations were found in both products.
Microscopy:
Both photomicrographs and time-lapse movies were obtained with a Nikon Eclipse E600 fluorescence microscope coupled to a cooled charge-coupled device camera (ORCA-ER; Hamamatsu, Bridgewater, NJ), and image processing and analysis were carried out with IPLab Spectrum software (Signal Analytics, Vienna, VA). To follow the dynamics of the actomyosin ring (labeled with Rlc1–GFP; Le Goff et al. 2000; Naqvi et al. 2000) and septum, time-lapse movies were produced from frames taken once every minute with a ×50 objective. Wild-type or sid2-250 cells were grown in rich medium at 25° and then mounted on a thin pad of agar made in YE on the slide with a coverslip applied on top. The slide was heated to 33° on the microscope stage with an objective heater (Bioptechs objective controller).
RESULTS
Isolation and characterization of ace2-35:
To identify potential targets and/or regulators of Sid2p, we screened for mutations that could suppress the temperature-sensitive growth defect of sid2-250 mutant cells (Jin and McCollum 2003). All but one of the suppressors (20 of 21) identified fell into a single complementation group and carried mutations in scw1+ (Jin and McCollum 2003). The remaining suppressor, sup35, showed cell-separation defects with a high percentage of cells with single or multiple septa (Figure 1A). DAPI staining revealed that each cell compartment contains one nucleus (data not shown), indicating a defect in cell separation after septum assembly. These cells did not show obvious temperature sensitivity or cold sensitivity (data not shown). We noted that the cell-separation defects of sup35 showed similarity to mutants in two transcription factors, sep1+ and ace2+ (Ribar et al. 1997; Martin-Cuadrado et al. 2003). Genetic crosses showed that sup35 was tightly linked to ace2+, with no sup35 ace2Δ∷kanR double mutants isolated from 42 complete tetrads dissected. This suggested that sup35 might be an allele of ace2+.
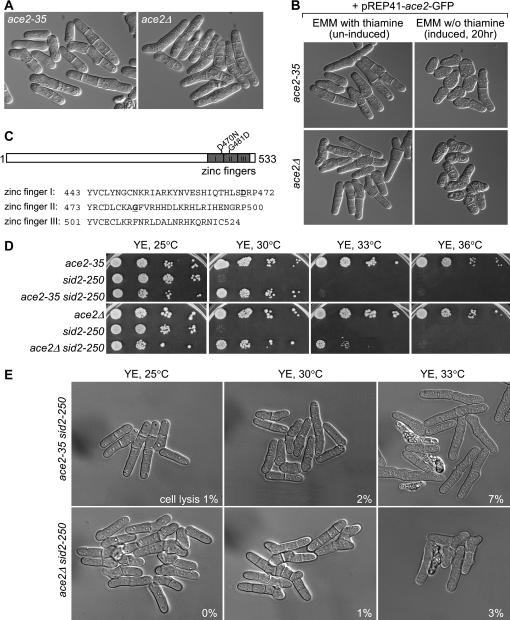
Figure 1.
A sid2-250 suppressor, ace2-35, is an allele of the ace2+ transcription factor. (A) The sid2-250 suppressor ace2-35 showed cell-separation defects similar to those of ace2Δ. ace2-35 (YDM1965) and ace2Δ (YDM2028) cells were grown in liquid YE medium and live cells were photographed using DIC microscopy. Quantitatively, 59% and 8% of ace2-35 cells show single septum and multiple septa, respectively, whereas 55% and 36% of ace2Δ cells show single septum and multiple septa, respectively. (B) Ectopic expression of ace2+ from a plasmid could rescue the cell-separation defects in both ace2-35 and ace2Δ cells. ace2-35 (YDM1965) and ace2Δ (YDM2028) cells transformed with plasmid pREP41–ace2–GFP were grown in EMM with thiamine or in the absence of thiamine to induce the expression of ace2+ under the nmt1 promoter. (C, top) Schematic indicating the position of the mutations in the ace2-35 mutant. (C, bottom) Amino acid sequences of three zinc-finger domains at the C terminus of Ace2p. The two amino acids that are mutated in the ace2-35 mutant are in boldface type and underlined. (D) ace2-35 was isolated as a suppressor of sid2-250. A serial dilution growth test was done with strains of ace2-35 (YDM1965), sid2-250 (YDM429), ace2-35 sid2-250 (YDM2025), ace2Δ (YDM2028), and ace2Δ sid2-250 (YDM2027). (E) Suppression of the lysis phenotype of sid2-250 cells by ace2-35 and ace2Δ in liquid cultures grown at low restrictive temperatures. Mutant cells of ace2-35 sid2-250 (YDM2025) and ace2Δ sid2-250 (YDM2027) were first grown in liquid rich medium at 25° to log phase and then shifted to 30° and 33° for 4 hr before images were collected using DIC microscopy. The percentages of lysed cells in each culture are shown.
To further confirm that sup35 represents a mutation in the gene ace2+, we expressed ace2+ from a plasmid in sup35 cells and examined whether the multiple septa phenotype can be rescued. It showed that slightly overexpressed ace2+ significantly reduced the number of cells with cell-separation defects, although some cells became round due to overproduced ace2+ (Figure 1B and Bahler 2005). Expression of ace2+ decreased the percentage of cells with multiple septa from 32 to 6% in ace2-35 cells and from 56 to 18% in ace2Δ cells.
Finally, we amplified the ace2+ gene and flanking regions from the sup35 mutant by PCR (see materials and methods), and sequencing of the PCR product revealed two point mutations (two nucleotide substitutions) within the ORF of the ace2+ gene, which result in two amino acid changes from Asp to Asn and from Gly to Asp at amino acids 470 and 481, respectively (i.e., D470N and G481D). Interestingly, these two mutations fell into the region with three tandem C2H2 zinc-finger motifs, which are conserved in the Ace2 transcription factor family (Figure 1C). Therefore, sup35 is a loss-of-function allele of ace2+ and we thereafter renamed sup35 as ace2-35.
ace2Δ can rescue sid2-250 slightly better than ace2-35 does:
Since ace2-35 is a loss-of-function allele of ace2+ and since it can rescue the growth defects of sid2-250 at restrictive temperature, we assumed that complete deletion of ace2+ (ace2Δ) would rescue sid2-250 as well. We constructed double mutants between ace2Δ and sid2-250, and serial dilution drop tests on plates at different temperatures showed that ace2Δ rescued the growth defects of the sid2-250 mutant slightly better than ace2-35 did, showing rescue at 30° and slightly at 33° whereas ace2-35 rescued only at 30° (Figure 1D). In liquid cultures, both ace2-35 and ace2Δ significantly decreased the percentage of lysed sid2-250 cells at low restrictive temperatures (compare Figure 1E and Figure 2A) with slightly fewer lysed cells in ace2Δ sid2-250 than in ace2-35 sid2-250. These data suggested that the ace2-35 mutant is hypomorphic, which is consistent with our observations that ace2-35 cells showed slightly fewer severe cell-separation defects than ace2Δ cells (Figure 1A).
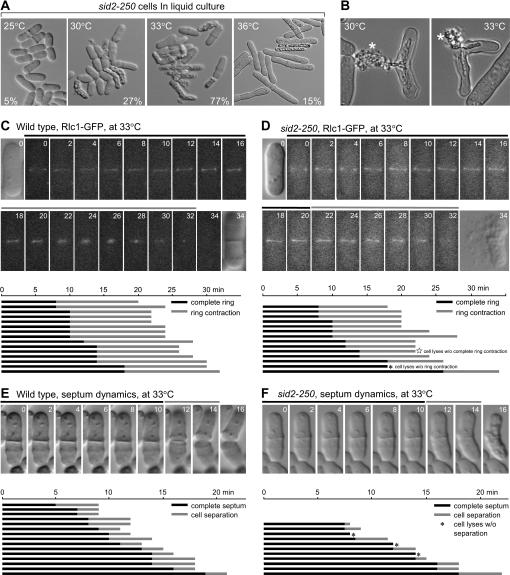
Figure 2.
Characterization of the cell lysis phenotype in sid2-250 cells at lower restrictive temperature. (A) sid2-250 (YDM429) cells show different lysis phenotypes at high and low restrictive temperatures. Mutant cells were first grown in liquid medium at 25° to log phase and then shifted to 30°, 33°, and 36° for 4 hr before images were collected using DIC microscopy. The percentages of lysed cells in each culture are shown. (B) Two representative cells of sid2-250 (YDM429) after being incubated at 30° and 33° for 4 hr. Both cells showed the cell lysis at the septum area, and the released cell contents are labeled with asterisks. (C and D) The dynamics of actomyosin ring formation were followed in (C) wild-type (YDM1264) and (D) sid2-250 (YDM1374) cells by monitoring the Rlc1–GFP signals at 33°. Live images were taken as described in materials and methods. The time point when the signal first appeared as a ring was set as T0 and numbers indicate the minutes afterward. The times of ring formation, maintenance, and contraction were measured from live cell movies. Bars denote the periods of ring formation and maintenance (solid) and ring contraction (shading). (C and D, top) Sample images from one wild-type and one sid2-250 cell selected at random, with the first and last images taken using DIC microscopy, and the rest showing Rlc1p–GFP fluorescence. (C and D, bottom) Actomyosin ring formation and contraction in (C) 15 wild-type and (D) 14 sid2-250 cells. All selected wild-type cells showed septum formation and all selected sid2-250 cells eventually lysed. (E and F) The septum dynamics were followed in (E) wild-type (YDM1264) and (F) sid2-250 (YDM1374) cells by DIC microscopy at 33°. The time point when the complete septum was assembled was set as T0 and numbers indicate the minutes afterward. Cells were judged to have complete septa when focusing up and down revealed no gaps in the middle. The times of septum maintenance and degradation were measured from live cell movies. Septum degradation was measured from the time cell separation begins to when the cells separate or lyse. Bars denote the periods of septum maintenance (solid) and septum degradation (shading). (E and F, top) Samples of (E) wild-type and (F) sid2-250 cells. (E and F, bottom) Septum formation and degradation in 15 wild-type and 11 sid2-250 cells. All selected wild-type cells showed successful septum degradation and all selected sid2-250 cells eventually lysed before or during septum degradation.
ace2Δ can rescue other SIN mutants:
We next tested whether the ace2Δ mutation specifically rescued sid2-250 or was capable of rescuing other SIN mutants. We constructed double mutants between ace2Δ and all the other available temperature-sensitive SIN mutants, including sid4-A1, cdc11-123, spg1-106, cdc7-24, sid1-125, sid1-239, cdc14-118, sid2-250, and mob1-1. Interestingly, serial dilution drop tests on plates at different temperatures showed that the rescue varied depending on the allele, with very strong mutant alleles, such as sid4-A1 and sid1-125, showing little or no rescue even at 30° (Table 2 and data not shown). cdc7-24 showed ambiguous rescue with variations among clones (Table 2). However, other mutants such as spg1-106, cdc14-118, sid2-250, and mob1-1 were rescued by ace2Δ at low but not high restrictive temperatures, which corresponded to when these single mutants died as short cells (also see below) (Table 2). This analysis suggested that the ace2Δ mutation was not able to bypass the SIN to promote cytokinesis but required some degree of residual SIN signaling to promote rescue.
TABLE 2.
Summary of rescue of SIN mutants by ace2-35 or ace2Δ
| 25° | 30° | 33° | 36° | |
|---|---|---|---|---|
| sid4-A1 | ++ | − | − | − |
| ace2Δ sid4-A1 | ++ | +/− | − | − |
| cdc11-123 | ++ | ++ | ++ | − |
| ace2Δ cdc11-123 | ++ | ++ | ++ | + |
| spg1-106 | ++ | − | − | − |
| ace2Δ spg1-106 | ++ | ++ | − | − |
| cdc7-24 | ++ | ++ | ++ | + |
| ace2Δ cdc7-24 | ++ | ++ | +/− | +/− |
| sid1-125 | ++ | − | − | − |
| ace2Δ sid1-125 | ++ | − | − | − |
| sid1-239 | ++ | ++ | ++ | + |
| ace2Δ sid1-239 | ++ | ++ | ++ | ++ |
| cdc14-118 | ++ | + | − | − |
| ace2Δ cdc14-118 | ++ | ++ | ++ | − |
| sid2-250 | ++ | − | − | − |
| ace2Δ sid2-250 | ++ | ++ | +/− | − |
| ace2-35 sid2-250 | ++ | ++ | − | − |
| mob1-1 | ++ | − | − | − |
| ace2Δ mob1-1 | ++ | + | + | − |
Growth was examined with a serial dilution drop test at different temperatures. ++, good growth; +, weak growth; +/−, weak growth with variations in growth in different clones; −, no growth.
Lysis phenotype in SIN mutants at low restrictive temperatures is due to the defects in septum synthesis:
Given that ace2Δ rescued sid2-250 at lower restrictive temperatures (30°–33°), where cells lysed as short cells, but not at a high restrictive temperature (36°), where cells became highly elongated (Figure 2A), we examined the sid2-250 mutant phenotype at different temperatures. We noted that although sid2-250 mutant cells lysed and died at temperatures ≥30°, they lysed in different manners at 30° and 36° (Figure 2A), a phenomenon also observed in other SIN mutants (see Figure 3A). At 30°–33°, sid2-250 mutant cells seemed to be able to assemble a septum and finish septation to a certain degree, and then lysed and released cell contents through ruptured cell wall in the septum region (Figure 2, A and B). At 36°, sid2-250 mutant cells could finish up to four rounds of nuclear divisions and finally lysed and gave no indications of septum assembly (Figure 2A and data not shown). Previous studies have shown that SIN mutants (including sid2-250) at a high restrictive temperature are capable of assembling but cannot maintain actomyosin rings upon completion of mitosis, thus leading to defects in septation (Gould and Simanis 1997; Balasubramanian et al. 1998; Guertin et al. 2002; Mishra et al. 2004). How well the actomyosin rings can be assembled and maintained at lower restrictive temperatures (such as 30°–33°) was not clear. To characterize the septation defects of sid2-250 mutant cells in greater detail at a lower restrictive temperature, the dynamics of actomyosin ring and septum formation were monitored using time-lapse microscopy.
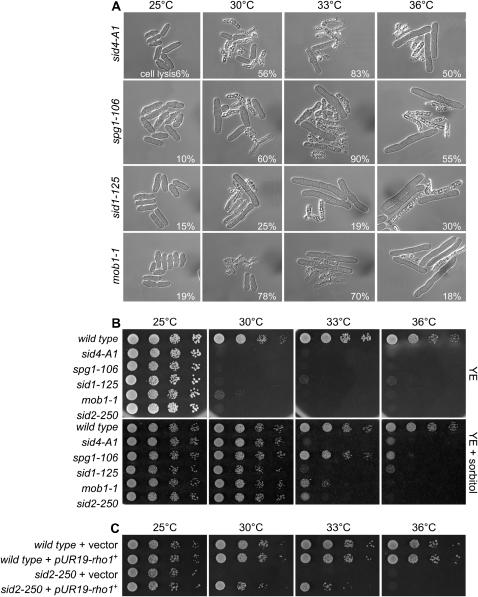
Figure 3.
The growth defect and lysis phenotype of SIN mutants can be rescued by osmotic stabilizer and overexpression of rho1+ can rescue sid2-250. (A) Other SIN mutants also show a lysis phenotype similar to that of sid2-250 at low restrictive temperatures. Selected SIN mutants [sid4-A1 (YDM116), spg1-106 (YDM430), sid1-125 (YDM445), and mob1-1 (YDM670)] were first grown in liquid rich medium at 25° to log phase and then shifted to 30,° 33°, and 36° for 4 hr before images were collected using DIC microscopy. The percentages of lysed cells in each culture are shown. (B) Wild-type (YDM105), sid2-250 (YDM429), and other selected SIN mutants (the same as in A) were grown in YE at 25° and then diluted and dropped on plates of YE or YE plus 1.2 m sorbitol. Plates were incubated at different temperatures as indicated for 3–5 days before photography. (C) Wild-type (YDM105) and sid2-250 (YDM429) cells transformed with either empty vector (pUR19) or pUR19-rho1+ were first grown in EMM and then diluted and dropped onto plates of YE.
We used wild-type and sid2-250 mutant cells expressing a GFP-tagged version of the actomyosin ring component Rlc1p (Le Goff et al. 2000; Naqvi et al. 2000) and at least 10 cells were imaged over time at 33°. We found that wild-type and sid2-250 cells were mostly comparable in ring formation, maintenance, and successful constriction (Figure 2, C and D). The appearance of the rings looked normal without any sign of fragmentation, although all sid2-250 cells that we took into our statistics eventually lysed (Figure 2D). Of 14 sid2-250 cells, we observed only one cell that lysed before ring constriction occurred, and another cell that lysed before ring constriction completely finished (Figure 2D). On average, after complete formation, the actomyosin ring persisted for ∼13 min in wild-type cells (15 cells) and 12.3 min in sid2-250 cells (13 cells) before the onset of constriction, indicating that ring formation and maintenance is not defective in sid2-250 cells at 33°. The ring constricted normally before cells lysed in most (12 of 14) of the sid2-250 cells (Figure 2D).
It has been established that after the mitotic spindle breaks down, the contractile ring constricts and disassembles while the septum is being deposited, and all these events depend on the SIN pathway. We wondered whether the sid2-250 cells could have defects in septum formation. Therefore we also followed the dynamics of septum formation and constriction by DIC microscopy at 33°. At a gross level, septum assembly looked normal in sid2-250 cells. After assembly, septa in sid2-250 and wild-type cells were maintained with a similar time range for 5–18 min before cell separation initiated (Figure 2, E and F). Sometimes cells with septa lysed before septum degradation and cell separation seemed to initiate (3 of 11 cells) (Figure 2F). However, we found that although most sid2-250 cells could initiate degradation of their septa normally, most cells lysed before they completed cell separation (8 of 11 cells) (Figure 2F). On the basis of these data, we concluded that the lysis phenotype in sid2-250 cells at lower restrictive temperatures (30°–33°) occurs after ring contraction and septum formation and during the process of septum cleavage or dissolution before full cell separation.
Although a septum can be formed in sid2-250 cells at lower restrictive temperatures, it might have defects. One line of evidence came from our observation that the growth defect and the cell lysis defect can be rescued by addition of the sorbitol in the media (Figure 3B). Sorbitol can act as an osmotic stabilizer and has been shown to rescue the cell lysis phenotype in mutants with a defective cell wall in both Saccharomyces cerevisiae and S. pombe (Ribas et al. 1991; Cid et al. 1995; Santos et al. 2003). In addition, we also observed the phenotypic rescue of other mutations in SIN pathway components by the presence of sorbitol, such as sid4-A1, spg1-106, sid1-125, and mob1-1 (Figure 3B), suggesting that SIN mutants have septum defects that can be stabilized by sorbitol.
In S. pombe, the Rho1p GTPase was identified as a regulatory component of the 1,3-β-d-glucan synthase and thus it is required for the synthesis of the major cell wall and primary septum polymer 1,3-β-d-glucan (Arellano et al. 1996). Interestingly, depletion of Rho1p or mutation of one of its exchange factors, Rgf3, caused a cell lysis phenotype (Arellano et al. 1997; Tajadura et al. 2004; Morrell-Falvey et al. 2005; Mutoh et al. 2005) that is extremely similar to that which we observed in the sid2-250 mutant cells at low restrictive temperatures. We decided to examine whether enhancing 1,3-β-d-glucan synthesis by slightly overexpressing Rho1p can rescue the lysis phenotype in sid2-250 mutant cells. Interestingly, sid2-250 cells showed growth at up to 33° upon the ectopic expression of Rho1 on a plasmid (Figure 3C). These data provided another piece of evidence that sid2-250 mutant cells at low restrictive temperatures have septum defects, which could be the main reason for the lysis phenotype.
Disruption of major targets of ace2+ can rescue the growth defects of sid2-250 at low restrictive temperatures:
The major structural components of the S. pombe cell wall are 1,3-β-glucan (50–54% of total polysaccharides) and 1,3-α-glucan (28–32%) (Kopecka et al. 1995; Humbel et al. 2001; Sugawara et al. 2003), and recent studies suggest that cell separation in S. pombe is mainly achieved through the concerted action of the endo-1,3-β-glucanase Eng1p and the 1,3-α-glucanase Agn1p whose expression is regulated by Ace2 (Alonso-Nunez et al. 2005). The finding that eng1Δ agn1Δ mutants show a separation defect similar to that seen in ace2Δ cells suggests that Eng1p and Agn1p might be the two main enzymatic activities required for cell separation in fission yeast. Our detailed microscopic analysis of the sid2-250 mutant lysis phenotype at lower restrictive temperatures strongly suggested that the release of cleavage enzymes, such as Eng1p and Agn1p, could trigger lysis of the sid2-250 mutant at 30°–33°. To test this hypothesis, we constructed eng1Δ sid2-250 and agn1Δ sid2-250 double mutants and tested for the possible rescue. We found that disruption of either enzyme individually did not give rise to good rescue even at 30°, although it seemed that the absence of Agn1 could give sid2-250 cells a slightly better chance to survive at 30° (Figure 4A). However, simultaneous removal of activity of both enzymes allowed cells to grow well at 30° although not so well at 33°. This confirmed that enzymatic activity of two glucanases on the weak cell walls in sid2-250 mutant cells plays a major role in the lysis of sid2-250 cells at low restrictive temperature.
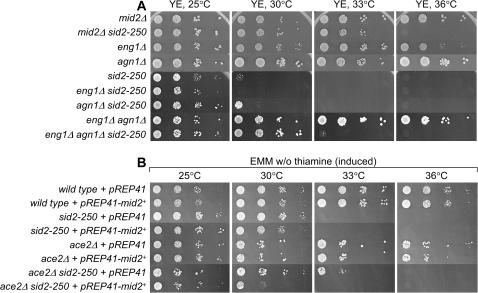
Figure 4.
Disruption of three major targets of ace2+ can rescue the growth defects of sid2-250 at lower restrictive temperature. (A) Serial dilution growth test was done with strains of mid2Δ (YDM1259), mid2Δ sid2-250 (YDM2055), eng1Δ (YDM1948), agn1Δ (YDM2801), sid2-250 (YDM429), eng1Δ sid2-250 (YDM2058), agn1Δ sid2-250 (YDM2802), eng1Δ agn1Δ (YDM2772), and eng1Δ agn1Δ sid2-250 (YDM2805). (B) Rescue of sid2-250 by ace2Δ can be reversed by overexpressing mid2+. Wild-type (YDM105), sid2-250 (YDM429), ace2Δ (YDM2028), and ace2Δ sid2-250 (YDM2027) cells transformed with either empty vector (pREP41) or the mid2+-expressing plasmid (pREP41–mid2+) were first grown in EMM plus thiamine medium and then washed, diluted, and dropped onto EMM plates without thiamine.
Because deletion of the Ace2p targets agn1+ and eng1+ did not rescue sid2-250 as well as ace2Δ did, we tested whether the loss of another target of Ace2 might contribute additionally to the rescue. One of the other targets of Ace2p is mid2+, an anillin homolog required for septin ring assembly and stability (Berlin et al. 2003; Tasto et al. 2003; Rustici et al. 2004; Alonso-Nunez et al. 2005). Mutant cells lacking mid2+ show a cell-separation defect similar to that of sep1Δ and ace2Δ but less severe. Our above observation that disruption of two targets of Ace2p (i.e., Agn1 and Eng1) can rescue sid2-250 cells from lysis prompted us to investigate whether disruption of mid2+ can also rescue sid2-250. Interestingly, we found that deletion of mid2+ can allow sid2-250 mutant cells to survive at 30°–33°, and actually mid2Δ can rescue sid2-250 slightly better than double-deletion eng1Δ agn1Δ (Figure 4A). We also found that rescue of sid2-250 by ace2Δ can be reversed by overexpressing Mid2p (Figure 4B), which confirmed that mid2+ is one of the major targets of Ace2p. Because Mid2p is important for septin ring assembly, we tested whether septin mutants would rescue sid2-250 cells. Fission yeast has four major septins (Spn1–4), which are homologs of the S. cerevisiae septins Cdc3p, Cdc10p, Cdc11p, and Cdc12p, respectively (Longtine et al. 1996; An et al. 2004). A recent study showed that the Spn4p–Spn1p subcomplex plays a more important role in septin complex assembly than the other two components (An et al. 2004). Interestingly, we found that disruption of septins could rescue sid2-250 mutant cells with variation in degree depending on which septin is absent (Table 3 and data not shown) and that spn4Δ seems to be the best rescuer of sid2-250 mutant.
TABLE 3.
Summary of rescue of the sid2-250 mutant by some cell-separation mutations
| Mutants in | Strain | 25° | 30° | 33° | 36° |
|---|---|---|---|---|---|
| Ace2 targets | mid2Δ sid2-250 | ++ | ++ | ++ | − |
| eng1Δ sid2-250 | ++ | − | − | − | |
| agn1Δ sid2-250 | ++ | +/− | − | − | |
| eng1Δ agn1Δ sid2-250 | ++ | ++ | +/− | − | |
| Septins | spn3Δ sid2-250 | ++ | + | − | − |
| spn4Δ sid2-250 | ++ | ++ | ++ | − | |
| Exocyst complex | sec8-1 sid2-250 | ++ | +/− | − | − |
| Rho GTPase | rho3Δ sid2-250 | ++ | +/− | − | − |
| rho4Δ sid2-250 | ++ | + | − | − | |
| PP2A regulatory subunit | par1Δ sid2-250 | ++ | +/− | − | − |
| par2Δ sid2-250 | ++ | − | − | − | |
| par1Δ par2Δ sid2-250 | ++ | + | − | − | |
| Transcription factor | sep1 sid2-250 | ++ | − | − | − |
Growth was examined with a serial dilution drop test at different temperatures. ++, good growth; +, weak growth; +/−, weak growth with variations in growth in different clones; −, no growth.
Other mutants with cell-separation defects can alleviate the lysis phenotype of sid2-250 cells:
In S. pombe, cell separation at the last stages of the cell cycle requires the dissolution of the primary septum. In recent years, numerous mutants showing complete or partial defects in cell separation have been isolated (see Introduction). Although in most cases the working mechanism of how these proteins affect cell separation remains elusive, it is possible that all these proteins are directly or indirectly involved in proper and timely degradation of the septum after cytokinesis. To determine whether general delays or defects in cell separation can rescue the lysis phenotype of sid2-250, we tested whether mutations of some of these genes can allow sid2-250 cells to grow. Our serial dilution growth test showed that some of these mutants, if not all, could at least partially rescue the lysis phenotype of sid2-250 (Table 3). In particular, mutation in one of the exocyst complex components, sec8-1, could slightly rescue sid2-250, consistent with a recent report showing that the delivery of dissolution enzymes to the septum is mediated by the exocyst complex (Martin-Cuadrado et al. 2005). Although the disruption of exocyst function does not show comparable rescue to that of mid2Δ, ace2Δ and double deletion of eng1Δ agn1Δ, it might be explained by the fact that the exocyst complex is involved in transporting numerous proteins for different purposes and is essential for viability (Wang et al. 2002). We did not observe rescue of sid2-250 by deletion of the transcription factor sep1+, which has ace2+ as one of its targets (Rustici et al. 2004; Alonso-Nunez et al. 2005), although sep1Δ shows very strong cell-separation defects. This might be explained by the fact that sep1+ regulates multiple targets, including sid2+, which may offset the effects of loss of ace2+ transcription.
DISCUSSION
Direct role for the SIN in cell wall assembly at the division septum:
The SIN pathway in S. pombe is required for coordinating mitosis and cytokinesis and for triggering the contraction of the actomyosin ring (reviewed by Bardin and Amon 2001; McCollum and Gould 2001; Guertin et al. 2002). Many SIN components have been identified to date and the absence of function of any of these proteins leads to failure of ring contraction and septum assembly. Previous studies have shown that SIN mutants (including sid2-250) at high restrictive temperature are capable of assembling but cannot maintain actomyosin rings upon completion of mitosis, thus leading to failure of septum deposition (Gould and Simanis 1997; Balasubramanian et al. 1998; Guertin et al. 2002; Mishra et al. 2004). Therefore, to date, it was clear that SIN plays a role in maintenance of the ring in late mitosis; however, its role in septum formation was less clear since the septum-synthesizing enzymes require the ring for localization (Cortes et al. 2002; Liu et al. 2002).
Here we show that although at high restrictive temperature most temperature-sensitive SIN mutants lyse after a few rounds nuclear division without cytokinesis, at lower restrictive temperatures, these cells seem capable of achieving nuclear division once and assembling a septum and then lysing prior to completing cell separation. In this study, our detailed microscopic analysis of formation and constriction of the actomyosin ring and septum in SIN mutants, in particular in the sid2-250 mutant, at low restrictive temperatures showed that these mutant cells can have stable actomyosin rings and can complete ring constriction and septum synthesis before their lysis during cell separation. Our observations show that the lysis phenotype of the SIN mutants is not due to failure of actomyosin ring maintenance or contraction. We propose that the lysis phenotype of SIN mutants at low restrictive temperatures is most likely due to defects in primary and/or secondary septum synthesis that lead to cell lysis when septum degradation and cell separation initiate. A few lines of evidence support this idea. For example, our data showed that a general delay in cell separation caused by compromised functions of a wide range of proteins, including Ace2p, some of Ace2p's targets (Mid2p, Agn1p, and Eng1p), septins, exocyst component Sec8p, and small GTPase Rho4p, all allow SIN mutant cells to survive the stress imposed by a weak cell wall and septum (Table 3), probably because these mutations slow down or block the action of septum-degrading enzymes on the septum. In addition, we also found that the presence of sorbitol in the medium could rescue the cell lysis phenotype of some mutations in SIN pathway components (Figure 3A), suggesting that SIN mutants might have septum defects, as sorbitol can act as an osmotic stabilizer and has been shown to rescue the cell lysis phenotype in mutants with a defective cell wall in both S. cerevisiae and S. pombe (Ribas et al. 1991; Cid et al. 1995; Santos et al. 2003). Furthermore, overexpression of the Rho1p GTPase can also rescue the lysis phenotype of sid2-250. Since Rho1p is required for the synthesis of the major cell wall and primary septum polymer 1,3-β-d-glucan (Arellano et al. 1996), it is plausible to assume that the overexpression of Rho1p can compensate for the defects in cell wall and septum in sid2-250 cells. Taken together, we favor the idea that the SIN pathway is indeed directly involved in septum formation, and examination of the sid2-250 mutant cells at lower restrictive temperature—where they have normal actomyosin ring formation, maintenance, and constriction—made this more apparent.
As discussed above, SIN mutants at the low restrictive temperature synthesize septa, but the septa probably have structural defects. However, because these studies are done at temperatures where there is still some residual SIN function, it is unclear whether the SIN is required for bulk septum synthesis. Although at high restrictive temperatures SIN mutants do not make septa, it is not clear if this is an indirect effect due to the disappearance of the actomyosin ring, which is required for localization of septum-synthesizing enzymes (Le Goff et al. 1999; Liu et al. 1999; Cortes et al. 2002), making this issue difficult to resolve.
Septins, Mid2, and exocyst complex function in cell separation:
The cell-separation defects of septins, Mid2p, and exocyst complex mutants, as well as our identification of them as SIN suppressors, indicate a role for these proteins in cell separation. A recent report showed that targeted secretion of the septum-degrading enzymes Agn1p and Eng1p depends on the exocyst, and their proper localization at the septum depends on the septins and Mid2p (Martin-Cuadrado et al. 2005). mid2+ is a target of the Ace2p transcription factor (Rustici et al. 2004; Alonso-Nunez et al. 2005). We show that expression of mid2+ from a heterologous promoter could reverse the ability of an ace2Δ mutant to suppress sid2-250, suggesting that ace2Δ suppression of sid2-250 is due to loss of mid2+ expression. This is surprising, given that we also find that double deletion of two other targets of Ace2p, agn1+ and eng1+, can rescue sid2-250 mutants. One explanation could be that because cell separation still occurs in ace2Δ cells, agn1+ and eng1+ transcription may not completely depend on Ace2p, and/or there are other septum-degrading enzymes that do not depend on Ace2p for their expression. If this is the case, then heterologously expressed Mid2p could recruit hydrolases to the septum region in the ace2Δ mutant.
What is the target for SIN in septum formation?
Most recent studies have focused on how the signaling is transduced within the SIN pathway and how nuclear and cell division is coordinated with the SIN. However, it remains unclear what downstream target(s) is being regulated by SIN signaling in septum formation.
It has been recently reported that depletion (shutoff) of the Rho1p guanine nucleotide exchange factor (GEF) Rgf3p leads to lysis phenotype similar to the depletion of Rho1p (Tajadura et al. 2004; Morrell-Falvey et al. 2005; Mutoh et al. 2005), and it is also extremely reminiscent of the lysis phenotype of SIN mutants at low restrictive temperature, where cells lyse after cytokinesis during the process of cell separation. In addition, the lysis of cells caused by switching off Rgf3p can be rescued by sorbitol, while Rho1p-depleted cells cannot, suggesting that Rgf3p controls a subset of the functions of Rho1p in cell wall biosynthesis (Arellano et al. 1997; Tajadura et al. 2004; Morrell-Falvey et al. 2005; Mutoh et al. 2005). More interestingly, both Rho1p and Rgf3p localize at the septum (Arellano et al. 1997; Tajadura et al. 2004; Morrell-Falvey et al. 2005; Mutoh et al. 2005), strongly suggesting that they are involved in cell wall biosynthesis at the septum. Thus, it is an intriguing possibility that the SIN targets Rho1 as one of its downstream effectors; this could be achieved by activating Rgf3, which is a specific Rho1 GEF for its function in cell wall biosynthesis (Tajadura et al. 2004). GTP-bound Rho1p can then directly or indirectly activate the α-1,3-d-glucan and β-1,3-d-glucan synthases and participate in the regulation of cell wall growth. The regulation of β-1,3-d-glucan synthase Bgs4p by Rho1p could be direct, as shutoff of Bgs4p causes a similar phenotype to shut off Rho1p and weak SIN mutants (Cortes et al. 2005). It is not known whether another β-1,3-d-glucan synthase subunit, Cps1/Bgs1, is also regulated by Rho1p. The regulation of α-1,3-d-glucan synthase Mok1p may be through the Rho1p and Pck pathway, since Pck1 and Pck2 kinases are Rho1 effectors and Mok1p is one of the downstream targets of Pck proteins (Katayama et al. 1999; Sayers et al. 2000). Both Rho1p and Rgf3p are also involved in actomyosin ring formation or maintenance (Arellano et al. 1997; Mutoh et al. 2005) as is the SIN; therefore it is also possible that the SIN could act through Rgf3p and Rho1p to affect the ring as well. Testing these different hypotheses will be important for future studies of the role of the SIN in cytokinesis.
Acknowledgments
We are grateful to Kathy Gould, Viesturs Simanis, Richard Hallberg, John Pringle, Mátyás Sipiczki, Beatriz Santos, and Carlos R. Vázquez de Aldana for providing strains and plasmids and Jürg Bähler for communicating microarray results. We thank the McCollum lab members for discussions. This work was supported by National Institutes of Health grant GM058406-07 to D. McCollum.
References
- Alonso-Nunez, M. L., H. An, A. B. Martin-Cuadrado, S. Mehta, C. Petit et al., 2005. Ace2p controls the expression of genes required for cell separation in Schizosaccharomyces pombe. Mol. Biol. Cell 16: 2003–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An, H., J. L. Morrell, J. L. Jennings, A. J. Link and K. L. Gould, 2004. Requirements of fission yeast septins for complex formation, localization, and function. Mol. Biol. Cell 15: 5551–5564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arellano, M., A. Duran and P. Perez, 1996. Rho 1 GTPase activates the (1–3)beta-D-glucan synthase and is involved in Schizosaccharomyces pombe morphogenesis. EMBO J. 15: 4584–4591. [PMC free article] [PubMed] [Google Scholar]
- Arellano, M., A. Duran and P. Perez, 1997. Localisation of the Schizosaccharomyces pombe rho1p GTPase and its involvement in the organisation of the actin cytoskeleton. J. Cell Sci. 110(Pt. 20): 2547–2555. [DOI] [PubMed] [Google Scholar]
- Arellano, M., P. M. Coll and P. Perez, 1999. RHO GTPases in the control of cell morphology, cell polarity, and actin localization in fission yeast. Microsc. Res. Tech. 47: 51–60. [DOI] [PubMed] [Google Scholar]
- Bahler, J., 2005. A transcriptional pathway for cell separation in fission yeast. Cell Cycle 4: 39–41. [DOI] [PubMed] [Google Scholar]
- Balasubramanian, M. K., D. McCollum, L. Chang, K. C. Wong, N. I. Naqvi et al., 1998. Isolation and characterization of new fission yeast cytokinesis mutants. Genetics 149: 1265–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardin, A. J., and A. Amon, 2001. Men and sin: What's the difference? Nat. Rev. Mol. Cell Biol. 2: 815–826. [DOI] [PubMed] [Google Scholar]
- Berlin, A., A. Paoletti and F. Chang, 2003. Mid2p stabilizes septin rings during cytokinesis in fission yeast. J. Cell Biol. 160: 1083–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib, E., J. Drgonova and T. Drgon, 1998. Role of small G proteins in yeast cell polarization and wall biosynthesis. Annu. Rev. Biochem. 67: 307–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cid, V. J., A. Duran, F. del Rey, M. P. Snyder, C. Nombela et al., 1995. Molecular basis of cell integrity and morphogenesis in Saccharomyces cerevisiae. Microbiol. Rev. 59: 345–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes, J. C., J. Ishiguro, A. Duran and J. C. Ribas, 2002. Localization of the (1,3)beta-D-glucan synthase catalytic subunit homologue Bgs1p/Cps1p from fission yeast suggests that it is involved in septation, polarized growth, mating, spore wall formation and spore germination. J. Cell Sci. 115: 4081–4096. [DOI] [PubMed] [Google Scholar]
- Cortes, J. C., E. Carnero, J. Ishiguro, Y. Sanchez, A. Duran et al., 2005. The novel fission yeast (1,3)beta-D-glucan synthase catalytic subunit Bgs4p is essential during both cytokinesis and polarized growth. J. Cell Sci. 118: 157–174. [DOI] [PubMed] [Google Scholar]
- Craven, R. A., D. J. Griffiths, K. S. Sheldrick, R. E. Randall, I. M. Hagan et al., 1998. Vectors for the expression of tagged proteins in Schizosaccharomyces pombe. Gene 221: 59–68. [DOI] [PubMed] [Google Scholar]
- Dekker, N., D. Speijer, C. H. Grun, M. van den Berg, A. de Haan et al., 2004. Role of the alpha-glucanase Agn1p in fission-yeast cell separation. Mol. Biol. Cell 15: 3903–3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drgonova, J., T. Drgon, D. H. Roh and E. Cabib, 1999. The GTP-binding protein Rho1p is required for cell cycle progression and polarization of the yeast cell. J. Cell Biol. 146: 373–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia, I., D. Jimenez, V. Martin, A. Duran and Y. Sanchez, 2005. The alpha-glucanase Agn1p is required for cell separation in Schizosaccharomyces pombe. Biol. Cell 97: 569–576. [DOI] [PubMed] [Google Scholar]
- Gould, K. L., and V. Simanis, 1997. The control of septum formation in fission yeast. Genes Dev. 11: 2939–2951. [DOI] [PubMed] [Google Scholar]
- Guertin, D. A., S. Trautmann and D. McCollum, 2002. Cytokinesis in eukaryotes. Microbiol. Mol. Biol. Rev. 66: 155–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbel, B. M., M. Konomi, T. Takagi, N. Kamasawa, S. A. Ishijima et al., 2001. In situ localization of beta-glucans in the cell wall of Schizosaccharomyces pombe. Yeast 18: 433–444. [DOI] [PubMed] [Google Scholar]
- Jiang, W., and R. L. Hallberg, 2000. Isolation and characterization of par1(+) and par2(+): two Schizosaccharomyces pombe genes encoding B′ subunits of protein phosphatase 2A. Genetics 154: 1025–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, Q. W., and D. McCollum, 2003. Scw1p antagonizes the septation initiation network to regulate septum formation and cell separation in the fission yeast Schizosaccharomyces pombe. Eukaryot. Cell 2: 510–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, B. F., B. Y. Yoo and G. B. Calleja, 1973. Cell division in yeasts: movement of organelles associated with cell plate growth of Schizosaccharomyces pombe. J. Bacteriol. 115: 358–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama, S., D. Hirata, M. Arellano, P. Perez and T. Toda, 1999. Fission yeast alpha-glucan synthase Mok1 requires the actin cytoskeleton to localize the sites of growth and plays an essential role in cell morphogenesis downstream of protein kinase C function. J. Cell Biol. 144: 1173–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecka, M., G. H. Fleet and H. J. Phaff, 1995. Ultrastructure of the cell wall of Schizosaccharomyces pombe following treatment with various glucanases. J. Struct. Biol. 114: 140–152. [DOI] [PubMed] [Google Scholar]
- Le Goff, X., A. Woollard and V. Simanis, 1999. Analysis of the cps1 gene provides evidence for a septation checkpoint in Schizosaccharomyces pombe. Mol. Gen. Genet. 262: 163–172. [DOI] [PubMed] [Google Scholar]
- Le Goff, X., F. Motegi, E. Salimova, I. Mabuchi and V. Simanis, 2000. The S. pombe rlc1 gene encodes a putative myosin regulatory light chain that binds the type II myosins myo3p and myo2p. J. Cell Sci. 113(Pt. 23): 4157–4163. [DOI] [PubMed] [Google Scholar]
- Le Goff, X., S. Buvelot, E. Salimova, F. Guerry, S. Schmidt et al., 2001. The protein phosphatase 2A B′-regulatory subunit par1p is implicated in regulation of the S. pombe septation initiation network. FEBS Lett. 508: 136–142. [DOI] [PubMed] [Google Scholar]
- Liu, J., H. Wang, D. McCollum and M. K. Balasubramanian, 1999. Drc1p/Cps1p, a 1,3-β-glucan synthase subunit, is essential for division septum assembly in Schizosaccharomyces pombe. Genetics 153: 1193–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J., X. Tang, H. Wang and M. Balasubramanian, 2000. Bgs2p, a 1,3-beta-glucan synthase subunit, is essential for maturation of ascospore wall in Schizosaccharomyces pombe. FEBS Lett. 478: 105–108. [DOI] [PubMed] [Google Scholar]
- Liu, J., X. Tang, H. Wang, S. Oliferenko and M. K. Balasubramanian, 2002. The localization of the integral membrane protein Cps1p to the cell division site is dependent on the actomyosin ring and the septation-inducing network in Schizosaccharomyces pombe. Mol. Biol. Cell 13: 989–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine, M. S., D. J. DeMarini, M. L. Valencik, O. S. Al-Awar, H. Fares et al., 1996. The septins: roles in cytokinesis and other processes. Curr. Opin. Cell Biol. 8: 106–119. [DOI] [PubMed] [Google Scholar]
- Martin-Cuadrado, A. B., E. Duenas, M. Sipiczki, C. R. Vazquez de Aldana and F. del Rey, 2003. The endo-beta-1,3-glucanase eng1p is required for dissolution of the primary septum during cell separation in Schizosaccharomyces pombe. J. Cell Sci. 116: 1689–1698. [DOI] [PubMed] [Google Scholar]
- Martin-Cuadrado, A. B., J. L. Morrell, M. Konomi, H. An, C. Petit et al., 2005. Role of septins and the exocyst complex in the function of hydrolytic enzymes responsible for fission yeast cell separation. Mol. Biol. Cell 16: 4867–4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maundrell, K., 1990. nmt1 of fission yeast. A highly transcribed gene completely repressed by thiamine. J. Biol. Chem. 265: 10857–10864. [PubMed] [Google Scholar]
- McCollum, D., and K. L. Gould, 2001. Timing is everything: regulation of mitotic exit and cytokinesis by the MEN and SIN. Trends Cell Biol. 11: 89–95. [DOI] [PubMed] [Google Scholar]
- Mishra, M., J. Karagiannis, S. Trautmann, H. Wang, D. McCollum et al., 2004. The Clp1p/Flp1p phosphatase ensures completion of cytokinesis in response to minor perturbation of the cell division machinery in Schizosaccharomyces pombe. J. Cell Sci. 117: 3897–3910. [DOI] [PubMed] [Google Scholar]
- Moreno, S., A. Klar and P. Nurse, 1991. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194: 795–823. [DOI] [PubMed] [Google Scholar]
- Morrell-Falvey, J. L., L. Ren, A. Feoktistova, G. D. Haese and K. L. Gould, 2005. Cell wall remodeling at the fission yeast cell division site requires the Rho-GEF Rgf3p. J. Cell Sci. 118: 5563–5573. [DOI] [PubMed] [Google Scholar]
- Mutoh, T., K. Nakano and I. Mabuchi, 2005. Rho1-GEFs Rgf1 and Rgf2 are involved in formation of cell wall and septum, while Rgf3 is involved in cytokinesis in fission yeast. Genes Cells 10: 1189–1202. [DOI] [PubMed] [Google Scholar]
- Nakano, K., T. Mutoh, R. Arai and I. Mabuchi, 2003. The small GTPase Rho4 is involved in controlling cell morphology and septation in fission yeast. Genes Cells 8: 357–370. [DOI] [PubMed] [Google Scholar]
- Naqvi, N. I., K. C. Wong, X. Tang and M. K. Balasubramanian, 2000. Type II myosin regulatory light chain relieves auto-inhibition of myosin-heavy-chain function. Nat. Cell Biol. 2: 855–858. [DOI] [PubMed] [Google Scholar]
- Ribar, B., A. Banrevi and M. Sipiczki, 1997. sep1+ encodes a transcription-factor homologue of the HNF-3/forkhead DNA-binding-domain family in Schizosaccharomyces pombe. Gene 202: 1–5. [DOI] [PubMed] [Google Scholar]
- Ribas, J. C., M. Diaz, A. Duran and P. Perez, 1991. Isolation and characterization of Schizosaccharomyces pombe mutants defective in cell wall (1–3)beta-D-glucan. J. Bacteriol. 173: 3456–3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustici, G., J. Mata, K. Kivinen, P. Lio, C. J. Penkett et al., 2004. Periodic gene expression program of the fission yeast cell cycle. Nat. Genet. 36: 809–817. [DOI] [PubMed] [Google Scholar]
- Santos, B., J. Gutierrez, T. M. Calonge and P. Perez, 2003. Novel Rho GTPase involved in cytokinesis and cell wall integrity in the fission yeast Schizosaccharomyces pombe. Eukaryot. Cell 2: 521–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayers, L. G., S. Katayama, K. Nakano, H. Mellor, I. Mabuchi et al., 2000. Rho-dependence of Schizosaccharomyces pombe Pck2. Genes Cells 5: 17–27. [DOI] [PubMed] [Google Scholar]
- Simanis, V., 2003. Events at the end of mitosis in the budding and fission yeasts. J. Cell Sci. 116: 4263–4275. [DOI] [PubMed] [Google Scholar]
- Sugawara, T., M. Sato, T. Takagi, T. Kamasaki, N. Ohno et al., 2003. In situ localization of cell wall alpha-1,3-glucan in the fission yeast Schizosaccharomyces pombe. J. Electron Microsc. 52: 237–242. [DOI] [PubMed] [Google Scholar]
- Sugiura, R., T. Toda, H. Shuntoh, M. Yanagida and T. Kuno, 1998. pmp1+, a suppressor of calcineurin deficiency, encodes a novel MAP kinase phosphatase in fission yeast. EMBO J. 17: 140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajadura, V., B. Garcia, I. Garcia, P. Garcia and Y. Sanchez, 2004. Schizosaccharomyces pombe Rgf3p is a specific Rho1 GEF that regulates cell wall beta-glucan biosynthesis through the GTPase Rho1p. J. Cell Sci. 117: 6163–6174. [DOI] [PubMed] [Google Scholar]
- Tasto, J. J., J. L. Morrell and K. L. Gould, 2003. An anillin homologue, Mid2p, acts during fission yeast cytokinesis to organize the septin ring and promote cell separation. J. Cell Biol. 160: 1093–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda, T., S. Dhut, G. Superti-Furga, Y. Gotoh, E. Nishida et al., 1996. The fission yeast pmk1+ gene encodes a novel mitogen-activated protein kinase homolog which regulates cell integrity and functions coordinately with the protein kinase C pathway. Mol. Cell. Biol. 16: 6752–6764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H., X. Tang, J. Liu, S. Trautmann, D. Balasundaram et al., 2002. The multiprotein exocyst complex is essential for cell separation in Schizosaccharomyces pombe. Mol. Biol. Cell 13: 515–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida, T., T. Toda and M. Yanagida, 1994. A calcineurin-like gene ppb1+ in fission yeast: mutant defects in cytokinesis, cell polarity, mating and spindle pole body positioning. J. Cell Sci. 107(Pt. 7): 1725–1735. [DOI] [PubMed] [Google Scholar]