Abstract
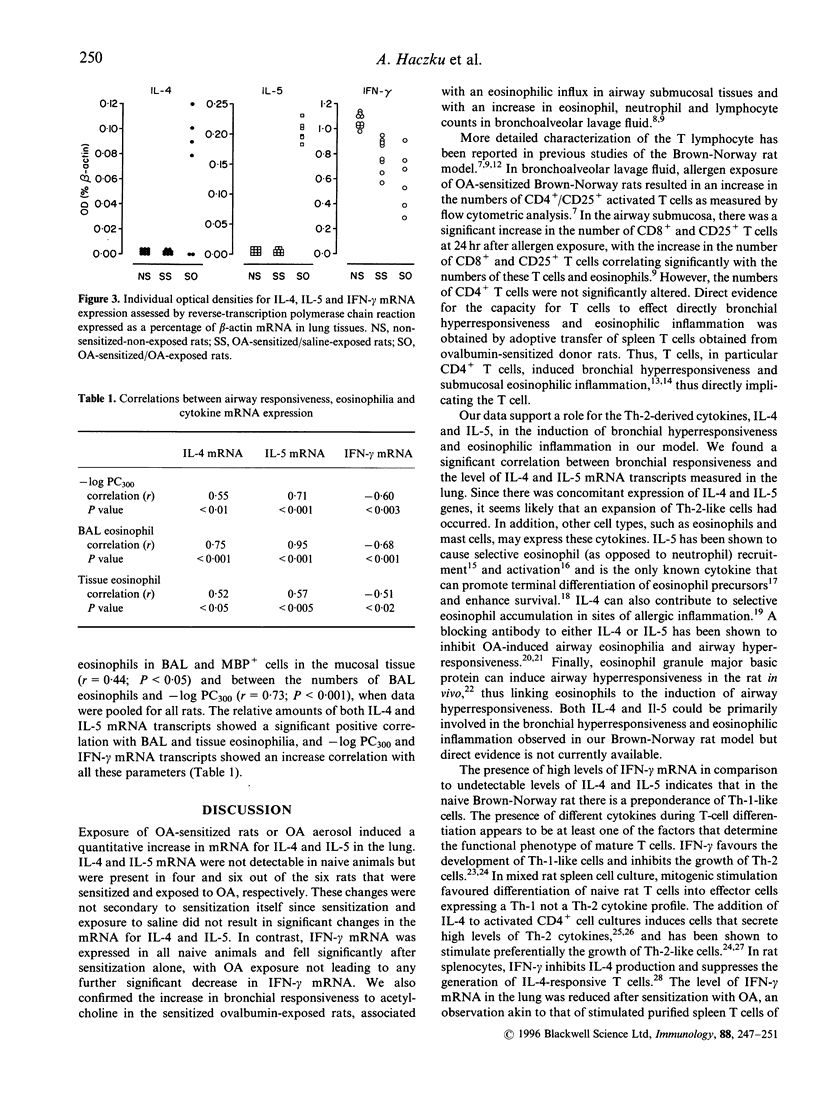
We determined the expression of Th-2 type cytokines, interleukin-4 (IL-4) and IL-5, and of the Th-1 type cytokine, interferon-gamma (IFN-gamma), in the Brown-Norway rat. Rats were intraperitoneally sensitized with ovalbumin and 21 days later were either exposed to ovalbumin or saline aerosol. The value -log PC300 (PC300 = concentration of acetylcholine needed to increase baseline lung resistance by 300%) was 2.49 +/- 0.15 in sensitized, exposed rats, was higher than in sensitized, saline-exposed or naive rats (1.54 +/- 0.27 and 1.63 +/- 0.06 respectively, P < 0.05). There was a significant increase in eosinophils in bronchoalveolar lavage fluid and in airway submucosal airway tissues in the sensitized exposed group. Reverse-transcriptase polymerase chain reaction was performed on total lung RNA using primers for IL-4, IL-5, IFN-gamma and beta-actin. IL-4 and IL-5 mRNA levels in control and sensitized saline-exposed rats were not detectable, but increased levels were found in sensitized and ovalbumin-exposed rats with levels of 0.25 +/- 0.01 and 0.98 +/- 0.02% of beta-actin mRNA as assessed by densitometric measurements. Expression of IFN-gamma mRNA was significantly reduced in sensitized and ovalbumin-exposed rats. As in asthmatic airways, there is an increased expression of Th-2 cytokines, IL-4 and IL-5, together with a reduction in the Th-1 cytokine, IFN-gamma, thus supporting a role for Th-2 cytokines in allergic eosinophilic inflammation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azzawi M., Bradley B., Jeffery P. K., Frew A. J., Wardlaw A. J., Knowles G., Assoufi B., Collins J. V., Durham S., Kay A. B. Identification of activated T lymphocytes and eosinophils in bronchial biopsies in stable atopic asthma. Am Rev Respir Dis. 1990 Dec;142(6 Pt 1):1407–1413. doi: 10.1164/ajrccm/142.6_Pt_1.1407. [DOI] [PubMed] [Google Scholar]
- Clutterbuck E. J., Hirst E. M., Sanderson C. J. Human interleukin-5 (IL-5) regulates the production of eosinophils in human bone marrow cultures: comparison and interaction with IL-1, IL-3, IL-6, and GMCSF. Blood. 1989 May 1;73(6):1504–1512. [PubMed] [Google Scholar]
- Diaz-Sanchez D., Noble A., Staynov D. Z., Lee T. H., Kemeny D. M. Elimination of IgE regulatory rat CD8+ T cells in vivo differentially modulates interleukin-4 and interferon-gamma but not interleukin-2 production by splenic T cells. Immunology. 1993 Apr;78(4):513–519. [PMC free article] [PubMed] [Google Scholar]
- Djukanović R., Roche W. R., Wilson J. W., Beasley C. R., Twentyman O. P., Howarth R. H., Holgate S. T. Mucosal inflammation in asthma. Am Rev Respir Dis. 1990 Aug;142(2):434–457. doi: 10.1164/ajrccm/142.2.434. [DOI] [PubMed] [Google Scholar]
- Elwood W., Lötvall J. O., Barnes P. J., Chung K. F. Characterization of allergen-induced bronchial hyperresponsiveness and airway inflammation in actively sensitized brown-Norway rats. J Allergy Clin Immunol. 1991 Dec;88(6):951–960. doi: 10.1016/0091-6749(91)90253-k. [DOI] [PubMed] [Google Scholar]
- Fernandez-Botran R., Sanders V. M., Mosmann T. R., Vitetta E. S. Lymphokine-mediated regulation of the proliferative response of clones of T helper 1 and T helper 2 cells. J Exp Med. 1988 Aug 1;168(2):543–558. doi: 10.1084/jem.168.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski T. F., Joyce J., Fitch F. W. Antiproliferative effect of IFN-gamma in immune regulation. III. Differential selection of TH1 and TH2 murine helper T lymphocyte clones using recombinant IL-2 and recombinant IFN-gamma. J Immunol. 1989 Jul 1;143(1):15–22. [PubMed] [Google Scholar]
- Gavett S. H., Chen X., Finkelman F., Wills-Karp M. Depletion of murine CD4+ T lymphocytes prevents antigen-induced airway hyperreactivity and pulmonary eosinophilia. Am J Respir Cell Mol Biol. 1994 Jun;10(6):587–593. doi: 10.1165/ajrcmb.10.6.8003337. [DOI] [PubMed] [Google Scholar]
- Greenbaum L. A., Horowitz J. B., Woods A., Pasqualini T., Reich E. P., Bottomly K. Autocrine growth of CD4+ T cells. Differential effects of IL-1 on helper and inflammatory T cells. J Immunol. 1988 Mar 1;140(5):1555–1560. [PubMed] [Google Scholar]
- Haczku A., Chung K. F., Sun J., Barnes P. J., Kay A. B., Moqbel R. Airway hyperresponsiveness, elevation of serum-specific IgE and activation of T cells following allergen exposure in sensitized Brown-Norway rats. Immunology. 1995 Aug;85(4):598–603. [PMC free article] [PubMed] [Google Scholar]
- Haczku A., Chung K. F., Sun J., Barnes P. J., Kay A. B., Moqbel R. Airway hyperresponsiveness, elevation of serum-specific IgE and activation of T cells following allergen exposure in sensitized Brown-Norway rats. Immunology. 1995 Aug;85(4):598–603. [PMC free article] [PubMed] [Google Scholar]
- Hamid Q., Azzawi M., Ying S., Moqbel R., Wardlaw A. J., Corrigan C. J., Bradley B., Durham S. R., Collins J. V., Jeffery P. K. Expression of mRNA for interleukin-5 in mucosal bronchial biopsies from asthma. J Clin Invest. 1991 May;87(5):1541–1546. doi: 10.1172/JCI115166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez A. F., Sanderson C. J., Gamble J. R., Campbell H. D., Young I. G., Vadas M. A. Recombinant human interleukin 5 is a selective activator of human eosinophil function. J Exp Med. 1988 Jan 1;167(1):219–224. doi: 10.1084/jem.167.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moqbel R., Barkans J., Bradley B. L., Durham S. R., Kay A. B. Application of monoclonal antibodies against major basic protein (BMK-13) and eosinophil cationic protein (EG1 and EG2) for quantifying eosinophils in bronchial biopsies from atopic asthma. Clin Exp Allergy. 1992 Feb;22(2):265–273. doi: 10.1111/j.1365-2222.1992.tb03082.x. [DOI] [PubMed] [Google Scholar]
- Noble A., Staynov D. Z., Kemeny D. M. Generation of rat Th2-like cells in vitro is interleukin-4-dependent and inhibited by interferon-gamma. Immunology. 1993 Aug;79(4):562–567. [PMC free article] [PubMed] [Google Scholar]
- Renzi P. M., Olivenstein R., Martin J. G. Inflammatory cell populations in the airways and parenchyma after antigen challenge in the rat. Am Rev Respir Dis. 1993 Apr;147(4):967–974. doi: 10.1164/ajrccm/147.4.967. [DOI] [PubMed] [Google Scholar]
- Robinson D. S., Hamid Q., Ying S., Tsicopoulos A., Barkans J., Bentley A. M., Corrigan C., Durham S. R., Kay A. B. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992 Jan 30;326(5):298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- Robinson D., Hamid Q., Bentley A., Ying S., Kay A. B., Durham S. R. Activation of CD4+ T cells, increased TH2-type cytokine mRNA expression, and eosinophil recruitment in bronchoalveolar lavage after allergen inhalation challenge in patients with atopic asthma. J Allergy Clin Immunol. 1993 Aug;92(2):313–324. doi: 10.1016/0091-6749(93)90175-f. [DOI] [PubMed] [Google Scholar]
- Schleimer R. P., Sterbinsky S. A., Kaiser J., Bickel C. A., Klunk D. A., Tomioka K., Newman W., Luscinskas F. W., Gimbrone M. A., Jr, McIntyre B. W. IL-4 induces adherence of human eosinophils and basophils but not neutrophils to endothelium. Association with expression of VCAM-1. J Immunol. 1992 Feb 15;148(4):1086–1092. [PubMed] [Google Scholar]
- Seder R. A., Paul W. E., Davis M. M., Fazekas de St Groth B. The presence of interleukin 4 during in vitro priming determines the lymphokine-producing potential of CD4+ T cells from T cell receptor transgenic mice. J Exp Med. 1992 Oct 1;176(4):1091–1098. doi: 10.1084/jem.176.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staynov D. Z., Lee T. H. Expression of interleukin-5 and granulocyte-macrophage colony-stimulating factor in human peripheral blood mononuclear cells after activation with phorbol myristate acetate. Immunology. 1992 Jan;75(1):196–201. [PMC free article] [PubMed] [Google Scholar]
- Swain S. L., Weinberg A. D., English M., Huston G. IL-4 directs the development of Th2-like helper effectors. J Immunol. 1990 Dec 1;145(11):3796–3806. [PubMed] [Google Scholar]
- Uchida D. A., Ackerman S. J., Coyle A. J., Larsen G. L., Weller P. F., Freed J., Irvin C. G. The effect of human eosinophil granule major basic protein on airway responsiveness in the rat in vivo. A comparison with polycations. Am Rev Respir Dis. 1993 Apr;147(4):982–988. doi: 10.1164/ajrccm/147.4.982. [DOI] [PubMed] [Google Scholar]
- Van Oosterhout A. J., Ladenius A. R., Savelkoul H. F., Van Ark I., Delsman K. C., Nijkamp F. P. Effect of anti-IL-5 and IL-5 on airway hyperreactivity and eosinophils in guinea pigs. Am Rev Respir Dis. 1993 Mar;147(3):548–552. doi: 10.1164/ajrccm/147.3.548. [DOI] [PubMed] [Google Scholar]
- Wang J. M., Rambaldi A., Biondi A., Chen Z. G., Sanderson C. J., Mantovani A. Recombinant human interleukin 5 is a selective eosinophil chemoattractant. Eur J Immunol. 1989 Apr;19(4):701–705. doi: 10.1002/eji.1830190420. [DOI] [PubMed] [Google Scholar]
- Waserman S., Olivenstein R., Renzi P., Xu L. J., Martin J. G. The relationship between late asthmatic responses and antigen-specific immunoglobulin. J Allergy Clin Immunol. 1992 Oct;90(4 Pt 1):661–669. doi: 10.1016/0091-6749(92)90140-w. [DOI] [PubMed] [Google Scholar]
- Watanabe A., Mishima H., Renzi P. M., Xu L. J., Hamid Q., Martin J. G. Transfer of allergic airway responses with antigen-primed CD4+ but not CD8+ T cells in brown Norway rats. J Clin Invest. 1995 Sep;96(3):1303–1310. doi: 10.1172/JCI118165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y., Hayashi Y., Sugama Y., Miura Y., Kasahara T., Kitamura S., Torisu M., Mita S., Tominaga A., Takatsu K. Highly purified murine interleukin 5 (IL-5) stimulates eosinophil function and prolongs in vitro survival. IL-5 as an eosinophil chemotactic factor. J Exp Med. 1988 May 1;167(5):1737–1742. doi: 10.1084/jem.167.5.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]