Abstract
Most land plants can form a root symbiosis with arbuscular mycorrhizal (AM) fungi for assimilation of inorganic phosphate from the soil. In contrast, the nitrogen-fixing root nodule symbiosis is almost completely restricted to the legumes. The finding that the two symbioses share common signaling components in legumes suggests that the evolutionarily younger nitrogen-fixing symbiosis has recruited functions from the more ancient AM symbiosis. The recent advances in cloning of the genes required for nodulation and AM symbioses from the two model legumes, Medicago truncatula and Lotus japonicus, provide a unique opportunity to address biological questions pertaining to the evolution of root symbioses in plants. Here, we report that nearly all cloned legume genes required for nodulation and AM symbioses have their putative orthologs in nonlegumes. The orthologous relationship can be clearly defined on the basis of both sequence similarity and microsyntenic relationship. The results presented here serve as a prelude to the comparative analysis of orthologous gene function between legumes and nonlegumes and facilitate our understanding of how gene functions and signaling pathways have evolved to generate species- or family-specific phenotypes.
IN natural ecosystems, many plants can grow on nutrient-poor soils by living together for mutual benefit with microorganisms, an example of a process called symbiosis. The association between arbuscular mycorrhizal (AM) fungi and >80% of land plants and that between rhizobial bacteria and legumes represent the two most important symbiotic interactions between the plant root and microbes. Through the AM symbiosis, AM fungi enhance nutrient availability to plants, in particular, inorganic phosphate, whereas, in the legume–rhizobia interaction, the rhizobia fix atmospheric nitrogen for use by the plant. Thus, these two symbioses are of critical importance in sustainable agriculture.
AM is an ancient symbiosis that originated >400 million years ago and may have played a key role in facilitating the movement of plants onto land (Remy et al. 1994; Redecker et al. 2000; Heckman et al. 2001). This symbiotic association takes place in all major land-plant lineages, including hornworts, liverworts, lycopods, and ferns (Brundrett 2002). At the onset of the symbioses, plants actively respond to AM fungi by altering both their cytoarchitecture and their transcriptional program. These host responses are likely triggered by diffusible fungal signals termed “Myc factors” but the structure of these molecules remains to be determined (Genre et al. 2005; Harrison 2005). AM fungi subsequently enter the plant root and differentiate within inner cortical cells, forming highly ramified fungal hyphae, called arbuscules (Harrison 2005). Concurrently, AM fungi develop extensive extraradical hyphae outside the plant root. The intraradical and extraradical hyphae constitute a single continuum, thereby facilitating the transfer of mineral nutrients from the soil to the plant.
In contrast to the ancient AM symbiosis, nitrogen-fixing symbiosis evolved more recently (∼60–70 million years ago) and is restricted to a clade within the Eurosid I, consisting of Fabales, Fagales, Cucurbitales, and Rosales (Fa Fa Cu Ro) (Soltis et al. 1999). In fact, the symbiosis may have arisen several times within this Fa Fa Cu Ro clade (Doyle 1998; Kistner and Parniske 2002; Doyle and Luckow 2003). Legume nodulation begins with an exchange of molecular signals between the host and bacteria (Dénarié et al. 1996; Long 1996; Spaink 2000). Flavonoid compounds released from legume roots attract the rhizobia to the root and trigger the expression of a range of rhizobial nodulation-related (nod) genes. Nod gene products synthesize and secrete specific lipochitooligosaccharides, called Nod factors, from the bacteria. Perception of Nod factors by the plant induces a suite of host responses, including the activation of host gene expression, calcium spiking, root hair deformation and curling, and cortical cell divisions (Downie and Walker 1999; Oldroyd and Downie 2004). These physiological and morphological changes ultimately lead to the formation of the root nodule, in which the bacteria find an ideal environment to fix atmospheric nitrogen.
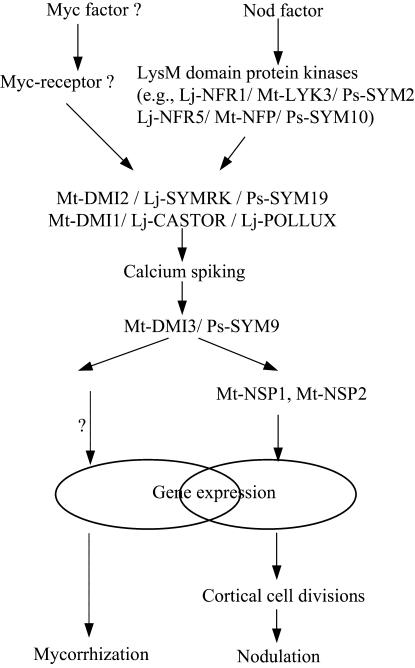
Interestingly, the AM and root nodule symbioses share common signaling components in legumes (Figure 1). A common set of host genes is induced in both rhizobial and AM symbioses and several legume mutants defective for nodulation are also defective in the AM interaction (Albrecht et al. 1999; Hirsch et al. 2001; Kistner and Parniske 2002). Furthermore, recent research has shown that the mechanism limiting the root nodule number also suppresses secondary mycorrhizal infections in root systems (Meixner et al. 2005). These observations support the hypothesis that the evolutionarily younger nitrogen-fixing symbiosis may have recruited functions from the more ancient AM symbiosis.
Figure 1.
Nodulation and endomycorrhization signaling pathways. Nod factors are perceived by LysM receptor kinases. Mycorrhizal signals (Myc factors) are also proposed to be perceived by yet unknown specific receptors. Downstream common signaling is mediated by DMI proteins, which activates GRAS family transcription factors (Mt-NSP1 and Mt-NSP2) required for the expression of early nodulation genes and the initiation of cortical cell divisions.
Tremendous progress has been made recently by cloning genes that are required for rhizobial and AM symbioses from the model legumes, Medicago truncatula and Lotus japonicus (Figure 1) (Oldroyd and Downie 2004; Riely et al. 2004; Oldroyd et al. 2005). Coupled with abundant sequence information from nonlegumes such as rice and Arabidopsis, we can now address several important biological questions pertaining to the evolution of the two symbioses in plants. Did the genes required for nitrogen-fixing root nodule symbiosis evolve specially in legumes or were genes involved in other aspects of developmental processes recruited to mediate legume nodulation? What are the functions of the nonlegume orthologs of the legume genes required for nodulation and AM symbioses? Are the nonlegume orthologs of the legume genes that are essential for both AM and nodulation symbioses also required for AM symbioses in nonlegumes? Answers to these questions will provide novel insights into the evolution of signaling pathways leading to species- or family-specific phenotypes and allow us to link comparative structural genomics with comparative plant biology.
This article reports the extent to which the legume genes required for nodulation and AM symbioses are conserved in nonlegume species. We define, where possible, the putative nonlegume orthologs of the target legume genes, analyze their sequence evolution, and compare their genomic organization across wide taxonomic boundaries. The results presented here serve as a prelude to the comparative analysis of orthologous gene function between legumes and nonlegumes.
MATERIALS AND METHODS
The full-length cDNA clones containing homologs of M. truncatula DMI genes were isolated from Physcomitrella patens (model moss) and soybean. RNA was isolated from P. patens protonemal filament and from Williams 82 soybean root tissues using a QIAGEN (Valencia, CA) RNeasy miniprep kit. One microgram of RNA was used in a 5′ and 3′ RACE amplification, using the SMART RACE cDNA amplification kit (BD Biosciences). Primers complementary to the P. patens and Glycine max sequences available in the NCBI GenBank database were used. The resulting PCR products were TA cloned into pCR2.1 (Invitrogen, San Diego) and independently amplified clones were sequenced. Other DNA sequence data were acquired from the NCBI GenBank database. Gene prediction was performed by FGENESH (http://www.softberry.com/berry.phtml?topic=gfind). Sequence alignments were performed using ClustalX (gap opening, 10; gap extension, 0.05; BLOSUM matrix) (Chenna et al. 2003) and manually curated. Phylogenetic trees were constructed by PAUP* 4.0 (Swofford 2003), using protein sequences. For each data set, parsimony heuristic searches were performed from 20 random addition sequence replicates followed by TBR branch swapping. Bootstrap values were determined on the basis of 500 (DMI1, DMI2, and DMI3) or 200 (LysM receptor kinases) simulated data sets. All bootstrap replicates were analyzed as described above. In all cases, a small number of trees (one to five) were found to be most parsimonious (MP) and the strict consensus topology of MP trees was identical to the majority rule consensus bootstrap tree.
RESULTS AND DISCUSSION
Nod factor receptors:
The exquisite sensitivity and high host specificity with which legume roots respond to Nod factors suggest that Nod factor signals are recognized by specific plant receptors (Cullimore et al. 2001; Geurts and Bisseling 2002; Amor et al. 2003; Cullimore and Dénarié 2003). Two L. japonicus genes, NFR1 and NFR5 (hereafter referred to as Lj-NFR1a and Lj-NFR5a, respectively), which are required for Nod factor perception, both encode LysM domain-containing receptor kinases (Madsen et al. 2003; Radutoiu et al. 2003) (Figure 1). The LysM domains have been implicated in binding polysaccharides, particularly glucosamine chains that are similar to the Nod factor backbone (Bateman and Bycroft 2000). Lj-NFR5a is proposed to be the ortholog of Pisum sativum Ps-SYM10 and M. truncatula Mt-NFP, on the basis of their mutant phenotypes and syntenic linkage map positions (Amor et al. 2003; Madsen et al. 2003). In M. truncatula, two LysM receptor kinases (LYKs), Mt-LYK3 and Mt-LYK4, mediate Nod factor-induced infection (Limpens et al. 2003), and they appear to be the orthologous counterparts of Lj-NFR1a and Ps-SYM2 (Figure 1). The fact that the Lj-nfr1a and Lj-nfr5a mutants lack all responses to Nod factors but are able to establish AM symbioses suggests that the Myc factors essential for AM symbioses are not recognized by the same receptors as Nod factors and that the Nod factor receptors act upstream of the common symbiotic pathway (Madsen et al. 2003; Radutoiu et al. 2003).
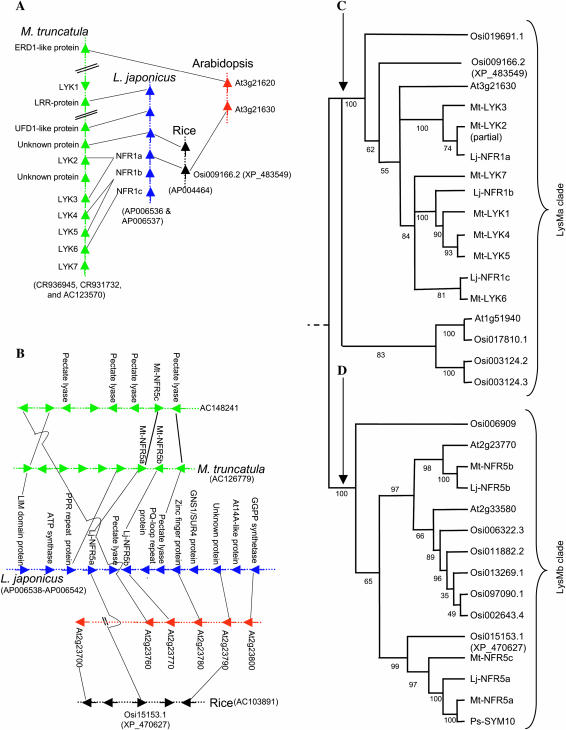
Sequence comparison of the Mt-LYK3/4 and Lj-NFR1a genomic regions reveals highly conserved microsynteny (Figure 2A). In both species, however, the orthologs were duplicated, with at least seven copies in M. truncatula (LYK1-7) and three copies in L. japonicus (NFR1a-c) within the sequenced genomic regions. Despite their involvement in legume root nodulation, Lj-NFR1a orthologs are not legume specific. BlastP analyses identified a single best-matched gene in Arabidopsis (At3g21630) and rice (XP_483549), the two widely diverged angiosperm species with whole-genome sequence available. At3g21630 and XP_483549 share highly conserved gene structure (11–12 exons) and sequence similarity (∼70%) with Lj-NFR1a. Furthermore, the Arabidopsis and rice regions show limited but informative synteny with the corresponding legume regions, indicating that these genes are descendants of a common ancestor (Figure 2A).
Figure 2.
Phylogeny and microsynteny of symbiotic LysM receptor-like kinases. (A) Microsynteny of NFR1 orthologous regions in M. truncatula, L. japonicus, rice, and Arabidopsis (the LysMa clade). (B) Microsynteny of NFR5 orthologous regions in M. truncatula, L. japonicus, rice, and Arabidopsis (the LysMb clade). (C and D) Strict consensus from the four most parsimonious trees of known LysM receptor kinase genes in legumes, A. thaliana, and rice. Proteins and clades have been named according to Shiu et al. (2004). The tree was rooted according to Shiu et al. (2004) as indicated by the dashed line. Numbers below the branches of the phylogenetic trees are the percentages of 200 bootstrap replications supporting the particular nodes (C, the LysMa clade; D, the LysMb clade).
Lj-NFR5a is quite different from Lj-NFR1a, showing <30% sequence identity. Lj-NFR5a has a simple gene structure of a single exon, in contrast to Lj-NFR1a, which consists of 12 exons. The close homologs of Lj-NFR5a can also be identified in Arabidopsis (At2g23770) and rice (XP_470627), which share the same gene structure with Lj-NFR5a. The Lj-NFR5a genomic region exhibits microsynteny with its counterpart in M. truncatula and the regions surrounding At2g23770 in Arabidopsis and XP_470627 in rice (Figure 2B). Similar to that observed for the Lj-NFR1a orthologous regions, Lj-NFR5a and its ortholog in M. truncatula both have a duplicated copy with a reverse transcriptional orientation (referred to as Lj-NFR5b and Mt-NFR5b, respectively). In addition, an additional copy in M. truncatula, namely Mt-NFR5c, is located on a duplicated segment (AC148241) of the Mt-NFR5a region (Figure 2B).
Phylogenetic analysis suggests that the major duplication events of the Lj-NFR1a (Figure 2C) and Lj-NFR5a (Figure 2D) orthologous regions predate the speciation of M. truncatula and L. japonicus, as evidenced by the fact that the between-species sequence similarity is, in some cases, higher than the within-species sequence similarity within the duplicated regions. However, the duplications appear to have continued after speciation for the NFR1 region in M. truncatula (Figure 2, A and C). Despite the presence of numerous paralogs in the Lj-NFR1a orthologous regions (Figure 2A), the orthologous relationship between Lj-NFR1a and Mt-LYK3 can be clearly defined, consistent with the observation that the knocked-down Mt-LYK3 roots showed a strong effect on rhizobial infection and nodule formation (Limpens et al. 2003; Figure 2, A and B). The same is also true for Lj-NFR5a (Figure 2, B and D), which is obviously orthologous to one of the two Medicago homologs, Mt-NFR5a, located on AC126779. There is evidence suggesting that Mt-NFR5a is likely the Mt-NFP (the putative ortholog of Lj-NFR5a), as a mutation was identified in the Mt-NFR5a gene of the M. truncatula nfp mutant (B. Riely, unpublished observation). For the Lj-NFR5a orthologous regions, the duplication events appear to predate the divergence of monocotyledonous and dicotyledonous plants, since the Arabidopsis gene At2g23770 is more similar to Mt-NFR5b and Lj-NFR5b, while the rice gene XP_470627 is more similar to Lj-NFR5a, Mt-NFR5a, and Ps-SYM10 (Figure 2D). These observations suggest that At2g23770 is an ancient paralog of Lj-NFR5a, while the rice gene XP_470627 is likely the putative ortholog of Lj-NFR5a. Such inference is further supported by the fact that the phylogenetic distances are consistent with their relative transcriptional orientation in each individual species (Figure 2, B and D).
This legume-specific gene duplication (i.e., both NFR1 and NFR5 orthologous regions) and legume-specific maintenance of ancient gene duplication (i.e., Mt-NFR5a and Lj-NFR5a) may have played an important role in gene neofunctionalization and/or subfunctionalization (Lynch and Conery 2000), leading to legume-specific root nodule symbiosis. Even within legume species, the function of orthologous genes continues to diverge, which is evidenced by the observation that Lj-NFR1a and Mt-LYK3 function differently in L. japonicus and M. truncatula, respectively. Lj-NFR1a functions as a Nod factor-signaling receptor that is required for all known Nod factor responses (Radutoiu et al. 2003), while Mt-LYK3 appears to be an entry receptor that facilitates rhizobial infection in the presence of a specific Nod factor structure (Limpens et al. 2003). The idea that gene loss/duplication may have driven such functional divergence is further support by the observation that Mt-LYK4, a tandem duplicate of Mt-LYK3, also plays a role in infection thread formation but not nodule formation (Limpens et al. 2003). It will be fascinating to learn the function of these homologous genes in both legumes and nonlegumes.
Common signaling components in root symbioses:
Three M. truncatula genes, Mt-DMI1, Mt-DMI2, and Mt-DMI3, are required for both AM and nodulation symbioses (Catoira et al. 2000) (Figure 1). Mt-DMI1 and its counterparts in L. japonicus, Lj-POLLUX and Lj-CASTOR, encode ion channel-like proteins that could mediate the early ion fluxes observed in root hairs responding to Nod factors (Ané et al. 2004; Imaizumi-Anraku et al. 2005). The Mt-DMI2 and its orthologs from pea (Ps-SYM19), alfalfa (Ms-NORK), and L. japonicus (Lj-SYMRK) encode receptor-like kinases with leucine-rich-repeat (LRR) domains in the predicted extracellular region (Endre et al. 2002; Stracke et al. 2002). Mt-DMI3 and its ortholog Ps-SYM9 encode proteins with strong similarity to Ca2+/calmodulin (CaM)-dependent protein kinases (Lévy et al. 2004; Mitra et al. 2004). Mt-DMI1 and Mt-DMI2 act upstream of calcium spiking, while Mt-DMI3 lies downstream of calcium spiking.
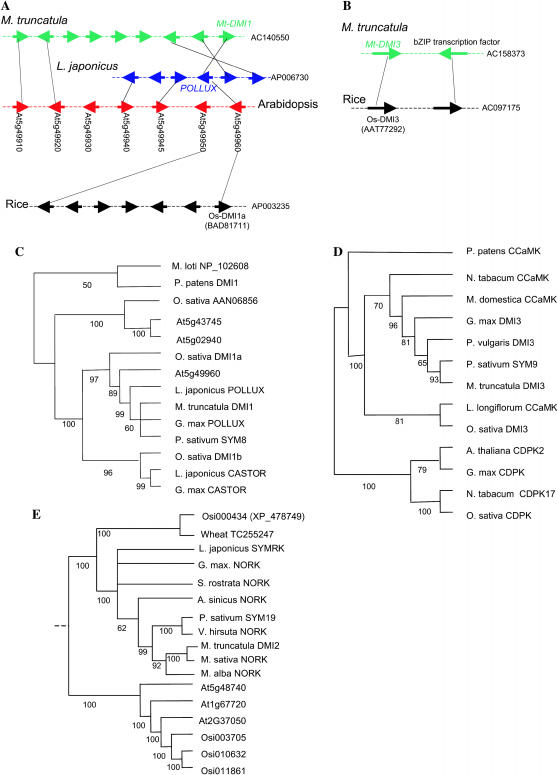
Mt-DMI1 and Mt-DMI3 are highly conserved across multiple plant taxa, suggestive of their possible roles in AM symbioses in nonlegumes. The putative orthologs of Mt-DMI1 can be defined unambiguously in L. japonicus (Lj-POLLUX), Arabidopsis (At5g49960), and rice (BAD81711, referred to as Os-DMI1a), on the basis of their highly conserved protein sequence (∼80% similarity) and microsyntenic relationship (Figure 3, A and C). In rice, however, we identified another homolog of Mt-DMI1 (XM_470380, referred to as Os-DMI1b in Figure 3C) which is located in a nonsyntenic region of the rice chromosome 3. Os-DMI1b appears to be orthologous to Lj-CASTOR (Figure 3C), but their syntenic relationship is unknown since the genomic region containing Lj-CASTOR has not been sequenced. The duplication of Mt-DMI1 homologs in rice and L. japonicus seems to have occurred before the monocot and eudicot divergence (Figure 3C).
Figure 3.
Microsynteny and phylogeny of DMI homologs in M. truncatula, L. japonicus, rice, and Arabidopsis. Trees (C, D, and E) are strict consensus from the most parsimonious (MP) trees. The numbers of MP trees found were five (C), four (E), and one (D). Numbers below the branches represent the percentages of 500 bootstrap replications supporting the particular nodes. The strict consensus tree of DMI1 homologs (C) was rooted using the bacterial Mesorhizobium loti and the moss P. patens homologs. The strict consensus tree of DMI2-like proteins (E) was rooted according to Shiu et al. (2004). The strict consensus tree of calcium- and calmodulin-dependent kinases (CCaMKs) (D) was rooted using the closest calcium-dependent kinase (CDPK) sequences.
Mt-DMI3 and its putative rice ortholog (AAT77292, referred to as Os-DMI3 in Figure 3, B and D) also maintain a syntenic relationship between the two genomes (Figure 3B). Mt-DMI3 homologs have been identified in many land plants (including soybean, bean, apple tree, and tobacco) but no close homolog of Mt-DMI3 could be identified in Arabidopsis (Figure 3D). The absence of a Mt-DMI3 ortholog in Arabidopsis may explain one of the reasons why Arabidopsis cannot establish symbiosis with AM fungi.
In contrast to Mt-DMI1 and Mt-DMI3, Mt-DMI2 is less globally conserved in nonlegumes. However, the kinase domain of Mt-DMI2 (∼270 amino acids long) is highly conserved (∼80% identity) across wide taxonomic species such as rice (XP_478749), maize (BM501199), and wheat (TC255247) (Figure 3E), but their orthologous relationship cannot be inferred due to a lack of microsynteny. In fact, the putative rice ortholog, XP_478749, lacks an extracellular domain that corresponds to the N terminus of the legume DMI2 orthologs (∼325 amino acids long). There are no Arabidopsis genes that show comparable sequence identity with the kinase domain of Mt-DMI2. Therefore, it is likely that the lineage giving rise to Arabidopsis lacks a Mt-DMI2 ortholog. This conclusion is further supported by the observation that the Mt-DMI2 region is syntenic with at least four segments of the Arabidopsis genome, but no Mt-DMI2 homologs could be detected in the syntenic segments (Zhu et al. 2005).
The finding of common genes required for both AM and nodulation symbioses in legumes has led to the hypothesis that the nitrogen-fixing root nodule symbiosis may have co-opted part of the mechanisms from the more ancient AM symbiosis. If this hypothesis holds true, then the orthologs of these common signaling components are expected to be present and function in AM symbioses in nonlegumes. The fact that DMI1 and DMI3 are conserved between legumes and nonlegumes appears to support this hypothesis. However, the DMI2 homologs are less conserved in nonlegumes, suggesting that they may be dispensable for AM symbioses in nonlegumes. The recent finding that legume mutants of DMI2 orthologs are leaky with respect to Myc− phenotypes is consistent with this hypothesis (Morandi et al. 2005; Kistner et al. 2005). Alternatively, AM symbioses may manifest relaxed structural requirements, allowing diverse DMI2 extracellular domains to retain biological function. Reverse genetic tools such as targeted induced local lesions in genomes (TILLING) and RNA interference (RNAi) are needed to elucidate the function of the DMI genes in nonlegumes and to address the evolutionary relationship between AM and root nodule symbioses (Limpens et al. 2004; Vandenbosch and Stacey 2003).
Nod factor response factors:
Nod factors induce the expression of many host genes through the activation of Nod factor response factors. Two genes in M. truncatula, Mt-NSP1 and Mt-NSP2, function downstream of Mt-DMI3 and are essential for all known Nod factor-induced transcriptional responses. Both genes encode GRAS family transcription factors, consistent with their roles in directly regulating gene expression (Kalo et al. 2005; Smit et al. 2005). Likewise Lj-NIN (orthologous to Ps-SYM35) is required for nodulation in L. japonicus (Schauser et al. 1999; Borisov et al. 2003). Lj-NIN encodes a transmembrane protein with a potential nuclear localization signal and a predicted DNA-binding domain and may also mediate symbiotic gene expression.
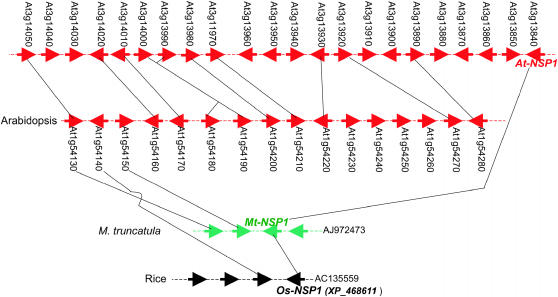
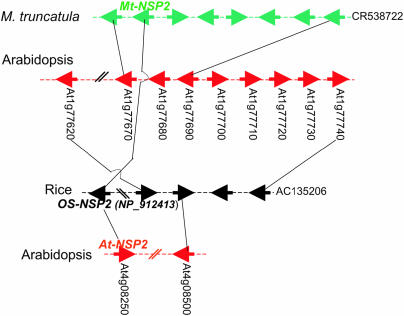
Mt-NSP1 and Mt-NSP2 both have putative orthologs in rice and Arabidopsis. Mt-NSP1 corresponds to At3g13840 in Arabidopsis and XP_468611 in rice; and Mt-NSP2 matches At4g08250 in Arabidopsis and NP_912413 in rice. Despite the high degeneracy of genome microstructure between species, the remnant of microsynteny can still be detected by constructing a synteny network across multiple species and the duplicated segments of Arabidopsis (Figure 4). For example, the syntenic relationship between Mt-NSP1 and the putative rice ortholog would not be detectable if we did not use the Arabidopsis duplicated regions as a bridge. This is also true for Mt-NSP2, for which the hidden microsynteny with rice and Arabidopsis can also be detected by the same strategy (Figure 5).
Figure 4.
Microsynteny of Mt-NSP1 orthologs in M. truncatula, rice, and Arabidopsis.
Figure 5.
Microsynteny of Mt-NSP2 orthologs in M. truncatula, rice, and Arabidopsis.
The evolution of Lj-NIN homologs in rice and Arabidopsis has been described (Schauser et al. 2005), but their orthologous relationship was not defined on the basis of their genomic organization across species. There are at least two additional NIN-like proteins in L. japonicus, Lj-NLP1 (AJ579910) and Lj-NLP2 (AJ579911). Lj-NIN and Lj-NLP1 are located in duplicated regions in L. japonicus, which are also syntenic to two M. truncatula homologous regions (CR936325 and CR931808), as well as to five Arabidopsis chromosomal segments surrounding five of a total of nine Arabidopsis homologs (i.e., At1g20640, At1g76350, At1g17150, At4g35270, and At4g38340) (data not shown). The Lj-NLP2 region, however, is syntenic with At2g43500 and At3g59580 regions. Two closest Lj-NIN homologs in rice, XP_470173 and BAD33743, are also located in the syntenic regions of Lj-NIN. The genomic organization of these homologous genes is consistent with the phylogenetic tree reported by Schauser et al. (2005), except for missing the gene, BAD33743, on rice chromosome 9. Segmental duplications have driven the evolution of the NIN-like genes in plants; and the duplications occurred both before and after speciation. Apparently, the function of the duplicated genes has been diverged, since mutation of one of the duplicated genes is sufficient to cause defective phenotypes in legume nodulation.
Genes regulating the root nodule number:
Legumes tightly regulate root nodule number to balance the cost of maintaining the symbiosis with their requirement for fixed nitrogen. This control is achieved by a mechanism known as autoregulation of nodulation (AON) whereby a shoot-derived signal exerts long-distance control of root nodule number. AON is regulated by a receptor-like kinase that has been isolated from soybean (Gm-NARK), L. japonicus (Lj-HAR1), M. truncatula (Mt-SUNN), and pea (Ps-SYM29) (Krusell et al. 2002; Nishimura et al. 2002b; Schnabel et al. 2003, 2005; Searle et al. 2003). All these genes are located in conserved regions that are also syntenic with at least seven duplicated segments in Arabidopsis (Zhu et al. 2005). The kinase is highly similar to the Arabidopsis CLAVATA1 (Clark et al. 1997) that controls stem cell proliferation in Arabidopsis but is located in a nonsyntenic region of the Arabidopsis CLAVATA1. Intriguingly, recent research has shown that a mutant of the Gm-NARK gene also reduces secondary infections by mycorrhizae, again implying overlap in the pathways mediating the two symbioses (Meixner et al. 2005). The recruitment of the CLAVATA1-like genes into the nodulation pathway can be due to gene duplication followed by change in gene expression, leading to tissue-specific expression and function in controlling the nodule number (Searle et al. 2003). It is likely that the ancestor of the CLAVATA1 gene may have regulated both stem cell proliferation and symbioses, and the descendent duplicated genes have evolved to partition the ancestral function by showing organ- and/or time-specific expression, one of which was to fit into the nodulation pathway.
A second regulatory system involves the plant hormone, ethylene. In M. truncatula, the sickle (skl) mutant exhibits, when compared with the wide-type plants, an ∼10-fold increase in numbers of root nodules (Penmetsa and Cook 1997; Penmetsa et al. 2003). The sickle mutant is also insensitive to ethylene. Mt-SKL encodes a component of the ethylene signaling pathway that fits into the legume nodulation pathway (R. V. Penmetsa and D. R. Cook, personal communications). It will be interesting to know if the same genes also regulate mycorrhizal infection.
Another example supporting that preexisting genes were recruited to control the nodule number is the gene ASTRAY in L. japonicus (Nishimura et al. 2002a). ASTRAY is a homolog of the Arabidopsis HY5, a transcription factor that regulates shoot photomorphogenesis and root architecture. In this case, however, ASTRAY has a legume-specific domain that is absent in HY5 but highly conserved in other legumes.
Conclusions:
The divergence of plant phenotypes that distinguish one species from another is still mysterious to plant biologists. From an evolutionary point of view, the unique property of legume symbiotic nitrogen fixation could result from a combination of the following mechanisms: (a) the symbiosis involves legume-specific genes that are lacking in nonlegumes and (b) genes required for other aspects of plant development have been recruited to function in a nodulation pathway. Comparative genome analyses reported in this article indicate that the putative orthologs of several genes known to be required for legume nodulation are present in nonlegumes, strongly arguing that the preexisting genes have been recruited to function in nitrogen-fixing root nodule symbioses (Gualtieri and Bisseling 2000; Szczyglowski and Amyot 2003). Such recruitment could be the result of divergence of orthologous gene function through change in gene regulation, family-specific gene amplification followed by subfunctionalization and selective gene loss, and acquisition of genes from other developmental processes into the nodulation pathway. Reverse genetic tools will be helpful to elucidate the function of putative nonlegume orthologs of legume genes required for nodulation and AM symbioses, which will advance our understanding of how gene functions and pathways have evolved to lead to species-specific phenotypes.
Acknowledgments
We thank Douglas R. Cook for his helpful comments on the manuscript. We are also grateful to Cécile Ané for her precious help in the phylogenetic analyses. This research is supported by a grant to H.Z. from the Kentucky Science and Engineering Foundation and by a grant to J.-M. A. from the Wisconsin Soybean Marketing Board. This article (05-06-132) is published with the approval of the Director of the Kentucky Agricultural Experiment Station.
References
- Albrecht, C., R. Geurts and T. Bisseling, 1999. Legume nodulation and mycorrhizae formation; two extremes in host specificity meet. EMBO J. 18: 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amor, B. B., S. L. Shaw, G. E. Oldroyd, F. Maillet, R. V. Penmetsa et al., 2003. The NFP locus of Medicago truncatula controls an early step of nod factor signal transduction upstream of a rapid calcium flux and root hair deformation. Plant J. 34: 495–506. [DOI] [PubMed] [Google Scholar]
- Ané, J. M., G. B. Kiss, B. K. Riely, R. V. Penmetsa, G. E. Oldroyd et al., 2004. Medicago truncatula DMI1 required for bacterial and fungal symbioses in legumes. Science 303: 1364–1367. [DOI] [PubMed] [Google Scholar]
- Bateman, A., and M. Bycroft, 2000. The structure of a LysM domain from E. coli membrane-bound lytic murein transglycosylase D (MltD). J. Mol. Biol. 299: 1113–1119. [DOI] [PubMed] [Google Scholar]
- Borisov, A. Y., L. H. Madsen, V. E. Tsyganov, Y. Umehara, V. A. Voroshilova et al., 2003. The Sym35 gene required for root nodule development in pea is an ortholog of nin from lotus japonicus. Plant Physiol. 131: 1009–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundrett, M. C., 2002. Coevolution of roots and mycorrhizas of land plants. New Phytol. 154: 275–304. [DOI] [PubMed] [Google Scholar]
- Catoira, R., C. Galera, F. de Billy, R. V. Penmetsa, E. P. Journet et al., 2000. Four genes of Medicago truncatula controlling components of a nod factor transduction pathway. Plant Cell 12: 1647–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenna, R., H. Sugawara, T. Koike, R. Lopez, T. J. Gibson et al., 2003. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 31: 3497–3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, S. E., R. W. Williams and E. M. Meyerowitz, 1997. The CLAVATA1 gene encodes a putative receptor kinase that controls shoot and floral meristem size in Arabidopsis. Cell 89: 575–585. [DOI] [PubMed] [Google Scholar]
- Cullimore, J., and J. Dénarié, 2003. Plant sciences. How legumes select their sweet talking symbionts. Science 302: 575–578. [DOI] [PubMed] [Google Scholar]
- Cullimore, J. V., R. Ranjeva and J. J. Bono, 2001. Perception of lipo-chitooligosaccharidic nod factors in legumes. Trends Plant Sci. 6: 24–30. [DOI] [PubMed] [Google Scholar]
- Dénarié, J., F. Debellé and J. C. Promé, 1996. Rhizobium lipo-chitooligosaccharide nodulation factors: signaling molecules mediating recognition and morphogenesis. Annu. Rev. Biochem. 65: 503–535. [DOI] [PubMed] [Google Scholar]
- Downie, J. A., and S. A. Walker, 1999. Plant responses to nodulation factors. Curr. Opin. Plant Biol. 2: 483–489. [DOI] [PubMed] [Google Scholar]
- Doyle, J. J., 1998. Phylogenetic perspectives on nodulation: an evolving view of plants and symbiotic bacteria. Trends Plant Sci. 3: 473–478. [Google Scholar]
- Doyle, J. J., and M. A. Luckow, 2003. The rest of the iceberg. Legume diversity and evolution in a phylogenetic context. Plant Physiol. 131: 900–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endre, G., A. Kereszt, Z. Kevei, S. Mihacea, P. Kalo et al., 2002. A receptor kinase gene regulating symbiotic nodule development. Nature 417: 962–966. [DOI] [PubMed] [Google Scholar]
- Genre, A., M. Chabaud, T. Timmers, P. Bonfante and D. G. Barker, 2005. Arbuscular mycorrhizal fungi elicit a novel intracellular apparatus in Medicago truncatula root epidermal cells before infection. Plant Cell 17: 3489–3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurts, R., and T. Bisseling, 2002. Rhizobium Nod factor perception and signalling. Plant Cell 14: 239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualtieri, G., and T. Bisseling, 2000. The evolution of nodulation. Plant Mol. Biol. 42: 181–194. [PubMed] [Google Scholar]
- Harrison, M. J., 2005. Signaling in the arbuscular mycorrhizal symbiosis. Annu. Rev. Microbiol. 59: 19–42. [DOI] [PubMed] [Google Scholar]
- Heckman, D. S., D. M. Geiser, B. R. Eidell, R. L. Stauffer, N. L. Kardos et al., 2001. Molecular evidence for the early colonization of land by fungi and plants. Science 293: 1129–1133. [DOI] [PubMed] [Google Scholar]
- Hirsch, A. M., M. R. Lum and J. A. Downie, 2001. What makes the rhizobia-legume symbiosis so special? Plant Physiol. 127: 1484–1492. [PMC free article] [PubMed] [Google Scholar]
- Imaizumi-Anraku, H., N. Takeda, M. Charpentier, J. Perry, H. Miwa et al., 2005. Plastid proteins crucial for symbiotic fungal and bacterial entry into plant roots. Nature 433: 527–531. [DOI] [PubMed] [Google Scholar]
- Kalo, P., C. Gleason, A. Edwards, J. Marsh, R. M. Mitra et al., 2005. Nodulation signaling in legumes requires NSP2, a member of the GRAS family of transcriptional regulators. Science 308: 1786–1789. [DOI] [PubMed] [Google Scholar]
- Kistner, C., and M. Parniske, 2002. Evolution of signal transduction in intracellular symbiosis. Trends Plant Sci. 7: 511–518. [DOI] [PubMed] [Google Scholar]
- Kistner, C., T. Winzer, A. Pitzschke, L. Mulder, S. Sato et al., 2005. Seven Lotus japonicus genes required for transcriptional reprogramming of the root during fungal and bacterial symbiosis. Plant Cell 17: 2217–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krusell, L., L. H. Madsen, S. Sato, G. Aubert, A. Genua et al., 2002. Shoot control of root development and nodulation is mediated by a receptor-like kinase. Nature 420: 422–426. [DOI] [PubMed] [Google Scholar]
- Lévy, J., C. Bres, R. Geurts, B. Chalhoub, O. Kulikova et al., 2004. A putative Ca2+ and calmodulin-dependent protein kinase required for bacterial and fungal symbioses. Science 303: 1361–1364. [DOI] [PubMed] [Google Scholar]
- Limpens, E., C. Franken, P. Smit, J. Willemse, T. Bisseling et al., 2003. LysM domain receptor kinases regulating rhizobial nod factor-induced infection. Science 302: 630–633. [DOI] [PubMed] [Google Scholar]
- Limpens, E., J. Ramos, C. Franken, V. Raz, B. Compaan et al., 2004. RNA interference in agrobacterium rhizogenes-transformed roots of Arabidopsis and Medicago truncatula. J. Exp. Bot. 55: 983–992. [DOI] [PubMed] [Google Scholar]
- Long, S. R., 1996. Rhizobium symbiosis: Nod factors in perspective. Plant Cell 8: 1885–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch, M., and J. S. Conery, 2000. The evolutionary fate and consequences of duplicate genes. Science 290: 1151–1155. [DOI] [PubMed] [Google Scholar]
- Madsen, E. B., L. H. Madsen, S. Radutoiu, M. Olbryt, M. Rakwalska et al., 2003. A receptor kinase gene of the LysM type is involved in legume perception of rhizobial signals. Nature 425: 637–640. [DOI] [PubMed] [Google Scholar]
- Meixner, C., J. Ludwig-Muller, O. Miersch, P. Gresshoff, C. Staehelin et al., 2005. Lack of mycorrhizal autoregulation and phytohormonal changes in the supernodulating soybean mutant nts1007. Planta 222: 709–715. [DOI] [PubMed] [Google Scholar]
- Mitra, R. M., C. A. Gleason, A. Edwards, J. Hadfield, J. A. Downie et al., 2004. A Ca2+ dependent protein kinase required for symbiotic nodule development: gene identification by transcript-based cloning. Proc. Natl. Acad. Sci. USA 101: 4701–4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morandi, D., E. Prado, M. Sagan and G. Duc, 2005. Characterisation of new symbiotic Medicago truncatula (Gaertn.) mutants, and phenotypic or genotypic complementary information on previously described mutants. Mycorrhiza 15: 283–289. [DOI] [PubMed] [Google Scholar]
- Nishimura, R., M. Ohmori and M. Kawaguchi, 2002. a The novel symbiotic phenotype of enhanced-nodulating mutant of lotus japonicus: Astray mutant is an early nodulating mutant with wider nodulation zone. Plant Cell Physiol. 43: 853–859. [DOI] [PubMed] [Google Scholar]
- Nishimura, R., M. Hayashi, G. J. Wu, H. Kouchi, H. Imaizumi-Anraku et al., 2002. b HAR1 mediates systemic regulation of symbiotic organ development. Nature 420: 426–429. [DOI] [PubMed] [Google Scholar]
- Oldroyd, G. E., and J. A. Downie, 2004. Calcium, kinases and nodulation signalling in legumes. Nat. Rev. Mol. Cell Biol. 5: 566–576. [DOI] [PubMed] [Google Scholar]
- Oldroyd, G. E., M. J. Harrison and M. Udvardi, 2005. Peace talks and trade deals. Keys to long-term harmony in legume-microbe symbioses. Plant Physiol. 137: 1205–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penmetsa, R. V., and D. R. Cook, 1997. A legume ethylene-insensitive mutant hyperinfected by its rhizobial symbiont. Science 275: 527–530. [DOI] [PubMed] [Google Scholar]
- Penmetsa, R. V., J. A. Frugoli, L. S. Smith, S. R. Long and D. R. Cook, 2003. Dual genetic pathways controlling nodule number in Medicago truncatula. Plant Physiol. 131: 998–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radutoiu, S., L. H. Madsen, E. B. Madsen, H. H. Felle, Y. Umehara et al., 2003. Plant recognition of symbiotic bacteria requires two LysM receptor-like kinases. Nature 425: 585–592. [DOI] [PubMed] [Google Scholar]
- Redecker, D., R. Kodner and L. E. Graham, 2000. Glomalean fungi from the Ordovician. Science 289: 1920–1921. [DOI] [PubMed] [Google Scholar]
- Remy, W., T. N. Taylor, H. Hass and H. Kerp, 1994. Four hundred-million-year-old vesicular arbuscular mycorrhizae. Proc. Natl. Acad. Sci. USA 91: 11841–11843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riely, B. K., J. M. Ané, R. V. Penmetsa and D. R. Cook, 2004. Genetic and genomic analyses in model legumes bring nod factor signaling to center stage. Curr. Opin. Plant Biol. 7: 408–413. [DOI] [PubMed] [Google Scholar]
- Schauser, L., A. Roussis, J. Stiller and J. Stougaard, 1999. A plant regulator controlling development of symbiotic root nodules. Nature 402: 191–195. [DOI] [PubMed] [Google Scholar]
- Schauser, L., W. Wieloch and J. Stougaard, 2005. Evolution of NIN-like proteins in Arabidopsis, rice, and lotus japonicus. J. Mol. Evol. 60: 229–237. [DOI] [PubMed] [Google Scholar]
- Schnabel, E., O. Kulikova, R. V. Penmetsa, T. Bisseling, D. R. Cook et al., 2003. An integrated physical, genetic and cytogenetic map around the sunn locus of Medicago truncatula. Genome 46: 665–672. [DOI] [PubMed] [Google Scholar]
- Schnabel, E., E. P. Journet, F. Carvalho-Niebel, G. Duc and F. Frugoli, 2005. The Medicago truncatula SUNN gene encoding a CLV1-like leucine-rich repeat receptor kinase regulates both nodule number and root length. Plant Mol. Biol. 58: 809–822. [DOI] [PubMed] [Google Scholar]
- Searle, I. R., A. E. Men, T. S. Laniya, D. M. Buzas, I. Iturbe-Ormaetxe et al., 2003. Long-distance signaling in nodulation directed by a CLAVATA1-like receptor kinase. Science 299: 109–112. [DOI] [PubMed] [Google Scholar]
- Shiu, S. H., W. M. Karlowski, R. Pan, Y. H. Tzeng, K. F. Mayer et al., 2004. Comparative analysis of the receptor-like kinase family in Arabidopsis and rice. Plant Cell 16: 1220–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit, P., J. Raedts, V. Portyanko, F. Debellé, C. Gough et al., 2005. NSP1 of the GRAS protein family is essential for rhizobial nod factor-induced transcription. Science 308: 1789–1791. [DOI] [PubMed] [Google Scholar]
- Soltis, P. S., D. E. Soltis and M. W. Chase, 1999. Angiosperm phylogeny inferred from multiple genes as a tool for comparative biology. Nature 402: 402–404. [DOI] [PubMed] [Google Scholar]
- Spaink, H. P., 2000. Root nodulation and infection factors produced by rhizobial bacteria. Annu. Rev. Microbiol. 54: 257–288. [DOI] [PubMed] [Google Scholar]
- Stracke, S., C. Kistner, S. Yoshida, L. Mulder, S. Sato et al., 2002. A plant receptor-like kinase required for both bacterial and fungal symbiosis. Nature 417: 959–962. [DOI] [PubMed] [Google Scholar]
- Swofford, D. L., 2003. PAUP*. Phylogenetic Analysis using Parsimony (*and Other Methods). Sinauer Associates, Sunderland, MA.
- Szczyglowski, K., and L. Amyot, 2003. Symbiosis, inventiveness by recruitment? Plant Physiol. 131: 935–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbosch, K., and G. Stacey, 2003. Summaries of legume genomics projects from around the globe. Community resources for crops and models. Plant Physiol. 131: 840–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, H., H. K. Choi, D. R. Cook and R. C. Shoemaker, 2005. Bridging model and crop legumes through comparative genomics. Plant Physiol. 137: 1189–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]