Abstract
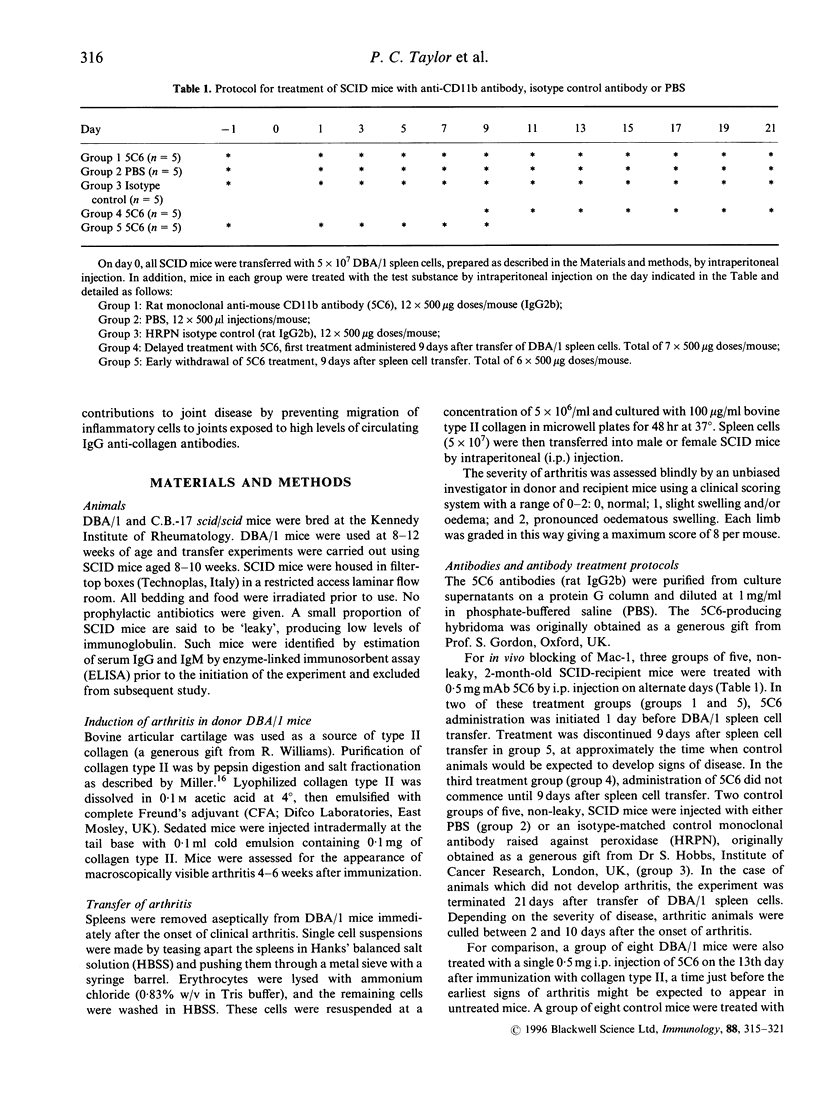
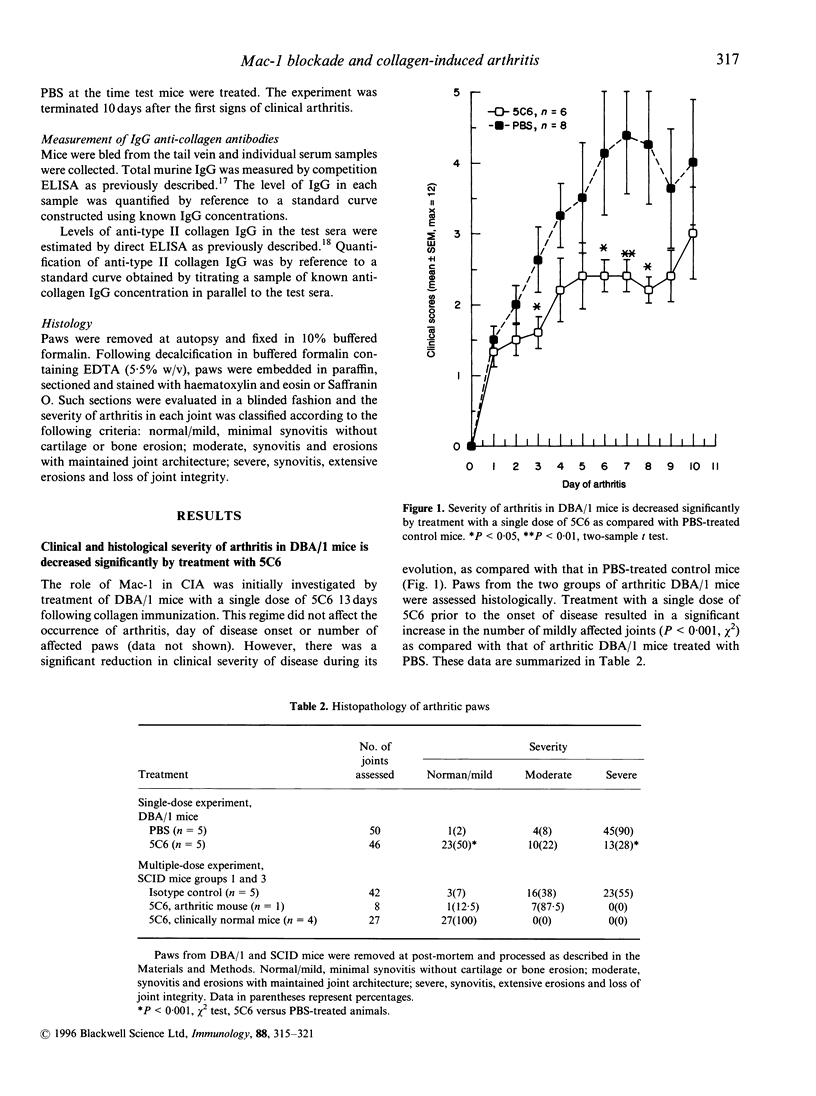
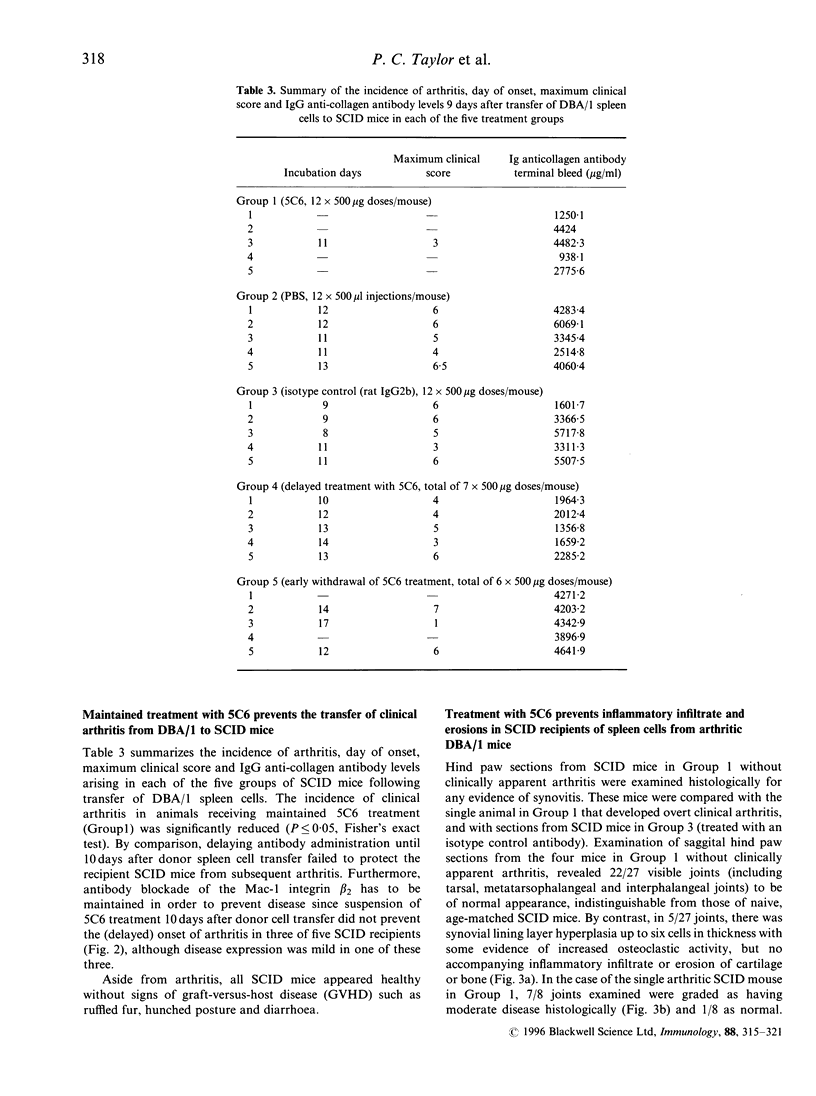
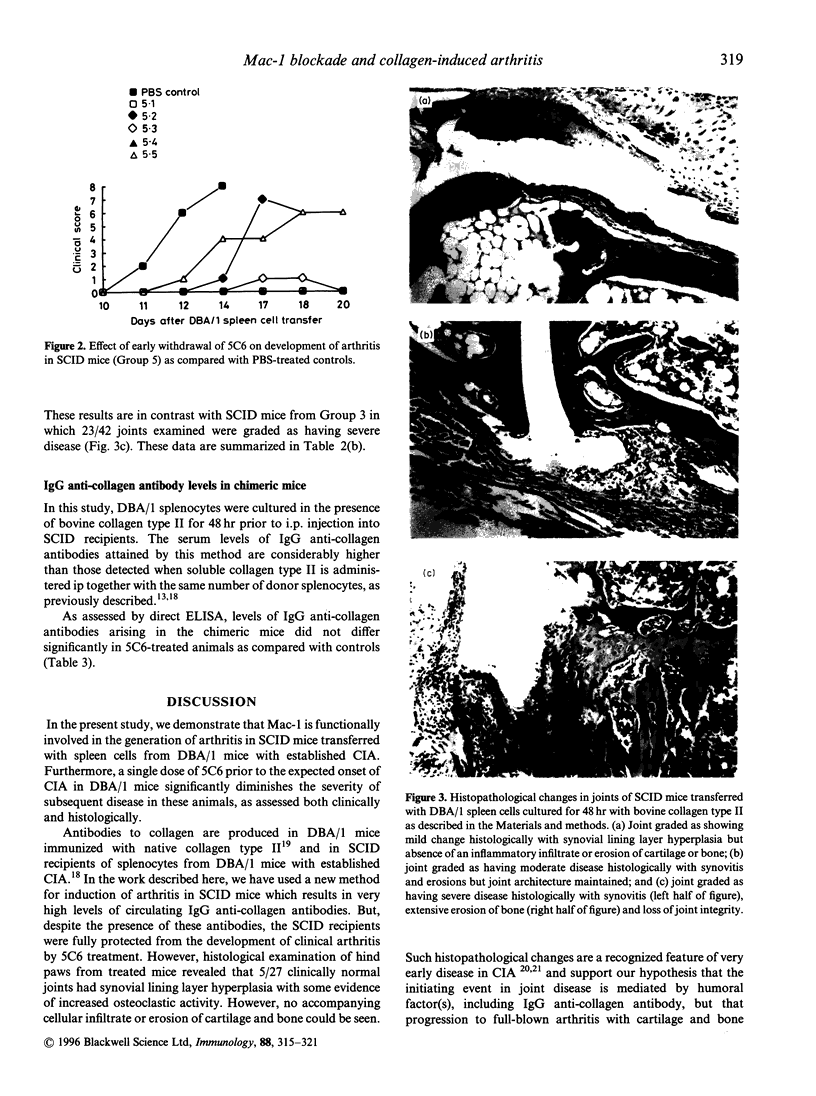
Collagen-induced arthritis in susceptible mice is widely accepted as an experimental model for human rheumatoid arthritis (RA). We have investigated the role of the Mac-1 integrin beta 2 in the development and maintenance of arthritis by means of in vivo administration of 5C6 monoclonal antibody (mAb) to block this receptor. Injection of a single dose of 5C6 mAb (0.5 mg, intraperitoneally) prior to the expected onset of collagen-induced arthritis in DBA/1 mice diminished the severity of subsequent disease in these animals, as assessed both clinically and histologically (P < 0.01, chi 2). In the DBA/1 to severe combined immunodeficiency (SCID) transfer model of arthritis, the incidence of clinical arthritis was significantly reduced in SCID mice receiving maintained 5C6 treatment commencing the day prior to administration of donor splenocytes. Histological evaluation of joints from animals without clinically evident arthritis confirmed the absence of an inflammatory infiltrate in 22/27 joints examined. In a minority of these joints, however, synovial hyperplasia was apparent. In contrast, delaying antibody administration until 10 days after donor spleen cell transfer failed to protect three of five SCID recipients. These results confirm a functional role for Mac-1 in the generation of collagen-induced arthritis in mice. Since mAb 5C6 is non-cytotoxic, its action must be by blockade of the interactions between Mac-1 and its natural ligand(s). Our findings support the hypothesis that cells expressing Mac-1 play an important role in the induction and maintenance of joint damage in collagen-induced arthritis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnaout M. A., Lanier L. L., Faller D. V. Relative contribution of the leukocyte molecules Mo1, LFA-1, and p150,95 (LeuM5) in adhesion of granulocytes and monocytes to vascular endothelium is tissue- and stimulus-specific. J Cell Physiol. 1988 Nov;137(2):305–309. doi: 10.1002/jcp.1041370214. [DOI] [PubMed] [Google Scholar]
- Arnaout M. A. Structure and function of the leukocyte adhesion molecules CD11/CD18. Blood. 1990 Mar 1;75(5):1037–1050. [PubMed] [Google Scholar]
- Berton G., Laudanna C., Sorio C., Rossi F. Generation of signals activating neutrophil functions by leukocyte integrins: LFA-1 and gp150/95, but not CR3, are able to stimulate the respiratory burst of human neutrophils. J Cell Biol. 1992 Feb;116(4):1007–1017. doi: 10.1083/jcb.116.4.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock W. E., Wright S. D. Role of the adherence-promoting receptors, CR3, LFA-1, and p150,95, in binding of Histoplasma capsulatum by human macrophages. J Exp Med. 1987 Jan 1;165(1):195–210. doi: 10.1084/jem.165.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper A., Rosen H., Blackwell J. M. Monoclonal antibodies that recognize distinct epitopes of the macrophage type three complement receptor differ in their ability to inhibit binding of Leishmania promastigotes harvested at different phases of their growth cycle. Immunology. 1988 Dec;65(4):511–514. [PMC free article] [PubMed] [Google Scholar]
- Graham I. L., Brown E. J. Extracellular calcium results in a conformational change in Mac-1 (CD11b/CD18) on neutrophils. Differentiation of adhesion and phagocytosis functions of Mac-1. J Immunol. 1991 Jan 15;146(2):685–691. [PubMed] [Google Scholar]
- Harlan J. M., Killen P. D., Senecal F. M., Schwartz B. R., Yee E. K., Taylor R. F., Beatty P. G., Price T. H., Ochs H. D. The role of neutrophil membrane glycoprotein GP-150 in neutrophil adherence to endothelium in vitro. Blood. 1985 Jul;66(1):167–178. [PubMed] [Google Scholar]
- Holmdahl R., Tarkowski A., Jonsson R. Involvement of macrophages and dendritic cells in synovial inflammation of collagen induced arthritis in DBA/1 mice and spontaneous arthritis in MRL/lpr mice. Autoimmunity. 1991;8(4):271–280. doi: 10.3109/08916939109007634. [DOI] [PubMed] [Google Scholar]
- Huitinga I., Damoiseaux J. G., Döpp E. A., Dijkstra C. D. Treatment with anti-CR3 antibodies ED7 and ED8 suppresses experimental allergic encephalomyelitis in Lewis rats. Eur J Immunol. 1993 Mar;23(3):709–715. doi: 10.1002/eji.1830230321. [DOI] [PubMed] [Google Scholar]
- Hutchings P., Rosen H., O'Reilly L., Simpson E., Gordon S., Cooke A. Transfer of diabetes in mice prevented by blockade of adhesion-promoting receptor on macrophages. Nature. 1990 Dec 13;348(6302):639–642. doi: 10.1038/348639a0. [DOI] [PubMed] [Google Scholar]
- Koo G. C., Rosen H., Sirotina A., Ma X. D., Shultz L. D. Anti-CD11b antibody prevents immunopathologic changes in viable moth-eaten bone marrow chimeric mice. J Immunol. 1993 Dec 15;151(12):6733–6741. [PubMed] [Google Scholar]
- Lecoanet-Henchoz S., Gauchat J. F., Aubry J. P., Graber P., Life P., Paul-Eugene N., Ferrua B., Corbi A. L., Dugas B., Plater-Zyberk C. CD23 regulates monocyte activation through a novel interaction with the adhesion molecules CD11b-CD18 and CD11c-CD18. Immunity. 1995 Jul;3(1):119–125. doi: 10.1016/1074-7613(95)90164-7. [DOI] [PubMed] [Google Scholar]
- Miller E. J. Structural studies on cartilage collagen employing limited cleavage and solubilization with pepsin. Biochemistry. 1972 Dec 19;11(26):4903–4909. doi: 10.1021/bi00776a005. [DOI] [PubMed] [Google Scholar]
- Nathan C., Srimal S., Farber C., Sanchez E., Kabbash L., Asch A., Gailit J., Wright S. D. Cytokine-induced respiratory burst of human neutrophils: dependence on extracellular matrix proteins and CD11/CD18 integrins. J Cell Biol. 1989 Sep;109(3):1341–1349. doi: 10.1083/jcb.109.3.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plater-Zyberk C., Clarke M. F., Lam K., Mumford P. A., Room G. R., Maini R. N. In vitro immunoglobulin synthesis by lymphocytes from patients with rheumatoid arthritis. I. Effect of monocyte depletion and demonstration of an increased proportion of lymphocytes forming rosettes with mouse erythrocytes. Clin Exp Immunol. 1983 Jun;52(3):505–511. [PMC free article] [PubMed] [Google Scholar]
- Ranges G. E., Sriram S., Cooper S. M. Prevention of type II collagen-induced arthritis by in vivo treatment with anti-L3T4. J Exp Med. 1985 Sep 1;162(3):1105–1110. doi: 10.1084/jem.162.3.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Gordon S. Monoclonal antibody to the murine type 3 complement receptor inhibits adhesion of myelomonocytic cells in vitro and inflammatory cell recruitment in vivo. J Exp Med. 1987 Dec 1;166(6):1685–1701. doi: 10.1084/jem.166.6.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Milon G., Gordon S. Antibody to the murine type 3 complement receptor inhibits T lymphocyte-dependent recruitment of myelomonocytic cells in vivo. J Exp Med. 1989 Feb 1;169(2):535–548. doi: 10.1084/jem.169.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H. Role of CR3 in induced myelomonocytic recruitment: insights from in vivo monoclonal antibody studies in the mouse. J Leukoc Biol. 1990 Nov;48(5):465–469. doi: 10.1002/jlb.48.5.465. [DOI] [PubMed] [Google Scholar]
- Ross G. D., Vetvicka V. CR3 (CD11b, CD18): a phagocyte and NK cell membrane receptor with multiple ligand specificities and functions. Clin Exp Immunol. 1993 May;92(2):181–184. doi: 10.1111/j.1365-2249.1993.tb03377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Stuart J. M., Dixon F. J. Serum transfer of collagen-induced arthritis in mice. J Exp Med. 1983 Aug 1;158(2):378–392. doi: 10.1084/jem.158.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart J. M., Townes A. S., Kang A. H. Nature and specificity of the immune response to collagen in type II collagen-induced arthritis in mice. J Clin Invest. 1982 Mar;69(3):673–683. doi: 10.1172/JCI110495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. C., Plater-Zyberk C., Maini R. N. The role of the B cells in the adoptive transfer of collagen-induced arthritis from DBA/1 (H-2q) to SCID (H-2d) mice. Eur J Immunol. 1995 Mar;25(3):763–769. doi: 10.1002/eji.1830250321. [DOI] [PubMed] [Google Scholar]
- Williams R. O., Plater-Zyberk C., Williams D. G., Maini R. N. Successful transfer of collagen-induced arthritis to severe combined immunodeficient (SCID) mice. Clin Exp Immunol. 1992 Jun;88(3):455–460. doi: 10.1111/j.1365-2249.1992.tb06471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]




