Abstract
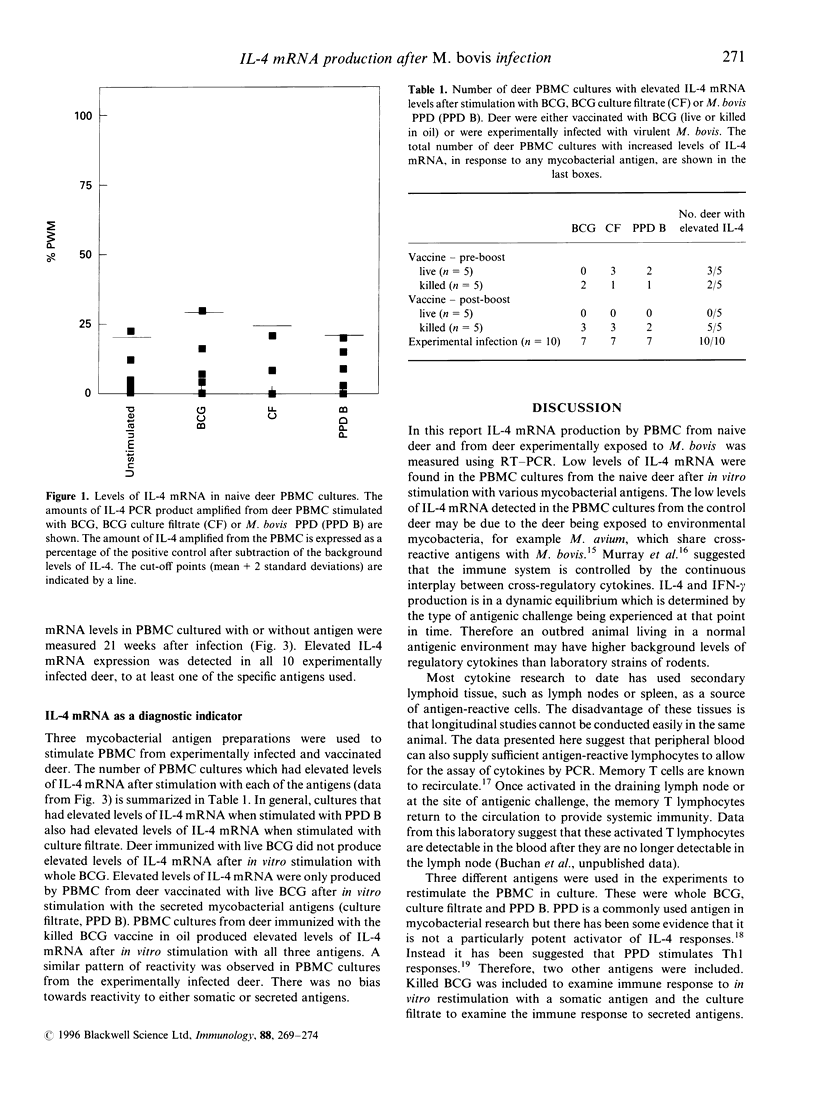
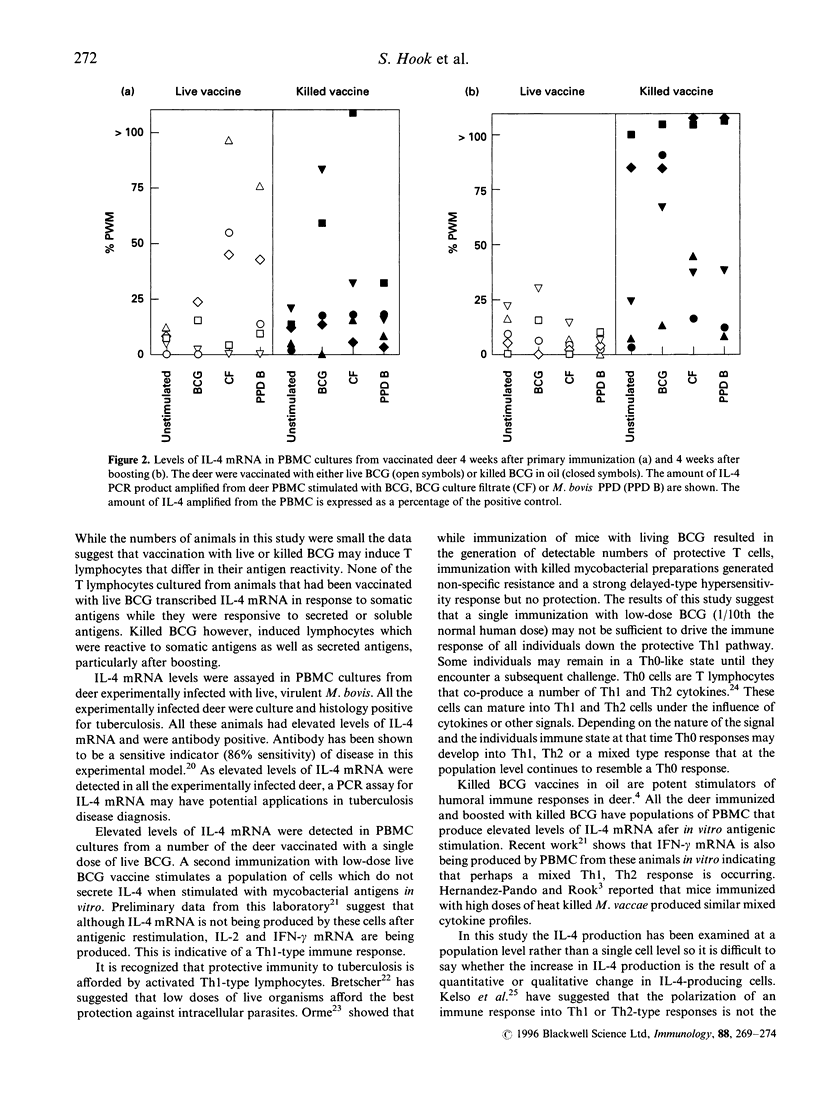
This study examines the expression of mRNA for the Th2 cytokine, interleukin-4 (IL-4). Peripheral blood mononuclear cells from deer infected with Mycobacterium bovis or vaccinated with live or killed M. bovis bacillus Calmette-Guérin (BCG) were cultured with mycobacterial antigens. IL-4 mRNA production was assayed using the polymerase chain reaction. Elevated levels of IL-4 mRNA were detected in response to at least one antigen preparation in all animals infected with M. bovis as compared with none of the non-infected control animals. After a primary immunization, elevated levels of IL-4 mRNA were detected in only a proportion of vaccinated animals and this did not correlate with whether the vaccine was live BCG or killed BCG in oil. After boosting, all the animals vaccinated with killed BCG in oil exhibited elevated IL-4 mRNA production whereas none of the animals vaccinated with live BCG showed elevated levels. The data suggest that IL-4 is turned off during the immune response to live BCG, that boosting of low-dose live BCG vaccine may be required to 'imprint' this signal and that this may be important in the development of protective immunity to tuberculosis. Killed BCG in adjuvant is not protective and as with experimental infection with virulent M. bovis it failed to switch off the IL-4 response. IL-4 may be useful as a diagnostic tool and as an in vitro marker of vaccine efficacy.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bretscher P. A. A strategy to improve the efficacy of vaccination against tuberculosis and leprosy. Immunol Today. 1992 Sep;13(9):342–345. doi: 10.1016/0167-5699(92)90168-7. [DOI] [PubMed] [Google Scholar]
- Buchan G. S., Griffin J. F. Tuberculosis in domesticated deer (Cervus elaphus): a large animal model for human tuberculosis. J Comp Pathol. 1990 Jul;103(1):11–22. doi: 10.1016/s0021-9975(08)80131-4. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Coffman R. L., Varkila K., Scott P., Chatelain R. Role of cytokines in the differentiation of CD4+ T-cell subsets in vivo. Immunol Rev. 1991 Oct;123:189–207. doi: 10.1111/j.1600-065x.1991.tb00611.x. [DOI] [PubMed] [Google Scholar]
- Daugelat S., Ladel C. H., Kaufmann S. H. Influence of mouse strain and vaccine viability on T-cell responses induced by Mycobacterium bovis bacillus Calmette-Guérin. Infect Immun. 1995 May;63(5):2033–2040. doi: 10.1128/iai.63.5.2033-2040.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine P. E. The BCG story: lessons from the past and implications for the future. Rev Infect Dis. 1989 Mar-Apr;11 (Suppl 2):S353–S359. doi: 10.1093/clinids/11.supplement_2.s353. [DOI] [PubMed] [Google Scholar]
- Firestein G. S., Roeder W. D., Laxer J. A., Townsend K. S., Weaver C. T., Hom J. T., Linton J., Torbett B. E., Glasebrook A. L. A new murine CD4+ T cell subset with an unrestricted cytokine profile. J Immunol. 1989 Jul 15;143(2):518–525. [PubMed] [Google Scholar]
- Gray D. Immunological memory. Annu Rev Immunol. 1993;11:49–77. doi: 10.1146/annurev.iy.11.040193.000405. [DOI] [PubMed] [Google Scholar]
- Griffin J. F., Buchan G. S. Aetiology, pathogenesis and diagnosis of Mycobacterium bovis in deer. Vet Microbiol. 1994 May;40(1-2):193–205. doi: 10.1016/0378-1135(94)90055-8. [DOI] [PubMed] [Google Scholar]
- Griffin J. F., Buchan G. S. Vaccination against tuberculosis: is BCG more sinned against than sinner? Immunol Cell Biol. 1993 Oct;71(Pt 5):431–442. doi: 10.1038/icb.1993.49. [DOI] [PubMed] [Google Scholar]
- Griffin J. F., Cross J. P., Chinn D. N., Rodgers C. R., Buchan G. S. Diagnosis of tuberculosis due to Mycobacterium bovis in New Zealand red deer (Cervus elaphus) using a composite blood test and antibody assays. N Z Vet J. 1994 Oct;42(5):173–179. doi: 10.1080/00480169.1994.35815. [DOI] [PubMed] [Google Scholar]
- Griffin J. F., Mackintosh C. G., Buchan G. S. Animal models of protective immunity in tuberculosis to evaluate candidate vaccines. Trends Microbiol. 1995 Nov;3(11):418–424. doi: 10.1016/s0966-842x(00)88994-5. [DOI] [PubMed] [Google Scholar]
- Hernandez-Pando R., Rook G. A. The role of TNF-alpha in T-cell-mediated inflammation depends on the Th1/Th2 cytokine balance. Immunology. 1994 Aug;82(4):591–595. [PMC free article] [PubMed] [Google Scholar]
- Hook S. M., Crawford A. M., Chinn D. N., Griffin J. F., Buchan G. S. Cloning and expression of the cervine interleukin 4 gene. Scand J Immunol. 1994 Jul;40(1):71–76. doi: 10.1111/j.1365-3083.1994.tb03435.x. [DOI] [PubMed] [Google Scholar]
- Kelso A., Groves P., Troutt A. B., Francis K. Evidence for the stochastic acquisition of cytokine profile by CD4+ T cells activated in a T helper type 2-like response in vivo. Eur J Immunol. 1995 May;25(5):1168–1175. doi: 10.1002/eji.1830250506. [DOI] [PubMed] [Google Scholar]
- Kollias G. V., Jr, Thoen C. O., Fowler M. E. Evaluation of comparative cervical tuberculin skin testing in cervids naturally exposed to mycobacteria. J Am Vet Med Assoc. 1982 Dec 1;181(11):1257–1262. [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Murray J. S., Madri J., Pasqualini T., Bottomly K. Functional CD4 T cell subset interplay in an intact immune system. J Immunol. 1993 May 15;150(10):4270–4276. [PubMed] [Google Scholar]
- Orme I. M., Roberts A. D., Griffin J. P., Abrams J. S. Cytokine secretion by CD4 T lymphocytes acquired in response to Mycobacterium tuberculosis infection. J Immunol. 1993 Jul 1;151(1):518–525. [PubMed] [Google Scholar]
- Saito S., Dorf M. E., Watanabe N., Tadakuma T. Preferential induction of IL-4 is determined by the type and duration of antigenic stimulation. Cell Immunol. 1994 Jan;153(1):1–8. doi: 10.1006/cimm.1994.1001. [DOI] [PubMed] [Google Scholar]
- Surcel H. M., Troye-Blomberg M., Paulie S., Andersson G., Moreno C., Pasvol G., Ivanyi J. Th1/Th2 profiles in tuberculosis, based on the proliferation and cytokine response of blood lymphocytes to mycobacterial antigens. Immunology. 1994 Feb;81(2):171–176. [PMC free article] [PubMed] [Google Scholar]
- Sánchez F. O., Rodríguez J. I., Agudelo G., García L. F. Immune responsiveness and lymphokine production in patients with tuberculosis and healthy controls. Infect Immun. 1994 Dec;62(12):5673–5678. doi: 10.1128/iai.62.12.5673-5678.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]


