Abstract
TAC1, a Candida albicans transcription factor situated near the mating-type locus on chromosome 5, is necessary for the upregulation of the ABC-transporter genes CDR1 and CDR2, which mediate azole resistance. We showed previously the existence of both wild-type and hyperactive TAC1 alleles. Wild-type alleles mediate upregulation of CDR1 and CDR2 upon exposure to inducers such as fluphenazine, while hyperactive alleles result in constitutive high expression of CDR1 and CDR2. Here we recovered TAC1 alleles from two pairs of matched azole-susceptible (DSY294; FH1: heterozygous at mating-type locus) and azole-resistant isolates (DSY296; FH3: homozygous at mating-type locus). Two different TAC1 wild-type alleles were recovered from DSY294 (TAC1-3 and TAC1-4) while a single hyperactive allele (TAC1-5) was isolated from DSY296. A single amino acid (aa) difference between TAC1-4 and TAC1-5 (Asn977 to Asp or N977D) was observed in a region corresponding to the predicted activation domain of Tac1p. Two TAC1 alleles were recovered from FH1 (TAC1-6 and TAC1-7) and a single hyperactive allele (TAC1-7) was recovered from FH3. The N977D change was seen in TAC1-7 in addition to several other aa differences. The importance of N977D in conferring hyperactivity to TAC1 was confirmed by site-directed mutagenesis. Both hyperactive alleles TAC1-5 and TAC1-7 were codominant with wild-type alleles and conferred hyperactive phenotypes only when homozygous. The mechanisms by which hyperactive alleles become homozygous was addressed by comparative genome hybridization and single nucleotide polymorphism arrays and indicated that loss of TAC1 heterozygosity can occur by recombination between portions of chromosome 5 or by chromosome 5 duplication.
CANDIDA albicans is an opportunistic pathogen that causes oral and systemic infections in immunocompromised patients as well as vaginal infections in immunocompetent women. To prevent and treat Candida infections, immunocompromised patients are often treated for a long time with antifungal agents among which is the class of azoles. As azoles are fungistatic, rather than fungicidal, C. albicans cells repetitively exposed to these antifungals can adapt to the drug pressure and eventually become resistant to azoles. The most important mechanism of resistance to azoles is the overexpression of multidrug transporters, encoded by either the major facilitator efflux pump CaMDR1 (multidrug resistance 1) or the ABC transporters CDR1 (candida drug resistance) and CDR2. Upregulation of CaMDR1 confers resistance to fluconazole, while upregulation of CDR1 and CDR2 confers resistance to multiple azoles (itraconazole, fluconazole, voriconazole). Understanding the transcriptional control of these genes, by both cis- and trans-acting effectors, is therefore important for determining how azole resistance and transport mechanisms are regulated in C. albicans.
CaMDR1 expression is controlled by at least two regulatory promoter cis-acting regions as reported recently by Harry et al. (2005). Several elements of CDR genes are important for the regulation of CDR1 and CDR2. A basal response element (BRE) is located between nt −860 and −810 in the CDR1 promoter, and a drug response element (DRE) is present in the promoters of both CDR1 and CDR2 (de Micheli et al. 2002). The BRE regulates basal expression of CDR1 (de Micheli et al. 2002), while the DRE sequence (5′-CGGAA/TATCGGATA-3′) is crucial for the upregulation of these genes in azole-resistant strains as well as for the transient upregulation of both genes in the presence of different drugs such as oestradiol, progesterone, or fluphenazine in azole-susceptible strains. In addition, another BRE (located between −243 and −234) and a negative regulatory element (NRE) located within the −289 region have been reported in CDR1 (Puri et al. 1999; Gaur et al. 2004). Finally, in the same gene, Karnani et al. (2004) identified SRE1 and SRE2 (steroid response elements) between −696 and −521.
Trans-acting factors regulating CDR1 and CDR2 were reported recently. C. G. Chen et al. (2004) described a potential activator of CDR1 identified by screening of a C. albicans genomic library expressed in a Saccharomyces cerevisiae strain, which contained a CDR1 promoter/lacZ fusion. This factor, CaNDT80, is a homolog to a meiosis-specific transcription factor in S. cerevisiae (C. G. Chen et al. 2004). Deletion of CaNDT80 in C. albicans conferred hypersensitivity to azoles and decreased the inducible expression of CDR1. Recently, our laboratory discovered Tac1p (transcriptional activator of CDR), a transcription factor belonging to the family of zinc-finger proteins with a Zn2Cys6 motif (Coste et al. 2004). Tac1p binds to the DRE, which contains two CGG triplets typical of the DNA-binding sites of Zn2Cys6 transcription factors. Tac1p is responsible for transient upregulation of both CDR genes in azole-susceptible strains in the presence of inducers. Interestingly, TAC1 is located close to (within ∼14 kb) the mating-type-like (MTL) locus. Previous studies reported a strong correlation between homozygosity at the mating-type locus and azole resistance in a number of clinical isolates (Rustad et al. 2002). In our previous study, we showed that a clinical azole-resistant strain (DSY296) that is homozygous at the mating-type locus contains a TAC1 allele that is sufficient to confer fluconazole resistance to a laboratory strain lacking TAC1 (Coste et al. 2004). This type of allele was defined as “hyperactive” because it caused constitutive high expression of CDR1 and CDR2 in a tac1Δ/Δ mutant. In contrast, TAC1 alleles of the matched azole-susceptible clinical strain (DSY294) or of a laboratory strain (CAF2-1), which are strains heterozygous at the mating-type locus, were not able to confer azole resistance to a tac1Δ/Δ mutant. These alleles were defined as “wild-type” alleles. Using C. albicans microarrays, we also showed that Tac1p regulates the expression of at least three other genes: RTA3, IFU5, and HSP12 (Coste et al. 2004; Karababa et al. 2004). Northern blot analysis showed that Tac1p also regulates PDR16 (D. Sanglard, unpublished data), a gene shown to be overexpressed in azole-resistant strains upregulating CDR1 and CDR2 (De Deken and Raymond 2004). Interestingly, all four of these Tac1p-regulated genes contain a putative DRE in their promoters.
In S. cerevisiae, the functional homolog of CDR1 and CDR2, PDR5, is known to be regulated by at least two Zn2Cys6 transcription factors (PDR1 and PDR3). These transcription factors bind as homo- and heterodimers to a cis-acting pleiotropic drug responsive element containing two CGG triplets (Katzmann et al. 1994; Carvajal et al. 1997). Point mutations in PDR1 and PDR3 lead to increased PDR5 expression and drug resistance (Carvajal et al. 1997; Nourani et al. 1997; Anderson et al. 2003). Carvajal et al. (1997) found that a F815S mutation (F815S) in the putative activation domain of Pdr1p is responsible for strong constitutive PDR5 expression. Point mutations in other regions of PDR1 also affect the regulation of PDR5 expression, but have a more moderate effect than the F815S mutation.
In this study, we analyzed TAC1 alleles of matched azole-susceptible and azole-resistant clinical C. albicans isolates. We identified a single point mutation (N977D) in both hyperactive TAC1 alleles, which is sufficient to confer hyperactivity as measured by upregulation of CDR1 and CDR2 and levels of drug resistance. We also show that hyperactive alleles carrying this mutation are codominant with other wild-type alleles such that only strains homozygous for hyperactive alleles show high expression levels of CDR1 and CDR2. We also show that homozygosity at MTL accompanies the acquisition of TAC1 homozygosity via at least two mechanisms, but that MTL homozygosity does not contribute to the azole resistance phenotype.
MATERIALS AND METHODS
Strains and media:
The C. albicans strains used in this study are listed in Table 1. These strains were grown either in complete medium YEPD (1% Bacto peptone, Difco Laboratories, Basel, Switzerland), 0.5% yeast extract (Difco), and 2% glucose (Fluka, Buchs, Switzerland) or in minimal medium yeast nitrogen base (Difco) and 2% glucose (Fluka). When grown on solid media, 2% agar (Difco) was added to either of the media. Escherichia coli DH5α was used as a host for plasmid constructions and propagation. DH5α was grown in Luria–Bertani broth (LB) or on LB plates, supplemented with ampicillin (0.1 mg/ml) when required.
TABLE 1.
Strains used in this study
| Strain | Parental strain | Genotype | Reference |
|---|---|---|---|
| CAF2-1 | SC5314 | ura3Δ∷imm434/URA3 | Fonzi and Irwin (1993) |
| CAF4-2 | CAF2-1 | ura3Δ∷imm434/ura3Δ∷imm434 | Fonzi and Irwin (1993) |
| DSY2875 | CAF4-2 | tac1-1Δ∷hisG/TAC1-2 | Coste et al. (2004) |
| DSY2903 | DSY2875 | tac1-1Δ∷hisG/tac1-2Δ∷hisG-URA-hisG | Coste et al. (2004) |
| DSY2906 | DSY2903 | tac1-1Δ∷hisG/tac1-2Δ∷hisG | Coste et al. (2004) |
| DSY2937-35 | DSY2906 | tac1-1Δ∷hisG/tac1-2Δ∷hisG, LEU2∷TAC1 | Coste et al. (2004) |
| DSY2925-47 | DSY2906 | tac1-1Δ∷hisG/tac1-2Δ∷hisG, LEU2∷TAC1-3 | Coste et al. (2004) |
| DSY2925-18 | DSY2906 | tac1-1Δ∷hisG/tac1-2Δ∷hisG, LEU2∷TAC1-4 | Coste et al. (2004) |
| DSY2984 | DSY2906 | tac1-1Δ∷hisG/tac1-2Δ∷hisG, LEU2∷TAC1-5 | This study |
| VTY9 | DSY2906 | tac1-1Δ∷hisG/tac1-2Δ∷hisG, LEU2∷TAC1-5D977N | This study |
| VTY21 | DSY2906 | tac1-1Δ∷hisG/tac1-2Δ∷hisG, LEU2∷TAC1-1N977D | This study |
| VTY28 | DSY2906 | tac1-1Δ∷hisG/tac1-2Δ∷hisG, LEU2∷TAC1-4N977D | This study |
| DSY3010-80 | DSY2906 | tac1-1Δ∷hisG/tac1-2Δ∷hisG,LEU2∷TAC1-6 | This study |
| DSY3010-113 | DSY2906 | tac1-1Δ∷hisG/tac1-2Δ∷hisG,LEU2∷TAC1-7-FH1 | This study |
| DSY3013 | DSY2906 | tac1-1Δ∷hisG/tac1-2Δ∷hisG,LEU2∷TAC1-7-FH3 | This study |
| ACY11 | DSY2906 | tac1-1Δ∷hisG/tac1-2Δ∷hisG,LEU2∷TAC1-6N977D | This study |
| ACY12 | DSY2906 | tac1-1Δ∷hisG/tac1-2Δ∷hisG,LEU2∷TAC1-7D977N | This study |
| DSY294 | Azole-susceptible clinical strain (MTLa/MTLα) | Coste et al. (2004) | |
| DSY3040 | DSY294 | ura3Δ∷FRT/ura3Δ∷FRT | This study |
| DSY3058 | DSY3040 | TAC1-4/tac1-3Δ∷hisG-URA3-hisG | This study |
| DSY3075 | DSY3058 | TAC1-4/tac1-3Δ∷hisG | This study |
| DSY3082 | DSY3075 | tac1-3Δ∷hisG/tac1-4Δ∷hisG-URA3-hisG | This study |
| DSY3089 | DSY3082 | tac1-3Δ∷hisG/tac1-4Δ∷hisG | This study |
| DSY3102-2 | DSY3089 | tac1-3Δ∷hisG/tac1-4Δ∷hisG, LEU2∷TAC1-5 | This study |
| DSY3287-1 | DSY3089 | tac1-3Δ∷hisG/tac1-4Δ∷hisG, LEU2∷TAC1-3 | This study |
| DSY 3288-3 | DSY3089 | tac1-3Δ∷hisG/tac1-4Δ∷hisG, LEU2∷TAC1-4 | This study |
| DSY3053-1 | DSY3040 | TAC1-4/tac1-3Δ∷hisG-URA3-hisG | This study |
| DSY3168-1 | DSY3053-1 | TAC1-4/tac1-3Δ∷hisG | This study |
| DSY3219-2 | DSY3168 | TAC1-4/tac1-3Δ∷hisG, tac1∷TAC1-5 | This study |
| DSY3220-1 | DSY3168 | TAC1-4/tac1-3Δ∷hisG, tac1∷TAC1-3 | This study |
| DSY3221-3 | DSY3168 | TAC1-4/tac1-3Δ∷hisG, tac1∷TAC1-4 | This study |
| DSY3053-2 | DSY3040 | TAC1-3/tac1-4Δ∷hisG-URA3-hisG | This study |
| DSY3168-2 | DSY3053-2 | TAC1-3/tac1-4Δ∷hisG | This study |
| DSY3222-2 | DSY3168-2 | TAC1-3/tac1-4Δ∷hisG, tac1∷TAC1-5 | This study |
| DSY3223-1 | DSY3168-2 | TAC1-3/tac1-4Δ∷hisG, tac1∷TAC1-3 | This study |
| DSY3224-1 | DSY3168-2 | TAC1-3/tac1-4Δ∷hisG, tac1∷TAC1-4 | This study |
| DSY296 | DSY294 | Azole-resistant clinical strain (MTLα/MTLα) | Coste et al. (2004) |
| DSY3041 | DSY296 | ura3Δ∷FRT/ura3Δ∷FRT | This study |
| DSY3059 | DSY3041 | TAC1-5/tac1-5Δ∷hisG-URA3-hisG | This study |
| DSY3076 | DSY3059 | TAC1-5/tac1-5Δ∷hisG | This study |
| DSY3210-1 | DSY3076 | TAC1-5/tac1-5Δ∷hisG, tac1∷TAC1-5 | This study |
| DSY3211-4 | DSY3076 | TAC1-5/tac1-5Δ∷hisG, tac1∷TAC1-3 | This study |
| DSY3215-1 | DSY3076 | TAC1-5/tac1-5Δ∷hisG, tac1∷TAC1-4 | This study |
| DSY3083 | DSY3076 | tac1-5Δ∷hisG/tac1-5Δ∷hisG-URA3-hisG | This study |
| DSY3090-8 | DSY3083 | tac1-5Δ∷hisG/tac1-5Δ∷hisG | This study |
| DSY3284-1 | DSY3090-8 | tac1-5Δ∷hisG/tac1-5Δ∷hisG, LEU2∷TAC1-5 | This study |
| DSY3285-1 | DSY3090-8 | tac1-5Δ∷hisG/tac1-5Δ∷hisG, LEU2∷TAC1-3 | This study |
| DSY3286-2 | DSY3090-8 | tac1-5Δ∷hisG/tac1-5Δ∷hisG, LEU2∷TAC1-4 | This study |
| FH1 | Azole-susceptible clinical strain (MTLa/MTLα) | Marr et al. (1997) | |
| FH3 | FH1 | Azole-resistant clinical strain (MTLα/MTLα) | Marr et al. (1997) |
| DSY3132-14 | FH1 | TAC1-7/tac1-6Δ∷FRT | This study |
| DSY3132-11 | FH1 | TAC1-6/tac1-7Δ∷FRT | This study |
| DSY3133-15 | FH3 | TAC1-7/tac1-7Δ∷FRT | This study |
| DSY3157-2 | FH1 | TAC1-6/TAC1-7/TAC1-7 | This study |
| DSY3301-4 | DSY3157-2 | TAC1-7/TAC1-7/TAC1-7 | This study |
Yeast transformation:
C. albicans cells from 0.2 ml stationary-phase culture were resuspended in 0.1 ml of a solution containing 200 mm lithium acetate (pH 7.5), 40% (w/v) PEG 8000, 15 mg/ml DTT, and 250 μg/ml denatured salmon sperm DNA. Transforming DNA (1–5 μg) was added to the yeast suspension, which was incubated for 60 min at 43.5°. Transformation mixtures were plated directly onto selective plates. For transformation involving the dominant marker SAT1, the transformation mixture was incubated at room temperature overnight in 1 ml YEPD and plated the day after on YEPD agar plates containing 200 μg/ml of nourseothricin (Werner Bioagent, Jena, Germany).
Drug susceptibility testing:
Drug susceptibility testing was performed by spotting cells onto solid agar plates containing the tested drugs. Yeast cultures were grown overnight in YEPD and diluted to a density of 1.5 × 107 cells/ml and serial 10-fold dilutions were performed to a final dilution step containing 1.5 × 103 cells/ml. Four microliters of each dilution were spotted onto YEPD plates with or without drugs. Plates were incubated for 48 hr at 35°.
Drug susceptibility testing was also performed in microtiter plates with twofold serial dilutions of fluconazole (range is from 128 to 0.06 μg/ml) or terbinafine (range is from 32 to 0.015 μg/ml). Yeast cultures were grown overnight in YEPD and inoculated at a density of 104 cells/ml in a total volume of 200 μl containing the serial dilution of fluconazole or terbinafine. Microtiter plates were incubated at 35° during 48 hr and optical densities read with a microtiter plate reader at a wavelength of 540 nm. The minimal inhibitory concentration (MIC) was determined as the drug concentration required to decrease the optical density of the drug-free culture by at least 50%.
Efflux of rhodamine 6G:
To measure the drug efflux capacity of C. albicans strains with specific TAC1 alleles and CDR1/CDR2 expression, rhodamine 6G (R6G) efflux was measured by fluorescence assays with whole cells. C. albicans cultures grown overnight in YEPD were diluted in 5 ml YEPD and allowed to grow at 30° under constant agitation until a density of 2 × 107 cells/ml was obtained. Cells were centrifuged, washed with 5 ml PBS (pH 7), and resuspended in 2 ml PBS. The cells were incubated for 1 hr at 30° under constant agitation in PBS to energy deprive cells. R6G was next added at a concentration of 10 μg/ml and the incubation was continued for 1 hr, thus facilitating R6G accumulation. After this incubation time, cells were sedimented by centrifugation, washed with PBS at 4°, and resuspended in a final volume of 200 μl PBS. Fifty microliters of individual strains were diluted in 150 μl PBS and aliquoted in a 96-well microtiter plate, which was placed in a SpectraMax Gemini fluorimeter with temperature control set at 30°. Baseline emission of fluorescence (excitation wavelength: 344 nm; emission wavelength: 555 nm) was recorded as relative fluorescence units (RFU) for 5 min and glucose (1% final concentration) was next added to each strain to initiate R6G efflux. As a negative control, no glucose was added to separate aliquots of each strain. Data points were recorded in duplicate for 60 min at 1-min intervals.
Immunoblots:
C. albicans cell extracts for immunoblotting were prepared by an alkaline extraction procedure from cells grown to midlog phase. Briefly, cells (5 OD540nm) were resuspended in an Eppendorf tube with 500 μl water and 150 μl of a solution containing 1.85 m NaOH and 7.5% β-mercaptoethanol. This mixture was incubated on ice for 10 min. Proteins were next precipitated with 150 μl of a 50% trichloroacidic acid solution and the suspension was left on ice for another 10 min. Precipitated proteins were sedimented by a centrifugation step at maximal speed in a microfuge for 15 min. The sediment was resuspended in 50 μl of loading buffer (40 mm Tris–HCl pH 6.8, 8 m urea, 5% SDS, 0.1 m EDTA, 1% β-mercaptoethanol, and 0.1 mg/ml bromophenol blue) and incubated at 37° for 10 min. Nonsolubilized material was cleared by a centrifugation step for 10 min. Ten microliters of solubilized yeast proteins were separated by 10% SDS–PAGE and transferred by Western blot on a nitrocellulose membrane. The membrane was stained by Ponceau reagent (0.25% Ponceau S in 40% methanol and 15% acetic acid) for 5 min to verify that protein extracts were evenly transferred. Immunodetection of Cdr1p and Cdr2p was performed with rabbit polyclonal anti-Cdr1p and anti-Cdr2p antibodies as described previously (de Micheli et al. 2002) by chemoluminescence with an ECL kit according to the recommendations of the manufacturer (Amersham Biosciences, Otelfingen, Switzerland).
Construction of gene disruption cassettes:
Four different TAC1 disruption cassettes were designed in this study. Three cassettes—C333, C357, and C343 in plasmids pDS1052, pDS1142, and pDS1102—were designed using the “Ura”-blaster system. C333 and C357 bear the deletion of a small portion of 271 bp between nt +1153 and +1424 with respect to the first ATG codon of TAC1. C343 was designed to delete a larger region of 1931 bp between nt +502 and +2433. C358 carried by the plasmid pDS1196 integrates the SAT1-flipper system (Reuss et al. 2004), in which a region of 1924 bp was deleted between nt +501 and +2425.
To construct these different deletion cassettes, the entire TAC1 ORF was first amplified from genomic DNA using the cloning primers CaZNC2–BamHI and CaZNC2–Xho (see supplemental Table S3 at http://www.genetics.org/supplemental/). For the construction of cassettes C333 and C343, TAC1 was amplified from the genomic DNA of CAF2-1 but for the construction of the cassette C357, TAC1 was amplified with genomic DNA from DSY2875 to specifically amplify the TAC1-2 allele. PCR fragments were cloned into pBluescript KS+ to yield pDS1048 and pDS1138 (supplemental Table S2 at http://www.genetics.org/supplemental/). For disruption with cassettes C333 and C357, pDS1048 and pDS1138 were digested with PstI and BglII and the 3.7-kb PstI–BglII fragment containing the “Ura”-blaster cassette from pMB7 was cloned into compatible sites to yield pDS1052 and pDS1142, respectively. For disruption with cassette C343, a deletion was created from pDS1048 using primers Znc2–BG2 and Znc2–PST (supplemental Table S3 at http://www.genetics.org/supplemental/). The obtained PCR fragment was digested with PstI and BglII and the 3.7-kb PstI–BglII fragment from pMB7 was inserted to obtain pDS1102 (supplemental Table S2 at http://www.genetics.org/supplemental/). For transformation in C. albicans, linear fragments were obtained by digestion of the plasmids with NsiI and XmnI, thus liberating cassettes C333 and C357, and by ApaI and SacI, liberating C343. For the cassette C358, TAC1 was amplified from CAF2-1 genomic DNA. The obtained PCR fragment was cloned into the pMTL21 (Chambers et al. 1998) to yield pDS1141. A deletion was created using pDS1141 as template with the primers Znc2–Apa and Znc2–SacII. Next, the ApaI–SacII fragment of pSFS2 comprising the SAT1-flipper cassette was cloned into the previously ApaI- and SacII-digested PCR fragment to obtain pDS1196. For the transformation in C. albicans, a linear fragment was obtained by digestion of the plasmid with SacI and SphI, thus liberating the C358 cassette. C. albicans strains DSY3053-1 and DSY3053-2 were obtained using the C357 disruption cassette. DSY3058 and DSY3059 were obtained using the C343 cassette. DSY3082, DSY3083, DSY2875, and DSY2903 were obtained using the C333 cassette. DSY3132-11, DSY3132-14, and DSY3133-15 were obtained using the C358 cassette.
To obtain ura3 mutants of the clinical isolates DSY294 and DSY296, the two URA3 alleles were deleted using the SAT1-flipping strategy (Reuss et al. 2004). For this purpose, the SAT1-flipper cassette was substituted for the MPAR-flipper cassette in the previously described plasmid pSFIU4 (Strauss et al. 2001) to result in pSFSU1, in which the SAT1 flipper is flanked by URA3 upstream and downstream sequences. The insert from this plasmid was then used to inactivate URA3 in the clinical C. albicans isolates by two rounds of targeted integration and subsequent FLP-mediated excision of the SAT1-flipper cassette, generating strains DSY3040 and DSY3041. Transformations were performed by electroporation and selection of nourseothricin-resistant transformants was performed as described (Reuss et al. 2004).
Construction of revertant strains:
The revertant strains from each homozygous mutant generated in this study were obtained by transformation of C. albicans ura3 derivatives with the pRC2312-derived plasmid pDS178 containing the URA3 and LEU2 markers as described previously (de Micheli et al. 2002). To generate revertants from tac1Δ/Δ mutant strains, TAC1 ORFs flanked by 500 bp were amplified from genomic DNA of strains SC5314, DSY294, DSY296, and FH1 and FH3 with primers Znc2-5–BamB and Znc2-3–Xho (supplemental Table S3 at http://www.genetics.org/supplemental) and inserted into pDS178 previously digested by BamHI and XhoI to yield pDS1097 (containing the TAC1-1 allele), pDS1098-1 and pDS1098-9 (containing the TAC1-4 and TAC1-3 alleles), pDS1099 (containing the TAC1-5 allele), pDS1045 (containing the TAC1-6 allele), and pDS1048 (containing the TAC1-7 allele), respectively. For each amplified allele, TAC1 was sequenced from several plasmids to rule out PCR artifacts. These plasmids either were linearized by SalI and transformed into C. albicans DSY2906, DSY3089, and DSY3090, allowing integration into the genomic LEU2 locus, or were linearized by BstBI, allowing integration in the TAC1 locus of strains DSY3168-1, DSY3168-2, and DSY3076. For each reintegration of TAC1 alleles, several independent transformants (approximately four to five) were tested for phenotypes (susceptibility assays, immunodetection of Cdr1p and Cdr2p) and correct integration. The transformants containing identical TAC1 alleles generally had the same phenotypes, but only a single revertant for individual alleles was selected and presented in this study. Integration at the TAC1 locus for strains DSY2906, DSY3089, and DSY3090 was also performed and yielded phenotypes comparable to those obtained by integration at the LEU2 locus (data not shown).
Southern blots:
Southern blots were performed as described previously (Sanglard et al. 1995). Radioactive signals were revealed by exposure to Kodak BioMax MR films (Amersham Biosciences, Otelfingen, Switzerland). Signals obtained in blotted membranes were quantified by counting of radioactivity with the help of an Instant Imager (Perkin-Elmer, Rotkreuz, Switzerland).
Site-directed mutagenesis:
For site-directed mutagenesis of TAC1-1, TAC1-4, and TAC1-5, the previously cloned alleles were amplified from pDS1097, pDS1098-1, and pDS1099, respectively, using the forward primer Zn2-5BAMB (Table S3) and with the reverse primers TAC1-Asn-Asp-977 (primer for pDS1097 and pDS1098-1) and TAC1-Asp-Asn-977 (primer for pDS1099). These primers introduce a modification of codon 977 from Asn to Asp in TAC1-1 and TAC1-4 and from Asp to Asn in TAC1-5. Amplified products were cloned in the pDS178 backbone yielding pVT21, pVT28, and pVT9 containing the mutated TAC1-1N977D, TAC1-4N977D, and TAC1-5D977N alleles, respectively. These plasmids were linearized by SalI and transformed into C. albicans DSY2906 as described above.
Single nucleotide polymorphism and comparative genomic hybridization:
Single nucleotide polymorphism (SNP) microarray hybridization and comparative genomic hybridization (CGH) were performed as described previously (Forche et al. 2005; Selmecki et al. 2005). Additional SNP markers were designed from specific regions of chromosome 5 using C. albicans genome data deposited at http://candida.bri.nrc.ca/candida/alignments/index.cfm?chr=5. The search for SNP markers not present on the microarrays was performed by amplification of specific regions of chromosome 5 from genomic DNA using different V5 and V3 primer pairs (see supplemental Table S3 at http://www.genetics.org/supplemental/) followed by sequencing the PCR products using a AB Prism, 3130 genetic analyzer (AB Applied Biosystems). Sequences were analyzed for polymorphisms using the Contig Express software (InforMax).
RESULTS
Analysis of TAC1 alleles isolated from azole-susceptible and azole-resistant C. albicans strains:
To analyze the different TAC1 alleles present in either azole-susceptible or azole-resistant strains, two sets of clinical strains were first chosen. A first set of matched strains reported previously (Sanglard et al. 1995, 1998) and consisting of the azole-susceptible strain DSY294 (also known as C43) and the azole-resistant strain DSY296 (also known as C56) originated from an HIV-positive patient with oropharyngeal candidiasis who was treated with fluconazole. A second set of strains described by Marr et al. (1998, 2001) consisted of azole-susceptible strains FH1 and FH2 and of strains FH3–FH8, which developed azole resistance. These strains were isolated from a bone marrow transplant patient suffering from invasive candidiasis and treated with fluconazole. FH1 and FH2 are heterozygous at the mating-type locus (Rustad et al. 2002). FH3–FH8 are homozygous at the mating-type locus (Rustad et al. 2002). TAC1 alleles of strains DSY294, DSY296, and FH1–FH8 were cloned and introduced in a tac1Δ/Δ mutant (strain DSY2906) derived from SC5314. The azole resistance phenotypes of the transformants were analyzed by drug susceptibility assays and immunoblotting detection of Cdr1p and Cdr2p.
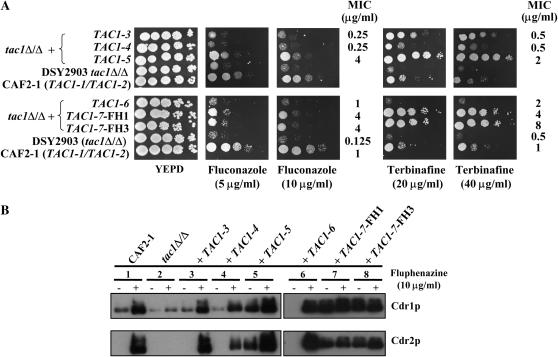
Sequencing of individual TAC1 alleles from DSY294 revealed two distinct alleles, TAC1-3 and TAC1-4 (Table 2). Both of these alleles had the properties of wild-type alleles when reintroduced in a tac1Δ/Δ mutant. First, they did not confer resistance to terbinafine or fluconazole (Figure 1A). Second, they did not result in constitutive high levels of Cdr1p or Cdr2p expression under normal growth conditions; rather, Cdr1p was detected at basal levels and Cdr2p could not be detected (Figure 1B, lanes 3 and 4). The Cdr1p and Cdr2p levels were comparable to those found in CAF2-1 and in the tac1Δ/Δ mutant (Figure 1B, lanes 1–4). However, Cdrp1 and Cdr2p were still inducible as in CAF2-1, since exposure of TAC1-3 or TAC1-4 revertant strains to fluphenazine led to high Cdr1p and Cdr2p levels (Figure 1B, lanes 1, 3, and 4). The identification of two distinct TAC1 alleles in DSY294 is consistent with the heterozygosity of DSY294 at the mating-type locus, given that TAC1 is located at a distance of ∼14 kb from this locus. In contrast, only a single TAC1 allele (TAC1-5, Table 2) was recovered from the matched azole-resistant strain DSY296. The isolation of a single TAC1 allele from DSY296 is consistent with homozygosity of this strain at the mating-type locus and suggests that a region >14 kb underwent loss of heterozygosity (LOH) during the acquisition of drug resistance.
TABLE 2.
TAC1 alleles present in the strains used in this study
| Polymorphism of TAC1 alleles (position of the nonsynonymous codons)
|
|||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain | Name of allele | Type of allele | 47 | 104 | 131 | 170 | 189 | 199 | 206 | 207 | 377 | 396 | 558 | 772 | 776 | 829 | 869 | 904 | 935 | 937 | 941 | 944 | 977 |
| CAF2-1 | TAC1-1 orf19.3188 (contig10-10170) | Wild type | TTA (L) | TTT (F) | CTA (L) | ATG (M) | TTT (F) | AGT (S) | CGT (R) | GTT (V) | GCT (A) | AAC (N) | ATT (I) | AAT (N) | GAC (D) | GAA (E) | CGA (R) | GAG (E) | TCA (S) | TCG (S) | CTG (S) | AAT (N) | AAT (N) |
| TAC1-2a orf19.10700 (contig10-20170) | Wild type | AAA (K) | GTC (V) | CTA (L) | ATG (M) | TTT (F) | AAT (N) | CAC (H) | GCT (A) | GCT (A) | AGC (S) | ATT (I) | AAA (K) | AAC (N) | CAA (Q) | CGA (R) | GAG (E) | TTA (L) | TCG (S) | CCG (P) | AAT (N) | AAT (N) | |
| DSY294 | TAC1-3 | Wild type | AAA (K) | TTT (F) | ATA (I) | ATG (M) | TCT (S) | AAT (N) | CAC (H) | GCT (A) | GCT (A) | AGC (S) | GTT (V) | AAA (K) | AAC (N) | GAA (E) | CGA (R) | GAG (E) | TCA (S) | TCG (S) | CTG (S) | AAT (N) | AAT (N) |
| TAC1-4 | Wild type | AAA (K) | TTT (F) | CTA (L) | GTG (V) | TTT (F) | AAT (N) | CAC (H) | GCT (A) | GTT (V) | AGC (S) | GTT (V) | AAA (K) | AAC (N) | CAA (Q) | CAA (Q) | GAG (E) | TCA (S) | TCG (S) | CTG (S) | AAT (N) | AAT (N) | |
| DSY296 | TAC1-5 | Hyperactive | AAA (K) | TTT (F) | CTA (L) | GTG (V) | TTT (F) | AAT (N) | CAC (H) | GCT (A) | GTT (V) | AGC (S) | GTT (V) | AAA (K) | AAC (N) | CAA (Q) | CAA (Q) | GAG (E) | TCA (S) | TCG (S) | CTG (S) | AAT (N) | GAT (D) |
| FH1 and FH2 | TAC1-6 | Wild type | AAA (K) | TTT (F) | ATA (I) | ATG (M) | TCT (S) | AAT (N) | CAC (H) | GCT (A) | GCT (A) | AGC (S) | GTT (V) | AAA (K) | AAC (N) | GAA (E) | CGA (R) | GGG (G) | TCA (S) | TTG (L) | CTG (S) | TAT (Y) | AAT (N) |
| TAC1-7 | Hyperactive | AAA (K) | TTT (F) | CTA (L) | ATG (M) | TTT (F) | AAT (N) | CAC (H) | GCT (A) | GCT (A) | AGC (S) | ATT (I) | AAT (N) | AAC (N) | GAA (E) | CGA (R) | GAG (E) | TCA (S) | TCG (S) | CCG (P) | AAT (N) | GAT (D) | |
| FH3 | TAC1-7 | Hyperactive | AAA (K) | TTT (F) | CTA (L) | ATG (M) | TTT (F) | AAT (N) | CAC (H) | GCT (A) | GCT (A) | AGC (S) | ATT (I) | AAT (N) | AAC (N) | GAA (E) | CGA (R) | GAG (E) | TCA (S) | TCG (S) | CCG (P) | AAT (N) | GAT (D) |
This allele is not corrected in CGD and contains a stop codon in the ORF.
Figure 1.
Analysis of drug resistance properties dependent on TAC1 alleles. (A) Drug susceptibility testing of C. albicans tac1Δ/Δ mutant and TAC1 revertant strains with different TAC1 alleles. Drug susceptibility assays were carried out by plating serial dilutions of overnight cultures onto YEPD agar plates containing different drugs as indicated. Plates were incubated for 48 hr at 35°. MIC assays were performed as described in materials and methods. (B) Immunodetection of Cdr1p and Cdr2p in tac1Δ/Δ mutant and TAC1 revertant strains with different TAC1 alleles. Protein extracts of each strain were separated on SDS-10% polyacrylamide gels and immunoblotted with rabbit polyclonal anti-Cdr1p and anti-Cdr2p as described previously (de Micheli et al. 2002). C. albicans strains were grown in liquid YEPD to midlog phase and exposed (+) or not (−) to fluphenazine (10 μg/ml) for 20 min. The following Ura+ strains correspond to the following genotypes: CAF2-1, TAC1-1/TAC1-2; DSY2903, tac1-1Δ/tac1-2Δ; DSY2925-47, tac1Δ/Δ + TAC1-3; DSY2925-18, tac1Δ/Δ + TAC1-4; DSY2984, tac1Δ/Δ + TAC1-5; DSY3010-80, tac1Δ/Δ + TAC1-6; DSY3010-113, tac1Δ/Δ + TAC1-7-FH1; DSY3013, tac1Δ/Δ + TAC1-7-FH3. For phenotypes and genotypes of the different strains of this study refer to supplemental Table S1 at http://www.genetics.org/supplemental/.
The TAC1-5 allele was considered to be a hyperactive allele. First, when reintroduced into a tac1Δ/Δ mutant, it conferred higher terbinafine resistance than that observed in strains containing the wild-type TAC1-3 and TAC1-4 alleles (Figure 1A). Second, it conferred constitutively high levels of Cdr1p and Cdr2p (Figure 1B, lane 5), which are comparable to levels seen in strains carrying wild-type alleles and exposed to fluphenazine (Figure 1B, lanes 1, 3, and 4).
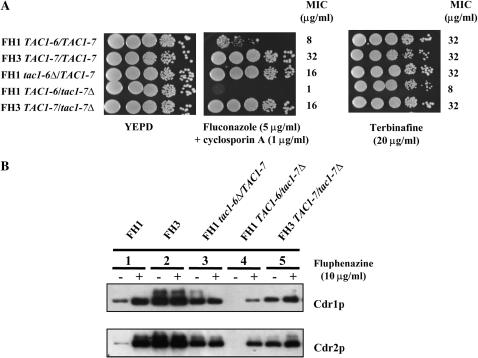
Similar analyses of TAC1 alleles were performed in FH1–FH8 strains. Two different TAC1 alleles were isolated from FH1 and FH2 (Table 2). One allele, TAC1-6, was wild type, and the other, TAC1-7, was hyperactive as defined above for TAC1 alleles of DSY294 and DSY296. TAC1-6 does not confer terbinafine resistance (Figure 1A) and does not express high levels of Cdr1p and Cdr2p under normal growth conditions (Figure 1B, lane 6). In contrast, TAC1-7 conferred terbinafine resistance (Figure 1A) and expressed constitutive high levels of Cdr1p and Cdr2p (Figure 1B, lane 7). When strains FH3–FH8 were screened for TAC1 alleles, all attempts to recover alleles different from TAC1-7 were unsuccessful. These results indicate that strains FH3–FH8 are homozygous for the TAC1-7 allele. Consistent with this, these strains were also homozygous at the MTL, suggesting that they underwent an LOH event encompassing >14 kb of chromosome 5.
Taken together, the analysis of TAC1 alleles from clinical strains identified five new alleles in addition to the TAC1-1 and TAC1-2 previously characterized from strain CAF2-1. Nucleotide polymorphisms in all these alleles are presented in Table 2. Because of the high diversity of TAC1 alleles, their nomenclature was modified (Table 2) as compared to results of our previous study (Coste et al. 2004). Among the five new alleles, three were defined as wild type and two as hyperactive. Furthermore, the two hyperactive alleles were the only alleles that were homozygous in two independent clinical, azole-resistant strains, a feature consistent with the linked homozygosity at the mating-type locus.
TAC1 alleles can be hyperactive through a mutation in the C-terminal domain:
TAC1 sequences were aligned to identify differences between wild-type and hyperactive alleles. Wild-type TAC1-4 and hyperactive TAC1-5 alleles differed by only one base in codon 977 (Table 2). This nonsynonymous point mutation changes asparagine (N) in TAC1-4 to aspartatic acid (D) in TAC1-5. Furthermore, none of the wild-type alleles contain this point mutation. Importantly, the TAC1-7 hyperactive allele contains the same nucleotide change that yields a N977D mutation in Tac1p. These results strongly suggest a relationship between the presence of the N977D mutation and the hyperactivity of TAC1-5 and TAC1-7. Nineteen other codons with nonsynonymous polymorphisms were detected among the seven allele sequences compared in this study (Table 2). Since these were present in both wild-type and hyperactive alleles, they were not associated with azole resistance.
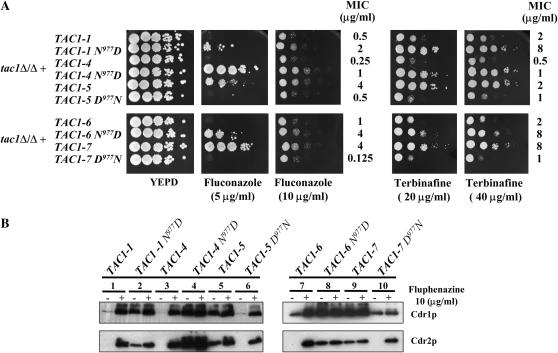
To test the role of the N977D mutation in TAC1 hyperactivity, Asp977 was introduced by site-directed mutagenesis into wild-type alleles TAC1-1, TAC1-4, and TAC1-6. The complementary experiment, replacing Asp977 with Asn977 in the hyperactive alleles TAC1-5 and TAC1-7, was also performed. The new alleles TAC1-1N977D, TAC1-4N977D, TAC1-6N977D, TAC1-5D977N, and TAC1-7D977N were then introduced into a tac1Δ/Δ mutant strain and the drug resistance phenotypes, as well as the Cdr1p and Cdr2p levels, were measured in cells with or without exposure to fluphenazine. The presence of Asp977 in the modified alleles TAC1-1N977D, TAC1-4N977D, and TAC1-6N977D increased resistance to fluconazole and terbinafine relative to the corresponding wild-type alleles (Figure 2A). Moreover, the presence of Asp977 in the modified alleles resulted in constitutive high levels of Cdr1p and Cdr2p (Figure 2B, lanes 2, 4, and 8). In contrast, expression of the corresponding wild-type alleles resulted in basal levels of Cdr1p. High levels of Cdr1p and Cdr2p expression were seen in strains carrying the wild-type alleles only upon fluphenazine exposure (Figure 2B, lanes 1, 3, and 7). When Asp977 was replaced by Asn977 in the hyperactive alleles (modified alleles TAC1-5D977N and TAC1-7D977N), the ability to grow on plates containing antifungal agents and the constitutive high levels of Cdr1p and Cdr2p were not maintained (Figure 2). Modified alleles TAC1-5D977N and TAC1-7D977N (Figure 2B, lanes 6 and 10) did not mediate constitutive high levels of Cdr1p and Cdr2p as compared to the nonmodified alleles TAC1-5 and TAC1-7 (Figure 2B, lanes 5 and 9). The phenotypes obtained by the strains carrying these modified hyperactive alleles were similar to those obtained with strains containing the wild-type alleles TAC1-1, TAC1-4, and TAC1-6 (Figure 2B, lanes 1, 3, and 7). Taken together, these analyses demonstrate that a single point mutation, the replacement of Asn by Asp at position 977 of Tac1p, is sufficient to modify the activity of the transcription factor into a hyperactive state.
Figure 2.
Effect of the mutation N977D on the properties of TAC1. (A) Drug susceptibility testing of C. albicans tac1Δ/Δ mutant and TAC1 revertant strains with different TAC1 alleles containing the N977D substitution. Drug susceptibility assays were carried out as described in Figure 1. MIC assays were performed as described in materials and methods. (B) Immunodetection of Cdr1p and Cdr2p in C. albicans tac1Δ/Δ mutant and TAC1 revertant strains containing different TAC1 alleles with the N977D substitution. See legend of Figure 1B for other details. The following strains correspond to the following genotypes: DSY2937-35, tac1Δ/Δ + TAC1-1; VTY21, tac1Δ/Δ + TAC1-1N977D; VTY28, tac1Δ/Δ + TAC1-4N977D; ACY11, tac1Δ/Δ + TAC1-6N977D; ACY12, tac1Δ/Δ + TAC1-7D977N; VTY9, tac1Δ/Δ + TAC1-5D977N. See legend of Figure 1 for other strains and genotype designations.
Role of TAC1 in drug susceptibility and Cdr1p/Cdr2p levels in clinical strains:
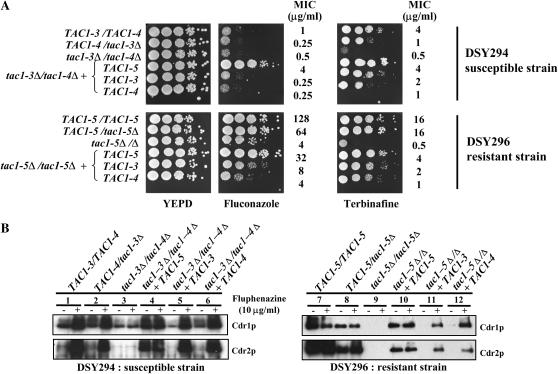
As mentioned above, strains DSY294 and DSY296 are matched azole-susceptible and azole-resistant isolates. To demonstrate that azole resistance was coupled with the presence of hyperactive alleles, TAC1 was inactivated in the background of these clinical strains. First, the ura3 auxotrophic marker was introduced into these strains using the dominant marker caSAT1 (Reuss et al. 2004). The deletion of TAC1 in DSY294 had a moderate effect on fluconazole MIC in both the heterozygous and the homozygous mutants (Figure 3A, top). The fluconazole MIC values decreased from 1 μg/ml for DSY294 to 0.25 and 0.5 μg/ml in both mutant strains. Furthermore, in the tac1Δ/Δ mutant, fluphenazine exposure did not increase Cdr1p and Cdr2p levels (Figure 3B, lane 3). This result is similar to observations of CAF2-1 and its tac1Δ/Δ derivative strain (Coste et al. 2004). These results highlight the crucial role of TAC1 in both basal and induced expression of CDR1 and CDR2 in this clinical strain. The deletion of one copy of TAC1-5 from DSY296 resulted in a slight decrease of fluconazole resistance (MIC varying from 128 to 64 μg/ml), while deletion of both TAC copies in DSY296 resulted in a significant reduction in fluconazole resistance (MIC varying from 128 to 4 μg/ml) (Figure 3A, bottom). A slight decrease in Cdr1p and Cdr2p levels was observed in TAC1-5/tac1-5Δ as compared to the wild-type strain (Figure 3B, right, lane 8), thus indicating that the TAC1-5 hyperactive allele copy number has an impact on expression levels of these proteins. In the tac1-5Δ/Δ homozygous mutant, Cdr1p/Cdr2p levels were almost undetectable both with and without fluphenazine exposure, demonstrating a direct relationship between the presence of TAC1 hyperactive alleles and azole resistance in these clinical strains (Figure 3B, lane 9). Interestingly, the MIC of fluconazole, but not of terbinafine, was higher in the tac1Δ/Δ DSY296-derived strain (4 μg/ml) than in the tac1Δ/Δ DSY294-derived strain (0.5 μg/ml). This discrepancy may be explained by the presence of a mutation in both ERG11 alleles in DSY296 (G464S) that alters binding of azoles to Erg11p and therefore contributes to fluconazole resistance (Sanglard et al. 1998).
Figure 3.
Analysis of TAC1 alleles from the clinical strains DSY294 and DSY296. (A) Drug susceptibility testing of C. albicans tac1Δ/Δ mutant and TAC1 revertant clinical strains containing specific TAC1 alleles. Drug susceptibility assays were carried out onto YEPD medium containing 2.5 μg/ml of fluconazole and 1 μg/ml cyclosporin A for the DSY294-derived strains and 5 μg/ml of fluconazole and 1 μg/ml cyclosporin A for the DSY296-derived strains. Cyclosporin A alone had no effect on the growth of these strains. All strains were spotted onto agar medium containing 20 μg/ml of terbinafine. Plates were incubated for 48 hr at 35°. MIC assays were performed as described in materials and methods. (B) Immunodetection of Cdr1p and Cdr2p in C. albicans tac1Δ/Δ mutant and TAC1 revertant clinical strains. See legend of Figure 1 for other details. The following strains correspond to the following genotypes: DSY294, TAC1-3/TAC1-4; DSY296, TAC1-5/TAC1-5; DSY3058, TAC1-4/tac1-3Δ; DSY3082; tac1-3Δ/tac1-4Δ; DSY3287-1, tac1-3Δ/tac1-4Δ + TAC1-3; DSY3288-3, tac1-3Δ/tac1-4Δ + TAC1-4; DSY3102-2, tac1-3Δ/tac1-4Δ + TAC1-5; DSY3059, TAC1-5/tac1-5Δ; DSY3083, tac1-5Δ/Δ; DSY3285-1, tac1-5Δ/Δ + TAC1-3; DSY3286-2, tac1-5Δ/Δ + TAC1-4; DSY33284-1, tac1-5Δ/Δ + TAC1-5.
To characterize the properties of TAC1 alleles from DSY294 and DSY296, the activity of TAC1-3, TAC1-4, and TAC1-5 was first assessed in the DSY294 and DSY296 backgrounds, which are mating type heterozygous and homozygous, respectively. Each type of TAC1 allele was reintroduced into the tac1Δ/Δ mutant strains derived from DSY294 and DSY296. TAC1 activity profiles for these alleles were similar to those observed in the CAF2-1 background. TAC1-3 and TAC1-4 did not confer resistance to fluconazole or terbinafine (Figure 3A) in either strain backgrounds. Consistently, they exhibited basal levels of Cdr1p and no Cdr2p and did mediate high Cdr1p/Cdr2p levels in the presence of fluphenazine (Figure 3B, lanes 5, 6, 11, and 12). TAC1-5 restored resistance to fluconazole and terbinafine in either strain backgrounds (Figure 3A) and constitutive high Cdr1p and Cdr2p levels (Figure 3B, lanes 4 and 10).
To correlate Cdr1p and Cdr2p levels with the capacity to efflux an ABC-transporter substrate, rhodamine 6G efflux was monitored in clinical strains DSY294 and DSY296 as well as in the tac1Δ/Δ derivatives (supplemental Figure S1, A and B, at http://www.genetics.org/supplemental/). The DSY296 strain exhibited higher rhodamine 6G efflux rates (average Vmax: 5.7 RFU/sec) than the DSY294 strain (average Vmax: 1.3 RFU/sec), which is consistent with the differences in Cdr1p and Cdr2p levels between the two strains. In the tac1Δ/Δ mutant strains from both isolates, rhodamine 6G efflux rates were similar to the rates observed in DSY294. The presence of TAC1-5 in the background of DSY294 tac1Δ/Δ mutants resulted in higher rhodamine 6G efflux rates (Vmax values between 3.8 and 4.1 RFU/sec) than those observed in DSY294, in tac1Δ/Δ mutants, and in TAC1-3 or TAC1-4 revertant strains (supplemental Figure S1A at http://www.genetics.org/supplemental/). Thus, the TAC1-5 hyperactive allele is necessary for increased Cdr1p/Cdr2p levels, which is also correlated to enhanced efflux rates and is independent of strain background. Importantly, since the MTL locus in DSY294 is heterozygous, these studies also show that MTL homozygosity does not have a detectable effect on azole resistance.
Codominance of TAC1 alleles with the N977D substitution:
Homozygosity at the mating-type locus was reported to correlate with the occurrence of azole resistance (Rustad et al. 2002). Hence, azole-resistant strains used in this study are homozygous at the mating-type locus and consequently contain a single TAC1 allele. It is possible that the development of the drug resistance phenotype necessitates hyperactive TAC1 alleles in a homozygous state, suggesting that these alleles are recessive to wild-type alleles. This rationale is supported by the profiles of TAC1 alleles in strain FH1: although this strain contains both a wild-type and a hyperactive TAC1 allele, this strain remains fluconazole susceptible.
TAC1 status in strains DSY294 and DSY296:
The above-described analyses were performed in tac1Δ/Δ homozygous mutant strains and therefore the dominance/recessivity relationships of TAC1 alleles could not be determined. To address this question, TAC1 alleles (TAC1-3–TAC1-5) were reintroduced in DSY294 and DSY296 TAC1/tac1Δ heterozygotes, thus generating heterozygous strains, each carrying two different TAC1 alleles.
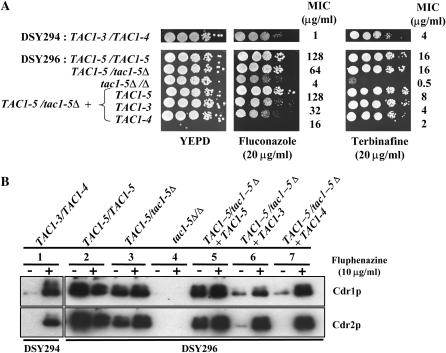
When the TAC1-5 allele was introduced at the genomic TAC1 locus in the heterozygous mutant strain TAC1-5/tac1-5Δ, the obtained revertant (TAC1-5/tac1-5Δ + TAC1-5) exhibited phenotypes similar to those observed in DSY296. The fluconazole and terbinafine MICs were similar in both strains: while the fluconazole MICs were 128 μg/ml for both strains, the terbinafine MICs were 16 and 8 μg/ml for DSY296 and the revertant, respectively (Figure 4A). Constitutive high levels of Cdr1p and Cdr2p were also similar between these strains (Figure 4B, lanes 2 and 5). When wild-type allele TAC1-3 or TAC1-4 was introduced into the TAC1-5/tac1-5Δ strain, the transformants had decreased resistance phenotypes compared to DSY296. Fluconazole MIC decreased from 128 μg/ml to 32 and 16 μg/ml for the TAC1-3 and TAC1-4 alleles, respectively; terbinafine MICs decreased from 16 to 4 and 2 μg/ml, respectively (Figure 4A). Moreover, constitutive high Cdr1p and Cdr2p levels were reduced in these heterozygotes compared to DSY296 (Figure 4B, lanes 2, 6, and 7). Cdr1p levels in these TAC1-5/tac1-5Δ +TAC1-3 and TAC1-5/tac1-5Δ +TAC1-4 strains were slightly higher than those observed in the azole-susceptible strain DSY294 (Figure 4B, lanes 1, 6, and 7). Cdr2p levels, although barely detectable in the heterozygote with TAC1-3, were not detectable in the heterozygote with TAC1-4 (Figure 4B, lanes 6 and 7) as in DSY294 (Figure 4B, lane 1). Induction of Cdr1p and Cdr2p expression by fluphenazine did occur in these heterozygotes. Thus these two heterozygous strains have phenotypes intermediate between that of the azole-susceptible strain DSY294 and the azole-resistant strain DSY296. For example, the heterozygotes with TAC1-5 and TAC1-3 alleles or TAC1-5 and TAC1-4 alleles have fluconazole MICs of 32 and 16 μg/ml, respectively, vs. 1 μg/ml for DSY294 (TAC1-3/TAC1-4) and 128 μg/ml for DSY296 (TAC1-5/TAC1-5). Similarly, DSY294-derived heterozygous strains TAC1-3/tac1-4Δ and tac1-3Δ/TAC1-4 transformed with the TAC1-5 allele had an intermediate phenotype in terms of drug resistance and Cdr1p and Cdr2p levels (data not shown). Taken together, our results suggest that the hyperactivity of the TAC1-5 allele is decreased in the presence of a wild-type allele. This implies that the hyperactive TAC1-5 allele is codominant with wild-type alleles TAC1-3 and TAC1-4. Moreover, these results confirm the codominance hypothesis of the azole resistance phenotype that was proposed from fusion experiments between DSY296 and an azole-susceptible strain with opposite mating type (Coste et al. 2004).
Figure 4.
Codominance of the TAC1-5 hyperactive allele. (A) Drug susceptibility testing of the C. albicans clinical isolates DSY294 and DSY296, heterozygous (TAC1-5/tac1-5Δ) and homozygous (tac1-5Δ/Δ) mutants derived from DSY296, and transformants of the heterozygous mutant in which a TAC1-3, TAC1-4, or TAC1-5 allele was reintroduced. See legend of Figure 1 for other details. (B) Immunodetection of Cdr1p and Cdr2p in the strains listed above. See legend of Figure 1 for other details. The following strains correspond to the following genotypes: DSY3211-4, TAC1-5/tac1-5Δ + TAC1-3; DSY3215-1, TAC1-5/tac1-5Δ + TAC1-4; DSY3210-1, TAC1-5/tac1-5Δ + TAC1-5. See the legend of Figure 3 for other strain and genotype designations.
TAC1 status in strains FH1 and FH3:
The development of drug resistance in strain FH1 is of interest because this strain contains both a wild-type allele (TAC1-6) and a hyperactive allele (TAC1-7). Consistent with this, strain FH1 exhibited intermediate levels of drug resistance and Cdr1p/Cdr2p levels. FH1 has MICs of 8 and 32 μg/ml for fluconazole and terbinafine, respectively (Figure 5A). Cdr1p and Cdr2p levels in normal growth conditions are higher in FH1 than those in CAF2-1 and DSY294 and are still inducible after fluphenazine exposure (Figure 5B, lane 1).
Figure 5.
Analysis of the TAC1 alleles from clinical strains FH1 and FH3. (A) Drug susceptibility testing of C. albicans FH1 and FH3 derivatives lacking one specific TAC1 allele. See legend of Figure 1 for other details. (B) Immunodetection of Cdr1p and Cdr2p in C. albicans FH1 and FH3 derivatives lacking one specific TAC1 allele. See legend of Figure1 for other details. The following strains correspond to the following genotypes: DSY3132-11, TAC1-6/tac1-7Δ; DSY3132-14, TAC1-7/tac1-6Δ; DSY3133-15, TAC1-7/tac1-7Δ; FH1, TAC1-6/TAC1-7; FH3, TAC1-7/TAC1-7.
To analyze the phenotypes of individual alleles, each TAC1 allele in strain FH1 was deleted. Whereas the FH1 strain possesses fluconazole and terbinafine MICs of 8 and 32 μg/ml, respectively (Figure 5A), the drug resistance of the TAC1-6/tac1-7Δ mutant decreased (fluconazole and terbinafine MICs of 1 and 8 μg/ml; Figure 5A). Both Cdr1p and Cdr2p levels decreased as well (Figure 5B, lanes 1 and 4). In contrast, when compared to FH1, the fluconazole resistance of the tac1-6Δ/TAC1-7 mutant increased from 8 to 16 μg/ml and Cdr1p and Cdr2p levels increased as well (Figure 5B, lanes 1 and 3). These phenotypes resemble those of FH3 and are almost identical to those observed in the FH3-derived TAC1-7/tac1-7Δ strain (Figure 5, A and B, lane 2).
In summary, the hyperactive TAC1-7 allele possesses codominant properties similar to those described for TAC1-5. The presence of TAC1-7 together with a wild-type allele results in intermediate drug resistance properties. Strains carrying two hyperactive alleles (i.e., DSY296 and FH3) exhibit the strongest phenotypes in terms of drug susceptibility and Cdr1p/Cdr2p levels. Therefore, development of high resistance to azoles in a TAC1 heterozygous strain carrying one hyperactive allele is due to LOH involving removal of the TAC1 wild-type copy and maintenance of the hyperactive allele. Since TAC1 is only 14 kb from the MTL, it appears that the mechanisms that result in LOH at TAC1 are often accompanied by LOH at MTL.
Stages in the development of azole resistance:
The mechanisms by which hyperactive alleles can become homozygous in azole-resistant strains remain unknown. One possibility is that, during azole exposure of a strain heterozygous at the TAC1 locus, the chromosome carrying a TAC1 hyperactive allele can be duplicated. This would then be followed by loss of one chromosome 5 copy with a wild-type TAC1 allele, thus resulting in homozygosity of the remaining chromosome 5 copies. Alternatively, the chromosome 5 copy with the wild-type TAC1 allele could be lost first and then the remaining copy could be duplicated. A third possibility is that the region containing TAC1 (and MTL) undergoes a local recombination event that results in gene conversion of a long region of the chromosome.
Development of in vitro fluconazole resistance:
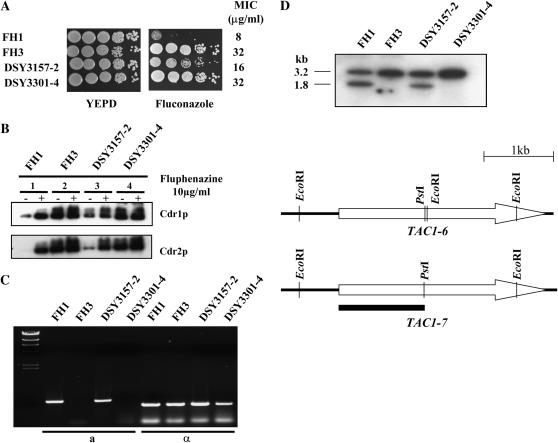
To ask how LOH and fluconazole resistance occurs, we followed the in vitro acquisition of fluconazole resistance in strain FH1 and analyzed the newly resistant strains for their chromosome 5 and TAC1 status. FH1 was spotted onto a YEPD plate supplemented with 10 μg/ml of fluconazole. The plate was incubated at 30° until resistant colonies appeared. The medium contained cyclosporine A to suppress residual growth of C. albicans in the presence of fluconazole as described (Marchetti et al. 2000). One resistant colony (DSY3157-2) was plated onto YEPD with 10 μg/ml fluconazole and fast-growing colonies were obtained. One of these, DSY3301-4, as well as strains FH1, FH3, and DSY3157-2, were analyzed for azole susceptibility and Cdr1p/Cdr2p protein levels. Strains FH1, DSY3157-2, and DSY3301-4 showed increasing fluconazole resistance (MIC of 8, 16, and 32 μg/ml, respectively, Figure 6A). These strains also exhibited increasing Cdr1p and Cdr2p levels as compared to FH1 under normal growth conditions (Figure 6B, lanes 1, 3 and 4). Furthermore, DSY3301-4 and FH3 had identical fluconazole MICs (32 μg/ml) and exhibited similar Cdr1p and Cdr2p levels in both the absence and the presence of fluphenazine (Figure 6B, lanes 2 and 4).
Figure 6.
Analysis of FH1-derived strains after in vitro fluconazole exposure. (A) Drug susceptibility testing of C. albicans FH3-, FH1-, and FH1-derived strains selected for their fluconazole resistance. DSY3157-2 was derived from a single colony of FH1 that arose after spotting onto YEPD medium with 10 μg/ml fluconazole. DSY3301-4 was derived from DSY3157-2 as a fast-growing colony onto medium with 10 μg/ml fluconazole. Drug susceptibility assays were carried out as described in Figure 1 onto YEPD medium containing 10 μg/ml fluconazole and 1 μg/ml cyclosporin A. Cyclosporin A alone had no effect on the growth of these strains. Plates were incubated for 48 hr at 35°. MIC assays were performed as described in materials and methods. (B) Immunodetection of Cdr1p and Cdr2p in C. albicans strains FH1, FH3, DSY3157-2, and DSY3301-4. See legend of Figure 1 for other details. (C) PCR analysis of the mating-type locus. PCR was performed as described (Rustad et al. 2002). “a” and “α” denote analysis performed to detect MTLa and MTLα loci, respectively. (D) Southern blot analysis of the TAC1 alleles in C. albicans FH-derivative strains. Genomic DNA of each strain was digested by EcoRI and Southern blot was performed as described in materials and methods. Radioactivity of signals was quantified as discussed in materials and methods. Restriction maps of both TAC1-6 and TAC1-7 alleles indicate restriction site polymorphism for EcoRI. The solid bar indicates the position of the labeled probe, which corresponded to the region located between the first TAC1 initiation codon and the PstI restriction site. Digestion of genomic DNA with EcoRI is expected to yield positive signals at 1.8 and 3.2 kb for TAC1-6 and TAC1-7, respectively.
Analysis of TAC1 and chromosome 5 status:
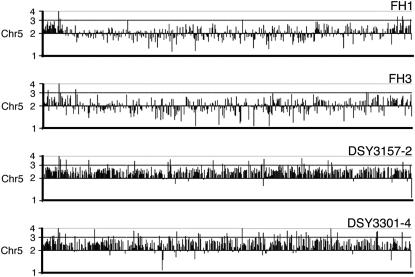
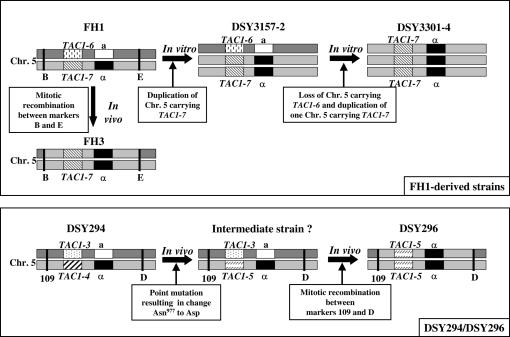
The stepwise increase of fluconazole resistance from FH1 to DSY3157-2 and DSY3301-4 could have occurred if TAC1-7 was duplicated in DSY3157-2 while TAC1-6 was maintained. In DSY3301-4, TAC1-6 could have been lost, leaving two TAC1-7 copies. To test these step hypotheses, we determined the status of the MTL loci. Both FH1 and DSY3157-2 were heterozygous (MTLa/MTLα) while both FH3 and DSY3301-4 were mating type homozygous (MTLα/MTLα) (Figure 6C). Southern blot analysis of the TAC1 loci of strains FH1, FH3, DSY3157-2, and DSY3301-4 confirmed that FH1 and DSY3157-2 contained two distinct alleles (1.8 and 3.2 kb), while FH3 contained only the 3.2-kb signal corresponding to TAC1-7. Interestingly, the signal ratio for TAC1-7:TAC1-6 was 0.98:1 in FH1 and 2.1:1 in DSY3157-2. This analysis is consistent with the idea that, in strain DSY3157-2, the genotype is TAC1-7/TAC1-7/TAC1-6. Comparative genome hybridization array analysis (Figure 7) supports the first part of this step hypothesis: strains FH1 and FH3 are disomic for all genes on chromosome 5, while chromosome 5 is trisomic in strain DSY3157-2. In addition, chromosome 5 is trisomic in DSY3301-4, suggesting that this strain carries three copies of TAC1-7, MTLα, and all of the other genes on chromosome 5. Thus, either the copy of chromosome 5 carrying MTLa and TAC1-6 was lost and another copy of a remaining chromosome 5 was duplicated or, alternatively, a region of chromosome 5 containing MTLa and TAC1-6 underwent gene conversion.
Figure 7.
CGH of FH-derived strains. The genomes of the tested strains were hybridized against the SC5314 genome according to the protocol published by Selmecki et al. (2005). Each gene on chromosome 5 is represented by its relative intensity as compared to signals obtained in SC5314.
Analysis of chromosome 5 alterations:
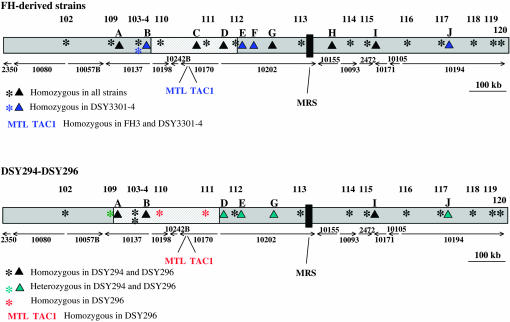
To distinguish between the above mechanisms of LOH in strain DSY3301-4, we conducted SNP microarray hybridization for 13 SNP loci evenly distributed across chromosome 5 (Forche et al. 2005). This analysis showed that 12 of these loci were homozygous in all four strains. One SNP locus (marker 104), located within the SNF1 ORF (orf19.1936), was heterozygous in strains FH1, FH3, and DSY3157-2. This locus became homozygous in strain DSY3301-4 (Figure 8, top).
Figure 8.
SNP analysis of chromosome 5. The map of chromosome 5 was obtained by assembly of the different contig sequences shown by arrows. An asterisk (*) indicates SNPs of chromosome 5 as measured on the SNP microarray: 102, 1855/2172; 103, HST3; 104, SNF1; 109, 1899/2008; 110, 1445/2395; 111, 1922/2344; 112, PDE1; 113, 1969/2162; 114, DPH5; 115, HEX1; 116, 2093/2390; 117, 1817/2082; 118, 1341/2493; 119, F16n1; 120, 2340/2493. “▴” indicates additional markers of chromosome 5: A, orf19.1976 (TRX1); B, orf19.1926; C, ZNC3; D, orf19.4225; E, orf19.4251; F, orf19.4288; G, orf19.1942 (TRR1); H, orf19.2646; I, orf19.6680; J, CRH12. Color codes indicate modifications in SNPs for individual strains. For the strains DSY294 and DSY296, sequences of the C, F, and H markers were not available. The hatched regions on chromosome 5 delimitate the maximal region of a recombination. MRS, major repeat sequence.
To obtain additional information, 10 more genes, not present on the SNP arrays (CRH12, orf19.4251, orf19.1926, orf19.4288, TRX1, TRR1, ZNC3, orf19.4225, orf19.2646, and orf19.6680, solid triangles in Figure 8), were sequenced to determine the allelic status in each of the four strains. Of these 10 genes, 6 were uninformative because they were homozygous for all four strains. The remaining 4 genes (CRH12, orf19.4251, orf19.1926, orf19.4288; see Table 3) were heterozygous in strains FH1, FH3, and DSY3157-2 and homozygous in strain DSY3301-4 (markers B, E, F, and J of Figure 8, top). Since the SNP loci that became homozygous in DSY3301-4 are distributed all along chromosome 5, this result is consistent with the idea that all of chromosome 5 became homozygous in DSY3301-4. It is likely that in DSY3301-4 a homolog carrying the TAC1-7 allele replaced the chromosome carrying TAC1-6.
TABLE 3.
Nucleotide polymorphisms in chromosome 5 in FH1–FH3 and derivative strains
| Genes and position of SNPs with respect to ATG
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| orf19.4251
|
orf19.1926
|
orf19.4288: |
CRH12
|
||||||||||
| Strains | 576 | 899 | 945 | 684 | 708 | 720 | 741 | 870 | 876 | 893 | 135 | 404 | 510 |
| FH1, FH3, DSY3157-2 | C or T | A or G | C or T | A or G | A or G | A or G | C or T | A or G | C or T | C or G | C or T | C or T | C or T |
| DSY3301-4 | T | A | T | A | G | G | T | A | T | C | C | T | C |
Interestingly, the mechanism by which FH3 became homozygous for TAC1-7 appears to be different from that of DSY3301-4; this strain shows LOH only between the MTL and TAC1 loci. Rather, it appears that a mitotic recombination event occurred between the two chromosome 5 copies of strain FH3, thus leading to gene conversion of the TAC1-6 and MTLa to TAC1-7 and MTLα (see Figure 9, top, for schematic).
Figure 9.
Schematic of chromosome 5 alterations in matched azole-susceptible and azole-resistant strains obtained from patients or developed in vitro. (Top) Chromosome 5 alterations in FH1-derived strains both in vitro and in vivo. Chromosome 5 containing TAC1-7 and MTLα was duplicated after in vitro fluconazole exposure in DSY3157-2; a chromosome 5 copy with TAC1-6 and MTLa was lost in DSY3301-4 and replaced by another chromosome 5 copy containing TAC1-7 and MTLα after a second fluconazole exposure; in FH3, a portion of chromosome 5 underwent mitotic recombination between markers B and E, resulting in TAC1-7 and MTLα homozygosity. (Bottom) Chromosome 5 alterations in DSY294 and DSY296 in vivo. In DSY296, a portion of chromosome 5 underwent mitotic recombination between markers 109 and D, resulting in TAC1-5 and MTLα homozygosity. An intermediate strain between DSY294 and DSY296 in which a single nucleotide change in TAC1-4 yielded TAC1-5 resulting in Asn977 to Asp could have existed. TAC1-3, -4, and -6 are defined as wild-type alleles; TAC1-5 and -7 are defined as hyperactive alleles. “a” and “α”designate MTL types. Position of markers 109, B, D, and E are indicated in Figure 8.
TAC1 and chromosome 5 status in clinical strains DSY294 and DSY296:
To ask about the mechanism by which strain DSY296 became azole resistant, we analyzed the organization of chromosome 5 homologs in this strain as well. Comparative genome hybridization array analysis indicated that both strains DSY294 and DSY296 carried two copies of chromosome 5 (data not shown). SNP microarray hybridization revealed that 10 of the 13 SNP markers on the array were homozygous in both strains. Markers 110 and 111 were homozygous only in DSY296 and marker 109 was heterozygous in both strains (Figure 8, bottom). Seven other genes (CRH12, orf19.4251, orf19.1926, TRX1, TRR1, orf19.4225 and orf19.6680) were amplified and sequenced along chromosome 5 as described above. DSY294 and DSY296 were homozygous for TRX1, orf19.1926, and orf19.6680 and heterozygous for orf19.4225, orf19.4251, TRR1, and CRH12 (Figure 8, bottom). Since strain DSY296 is heterozygous for five polymorphisms tested here (marker 109, orf19.4225, orf19.4251, TRR1 and CRH12), we conclude that LOH at TAC1 and MTL was not caused by duplication of all of chromosome 5. Rather, it appears that mitotic recombination between two copies of chromosome 5 likely resulted in LOH at these loci (see Figure 9, bottom, for schematic). We propose that an intermediate strain, carrying one TAC1-3 and one TAC1-5 allele (derived from TAC1-4), underwent mitotic recombination and gene conversion leading to homozygosis of the TAC1-5 alleles in strain DSY296.
Taken together, our results show that increased antifungal drug resistance due to constitutively high expression of the ABC transporters Cdr1p and Cdr2p can be achieved when TAC1 alleles carry the N977D mutation and become homozygous. Homozygosis can occur either by mitotic recombination between chromosome 5 copies that results in gene conversion or by the presence of extra copies of chromosome 5 carrying the hyperactive TAC1 allele accompanied by loss of chromosome 5 carrying the wild-type TAC1 allele.
DISCUSSION
A mutation in TAC1 is involved in azole resistance:
In this work, we established for the first time a link between a point mutation in a transcription factor and the constitutive high expression of the multidrug transporters Cdr1p and Cdr2p responsible for antifungal drug resistance. It was previously shown that azole resistance can be due to point mutations in ERG11, which encodes the enzyme target of azoles. It is believed that these mutations can alter the affinity of azoles for their target and therefore can participate in the development of resistance (Sanglard et al. 1998; Perea et al. 2001). Among azole-resistant strains with altered ERG11 alleles, Marichal et al. (1999) observed three “hot spots” localized in the region 105–165, 266–287, and 405–488 of Erg11p. Resistance to other antifungal drugs such as 5-fluorocytosine (5-FC) and caspofungin was also shown to be due to point mutations in specific genes (Dodgson et al. 2004; Park et al. 2005). Resistance to 5-FC is restricted to clade I and due to a single point mutation, C301T, in the FUR1 gene, encoding a phosphoribosyltransferase. This nonsynonymous mutation changes arginine to cysteine at position 101 of Fur1p (Dodgson et al. 2004). Recently, Park et al. (2005) showed that the modification of the serine 645 of CaFks1p, a subunit of the 1,3-β-d-glucan synthase, is sufficient to confer reduced susceptibility to echinocandins in C. albicans.
The single nucleotide mutation in codon 977 (A to G at nt 2929) in TAC1 corresponds to a nonsynonymous modification from Asn to Asp in Tac1p. This mutation is located within a putative C-terminal activation domain of the transcription factor. The importance of this region for transcriptional activity was confirmed by preliminary experiments that deleted the C-terminal region from aa 801 to the C-terminal end of Tac1p. This deletion resulted in a truncated Tac1p unable to activate the expression of at least CDR2 in presence of fluphenazine (D. Sanglard, unpublished results). Further experiments will be needed to elucidate how the N977D point mutation transforms Tac1p into a hyperactive state. Several mutations in the C-terminal activation domain region of the S. cerevisiae transcription factors Pdr1p or Pdr3p are also responsible for antifungal drug resistance and for upregulation of PDR5. These include pdr1-3 (F815S; Carvajal et al. 1997), pdr1-8 (L1036W; Carvajal et al. 1997), pdr1-12 (L1044Q; Wendler et al. 1997), PDR1-101 (T879M; Reid et al. 1997), PDR1 (R821H; Tuttle et al. 2003), pdr3-17 (G834D), pdr3-18 (G834S), pdr3-19 (L837S), and pdr3-20 (G957N; Carvajal et al. 1997). Gao et al. (2004) showed that a strain carrying the hyperactive pdr1-3 allele expressed PDR5 in a drug-independent manner. This can be associated with enhanced promoter occupancy of coactivator complexes, including SAGA, Mediator, chromatin-remodeling SWI/SNF complex, and TATA-binding protein. Using chromatin immunoprecipitation, loss of contacts between histones and DNA was demonstrated at PDR5 promoter and coding sequences (Gao et al. 2004). Other mechanisms to activate zinc-finger regulators have also been described, including nuclear-cytoplasmic shuffling (Gorner et al. 1998; Santos and de Larrinoa 2005), dimerization (Rottensteiner et al. 1997), DNA binding, phosphorylation (Sadowski et al. 1996; Kren et al. 2003), and unmasking of the activation domain (Sadowski et al. 1996). Auto-induction also can be envisaged as in the case of PDR3 (Delahodde et al. 1995). Tac1p is present in the nucleus of cells in normal growth conditions (Coste et al. 2004). Thus, it is likely that Tac1p hyperactivity involves constitutive binding to the DRE in the promoter of CDR1 and CDR2, constitutive phosphorylation of the protein, and/or a change in the conformation of the protein, which can lead to the constant unmasking of the activation domain.
TAC1 hyperactive alleles are codominant with wild-type alleles:
Much like PDR1 F815S and other PDR1 gain-of-function alleles, hyperactive TAC1 N977D alleles are codominant with wild-type alleles. This suggests that high levels of antifungal drug resistance cannot be achieved in the presence of wild-type alleles. Rather, homozygosis of the hyperactive alleles is necessary for the development of high levels of azole resistance. Codominance of these alleles probably reflects the fact that Tac1p is a transcription factor belonging to the Zn2–Cys6 family, which often dimerize to bind a cis-acting element (Mamnun et al. 2002). When two different TAC1 alleles are expressed in the same cell, heterodimer formation is likely. A corollary to this hypothesis, which remains to be tested, is that heterodimers containing one hyperactive and one wild-type Tac1p cannot direct high levels of basal Cdr1p and Cdr2p expression.
Codominance is a feature shared with other alleles of genes involved in antifungal resistance such as ERG11 or FUR1. White (1997) showed that the R467K mutation in ERG11 alone is not sufficient to confer azole resistance. Loss of allelic variation in ERG11 is required to confer strong azole resistance (White 1997). This observation was also made independently for other ERG11 alleles in our laboratory (Sanglard et al. 1998). Dodgson et al. (2004) found a semidominant relationship between the hyperactive and wild-type alleles of FUR1 for the development of 5-FC resistance.
Duplication of and recombination between chromosome 5 homologs as mechanisms resulting in TAC1 homozygosity:
To observe strong drug resistance, the codominance of TAC1 N977D alleles requires of loss of allelic variation. This property of TAC1 may explain the link between MTL homozygosity and antifungal drug resistance. In this study, we reconstituted the loss of allelic variation at the TAC1 locus in vitro. Drug resistance increased in two steps: the first step corresponded to a duplication of the TAC1-7 hyperactive allele along with all other chromosome 5-linked genes; the second step involved the replacement of the remaining wild-type TAC1-6 allele by the hyperactive TAC1-7 allele while the strain remained trisomic for chromosome 5. SNP analysis suggests that the mechanism by which this occurred was loss of the MTLa chromosome 5 homolog and gain of a third copy of the MTLα homolog. The order of these events is not clear, but appears to have involved all of chromosome 5.
We also observed other mechanisms of LOH: duplication and exchanges of parts of chromosome 5. Clinical strain FH3 did not undergo LOH for all investigated SNP markers and for 10 other genes situated on chromosome 5 (see Figure 8). In FH3, the LOH that led to homozygosity of TAC1 and of MTL includes flanking regions that compose a 250-kb region delimitated by heterozygous loci orf19.1926 and orf19.4251 (Figure 9, hatched region of chromosome 5). Similarly, SNP analysis of strains DSY294 and DSY296 revealed that a mitotic recombination event in DSY296 comprising an ∼300-kb fragment bordered by heterozygous loci 1899/2008 and orf19.4225 resulted in LOH at TAC1 and MTL. This homozygosity was not restricted to the MTLa/α genes but extended into the PAP genes within MTL as well (data not shown). This observation is not in agreement with the findings of Goldman et al. (2004), which indicated that recombination occurring within the MTL locus of azole-resistant clinical isolates leads to homozygosity for the a/α genes but not to other genes of the MTL locus.
The experiments undertaken with strains DSY3157-2 and DSY3301-4 also revealed that chromosome 5 can become trisomic prior to the loss of one chromosome 5 copy. These results are partially consistent with those presented by Wu et al. (2005), who showed that MTL homozygosity can occur by loss of one copy of chromosome 5 followed by duplication of the remaining copy. Our work suggests that several mechanisms contribute to LOH, which is consistent with the recognized elasticity of the C. albicans genome (Iwaguchi et al. 2001; X. Chen et al. 2004; Iwaguchi et al. 2004). Although the number of isolates investigated here was limited, LOH at chromosome 5 was obtained by mitotic recombination in the investigated clinical isolates, whereas it was obtained by chromosome 5 loss and duplications in laboratory conditions. This difference might reflect a fitness cost for such events under the conditions encountered in the host. Therefore chromosome 5 mitotic recombinations rather might be selected in vivo. Additional azole-resistant clinical isolates should be investigated to address this question.
Association among azole resistance, TAC1, and the mating-type locus:
The short distance between TAC1 and MTL has interesting consequences. Under selective drug pressure, codominance of TAC1 favors LOH at this locus and indirectly contributes to the appearance of MTL homozygous yeast strains. Importantly, our work with different TAC1 alleles in different strain backgrounds that were heterozygous or homozygous for MTL did not detect any contribution of MTL in drug resistance. Nonetheless, selective pressure for LOH at the TAC1 locus appears to facilitate the exchange of genetic material within a yeast population. For example, mating-competent strains that emerge from LOH in this region have the capacity to mate with cells of the opposite mating type. Even though mating in a C. albicans population under in vivo conditions has been reported, evidence for genetic rearrangement and exchange after mating is still sparse. However, the link between TAC1 and mating-type locus enables us to test the effect of drug pressure on the ability to propagate a drug resistance genotype.
Genetic transfer of drug resistance in bacteria is a common feature; however, genetic transfer in fungi and especially in C. albicans has not yet been reported. Animal models will be used in future studies to test this hypothesis. Interestingly, in some clinical isolates, azole resistance mediated by upregulation of CDR1 and CDR2 is not linked to homozygosity at the mating-type locus (D. Sanglard, unpublished results). The TAC1 alleles of these isolates are probably dominant and may carry mutation(s) different from the N977D mutation described here. A catalog of existing mutations in TAC1 alleles in the population of azole-resistant isolates is necessary to identify and characterize other mutations responsible for Tac1p hyperactivity as well as their distribution in the population of clinical strains. First, this catalog will help to establish critical domains necessary for TAC1 hyperactivity and, second, it will assist the design of molecular diagnostic tools for the detection of such alleles.
Acknowledgments
This work was supported by a grant (no. 3200B0-100747/1) from the Swiss Research Foundation to D.S. Sequence data for C. albicans were obtained from the Stanford Genome Technology Center website at http://www-sequence.stanford.edu/group/candida. Sequencing of C. albicans was accomplished with the support of the National Institute of Dental Research and the Burroughs Wellcome Fund. Joachim Morschhäuser was supported by the European Community project QLK2-CT-2001-02377. Anna Selmecki was supported by an Integrative Fellowship from the Center for Microbial and Plant Genomics, University of Minnesota. Anja Forche and Judith Berman were supported by grant AI62427 from the National Institutes of Health.
References
- Anderson, J. B., C. Sirjusingh, A. B. Parsons, C. Boone, C. Wickens et al., 2003. Mode of selection and experimental evolution of antifungal drug resistance in Saccharomyces cerevisiae. Genetics 163: 1287–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvajal, E., H. B. van den Hazel, A. Cybularz-Kolaczkowska, E. Balzi and A. Goffeau, 1997. Molecular and phenotypic characterization of yeast PDR1 mutants that show hyperactive transcription of various ABC multidrug transporter genes. Mol. Gen. Genet. 256: 406–415. [DOI] [PubMed] [Google Scholar]
- Chambers, S. P., S. E. Prior, D. A. Barstow and N. P. Minton, 1988. The pMTL nic- cloning vectors. I. Improved pUC polylinker regions to facilitate the use of sonicated DNA for nucleotide sequencing. Gene 68: 139–149. [DOI] [PubMed] [Google Scholar]
- Chen, C. G., Y. L. Yang, H. I. Shih, C. L. Su and H. J. Lo, 2004. CaNdt80 is involved in drug resistance in Candida albicans by regulating CDR1. Antimicrob. Agents Chemother. 48: 4505–4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X., B. B. Magee, D. Dawson, P. T. Magee and C. A. Kumamoto, 2004. Chromosome 1 trisomy compromises the virulence of Candida albicans. Mol. Microbiol. 51: 551–565. [DOI] [PubMed] [Google Scholar]
- Coste, A. T., M. Karababa, F. Ischer, J. Bille and D. Sanglard, 2004. TAC1, transcriptional activator of CDR genes, is a new transcription factor involved in the regulation of Candida albicans ABC transporters CDR1 and CDR2. Eukaryot. Cell 3: 1639–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Deken, X., and M. Raymond, 2004. Constitutive activation of the PDR16 promoter in a Candida albicans azole-resistant clinical isolate overexpressing CDR1 and CDR2. Antimicrob. Agents Chemother. 48: 2700–2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delahodde, A., T. Delaveau and C. Jacq, 1995. Positive autoregulation of the yeast transcription factor Pdr3p, which is involved in control of drug resistance. Mol. Cell. Biol. 15: 4043–4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Micheli, M., J. Bille, C. Schueller and D. Sanglard, 2002. A common drug-responsive element mediates the upregulation of the Candida albicans ABC transporters CDR1 and CDR2, two genes involved in antifungal drug resistance. Mol. Microbiol. 43: 1197–1214. [DOI] [PubMed] [Google Scholar]
- Dodgson, A. R., K. J. Dodgson, C. Pujol, M. A. Pfaller and D. R. Soll, 2004. Clade-specific flucytosine resistance is due to a single nucleotide change in the FUR1 gene of Candida albicans. Antimicrob. Agents Chemother. 48: 2223–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzi, W. A., and M. Y. Irwin, 1993. Isogenic strain construction and gene mapping in Candida albicans. Genetics 134: 717–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forche, A., G. May and P. T. Magee, 2005. Demonstration of loss of heterozygosity by single-nucleotide polymorphism microarray analysis and alterations in strain morphology in Candida albicans strains during infection. Eukaryot. Cell 4: 156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, C., L. Wang, E. Milgrom and W. C. Shen, 2004. On the mechanism of constitutive Pdr1 activator-mediated PDR5 transcription in Saccharomyces cerevisiae: evidence for enhanced recruitment of coactivators and altered nucleosome structures. J. Biol. Chem. 279: 42677–42686. [DOI] [PubMed] [Google Scholar]
- Gaur, N. A., N. Puri, N. Karnani, G. Mukhopadhyay, S. K. Goswami et al., 2004. Identification of a negative regulatory element which regulates basal transcription of a multidrug resistance gene CDR1 of Candida albicans. FEMS Yeast Res. 4: 389–399. [DOI] [PubMed] [Google Scholar]
- Goldman, G. H., M. E. da Silva Ferreira, E. dos Reis Marques, M. Savoldi, D. Perlin et al., 2004. Evaluation of fluconazole resistance mechanisms in Candida albicans clinical isolates from HIV-infected patients in Brazil. Diagn. Microbiol. Infect. Dis. 50: 25–32. [DOI] [PubMed] [Google Scholar]
- Gorner, W., E. Durchschlag, M. T. Martinez-Pastor, F. Estruch, G. Ammerer et al., 1998. Nuclear localization of the C2H2 zinc finger protein Msn2p is regulated by stress and protein kinase A activity. Genes Dev. 12: 586–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harry, J. B., B. G. Oliver, J. L. Song, P. M. Silver, J. T. Little et al., 2005. Drug-induced regulation of the MDR1 promoter in Candida albicans. Antimicrob. Agents Chemother. 49: 2785–2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaguchi, S. I., M. Sato, B. B. Magee, P. T. Magee, K. Makimura et al., 2001. Extensive chromosome translocation in a clinical isolate showing the distinctive carbohydrate assimilation profile from a candidiasis patient. Yeast 18: 1035–1046. [DOI] [PubMed] [Google Scholar]
- Iwaguchi, S., M. Suzuki, N. Sakai, Y. Nakagawa, P. T. Magee et al., 2004. Chromosome translocation induced by the insertion of the URA blaster into the major repeat sequence (MRS) in Candida albicans. Yeast 21: 619–634. [DOI] [PubMed] [Google Scholar]
- Karababa, M., A. T. Coste, B. Rognon, J. Bille and D. Sanglard, 2004. Comparison of gene expression profiles of Candida albicans azole-resistant clinical isolates and laboratory strains exposed to drugs inducing multidrug transporters. Antimicrob. Agents Chemother. 48: 3064–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnani, N., N. A. Gaur, S. Jha, N. Puri, S. Krishnamurthy et al., 2004. SRE1 and SRE2 are two specific steroid-responsive modules of Candida drug resistance gene 1 (CDR1) promoter. Yeast 21: 219–239. [DOI] [PubMed] [Google Scholar]
- Katzmann, D. J., P. E. Burnett, J. Golin, Y. Mahe and W. S. Moye-Rowley, 1994. Transcriptional control of the yeast PDR5 gene by the PDR3 gene product. Mol. Cell. Biol. 14: 4653–4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kren, A., Y. M. Mamnun, B. E. Bauer, C. Schuller, H. Wolfger et al., 2003. War1p, a novel transcription factor controlling weak acid stress response in yeast. Mol. Cell. Biol. 23: 1775–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamnun, Y. M., R. Pandjaitan, Y. Mahe, A. Delahodde and K. Kuchler, 2002. The yeast zinc finger regulators Pdr1p and Pdr3p control pleiotropic drug resistance (PDR) as homo- and heterodimers in vivo. Mol. Microbiol. 46: 1429–1440. [DOI] [PubMed] [Google Scholar]
- Marchetti, O., J. M. Entenza, D. Sanglard, J. Bille, M. P. Glauser et al., 2000. Fluconazole plus cyclosporine: a fungicidal combination effective against experimental endocarditis due to Candida albicans. Antimicrob. Agents Chemother. 44: 2932–2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marichal, P., L. Koymans, S. Willemsens, D. Bellens, P. Verhasselt et al., 1999. Contribution of mutations in the cytochrome P450 14α-demethylase (Erg11p, Cyp51p) to azole resistance in Candida albicans. Microbiology 145: 2701–2713. [DOI] [PubMed] [Google Scholar]
- Marr, K. A., T. C. White, J. A. van Burik and R. A. Bowden, 1997. Development of fluconazole resistance in Candida albicans causing disseminated infection in a patient undergoing marrow transplantation. Clin. Infect Dis. 25: 908–910. [DOI] [PubMed] [Google Scholar]
- Marr, K. A., C. N. Lyons, T. R. Rustad, R. A. Bowden and T. C. White, 1998. Rapid, transient fluconazole resistance in Candida albicans is associated with increased mRNA levels of CDR. Antimicrob. Agents Chemother. 42: 2584–2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr, K. A., C. N. Lyons, K. Ha, T. R. Rustad and T. C. White, 2001. Inducible azole resistance associated with a heterogeneous phenotype in Candida albicans. Antimicrob. Agents Chemother. 45: 52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nourani, A., D. Papajova, A. Delahodde, C. Jacq and J. Subik, 1997. Clustered amino acid substitutions in the yeast transcription regulator Pdr3p increase pleiotropic drug resistance and identify a new central regulatory domain. Mol. Gen. Genet. 256: 397–405. [DOI] [PubMed] [Google Scholar]
- Park, S., R. Kelly, J. N. Kahn, J. Robles, M. J. Hsu et al., 2005. Specific substitutions in the echinocandin target Fks1p account for reduced susceptibility of rare laboratory and clinical Candida sp. isolates. Antimicrob. Agents Chemother. 49: 3264–3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea, S., J. L. Lopez-Ribot, W. R. Kirkpatrick, R. K. McAtee, R. A. Santillan et al., 2001. Prevalence of molecular mechanisms of resistance to azole antifungal agents in Candida albicans strains displaying high-level fluconazole resistance isolated from human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 45: 2676–2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri, N., S. Krishnamurthy, S. Habib, S. E. Hasnain, S. K. Goswami et al., 1999. CDR1, a multidrug resistance gene from Candida albicans, contains multiple regulatory domains in its promoter and the distal AP-1 element mediates its induction by miconazole. FEMS Microbiol. Lett. 180: 213–219. [DOI] [PubMed] [Google Scholar]
- Reid, R. J., E. A. Kauh and M. A. Bjornsti, 1997. Camptothecin sensitivity is mediated by the pleiotropic drug resistance network in yeast. J. Biol. Chem. 272: 12091–12099. [DOI] [PubMed] [Google Scholar]
- Reuss, O., A. Vik, R. Kolter and J. Morschhauser, 2004. The SAT1 flipper, an optimized tool for gene disruption in Candida albicans. Gene 341: 119–127. [DOI] [PubMed] [Google Scholar]
- Rottensteiner, H., A. J. Kal, B. Hamilton, H. Ruis and H. F. Tabak, 1997. A heterodimer of the Zn2Cys6 transcription factors Pip2p and Oaf1p controls induction of genes encoding peroxisomal proteins in Saccharomyces cerevisiae. Eur. J. Biochem. 247: 776–783. [DOI] [PubMed] [Google Scholar]
- Rustad, T. R., D. A. Stevens, M. A. Pfaller and T. C. White, 2002. Homozygosity at the Candida albicans MTL locus associated with azole resistance. Microbiology 148: 1061–1072. [DOI] [PubMed] [Google Scholar]
- Sadowski, I., C. Costa and R. Dhanawansa, 1996. Phosphorylation of Ga14p at a single C-terminal residue is necessary for galactose-inducible transcription. Mol. Cell. Biol. 16: 4879–4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanglard, D., K. Kuchler, F. Ischer, J. L. Pagani, M. Monod et al., 1995. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob. Agents Chemother. 39: 2378–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanglard, D., F. Ischer, L. Koymans and J. Bille, 1998. Amino acid substitutions in the cytochrome P450 lanosterol 14α-demethylase (CYP51A1) from azole-resistant Candida albicans clinical isolates contributing to the resistance to azole antifungal agents. Antimicrob. Agents Chemother. 42: 241–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos, M., and I. F. de Larrinoa, 2005. Functional characterization of the Candida albicans CRZ1 gene encoding a calcineurin-regulated transcription factor. Curr. Genet. 48: 88–100. [DOI] [PubMed] [Google Scholar]
- Selmecki, A., S. Bergmann and J. Berman, 2005. Comparative genome hybridization reveals widespread aneuploidy in Candida albicans laboratory strains. Mol. Microbiol. 55: 1553–1565. [DOI] [PubMed] [Google Scholar]
- Strauss, A., S. Michel and J. Morschhauser, 2001. Analysis of phase-specific gene expression at the single-cell level in the white-opaque switching system of Candida albicans. J. Bacteriol. 183: 3761–3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttle, M. S., D. Radisky, L. Li and J. Kaplan, 2003. A dominant allele of PDR1 alters transition metal resistance in yeast. J. Biol. Chem. 278: 1273–1280. [DOI] [PubMed] [Google Scholar]
- Wendler, F., H. Bergler, K. Prutej, H. Jungwirth, G. Zisser et al., 1997. Diazaborine resistance in the yeast Saccharomyces cerevisiae reveals a link between YAP1 and the pleiotropic drug resistance genes PDR1 and PDR3. J. Biol. Chem. 272: 27091–27098. [DOI] [PubMed] [Google Scholar]
- White, T. C., 1997. The presence of an R467K amino acid substitution and loss of allelic variation correlate with an azole-resistant lanosterol 14-alpha-demethylase in Candida albicans. Antimicrob. Agents Chemother. 41: 1488–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, W., C. Pujol, S. R. Lockhart and D. R. Soll, 2005. Chromosome loss followed by duplication is the major mechanism of mating-type locus homozygosis in Candida albicans. Genetics 169: 1311–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]