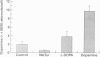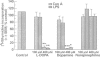Abstract
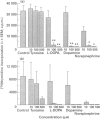
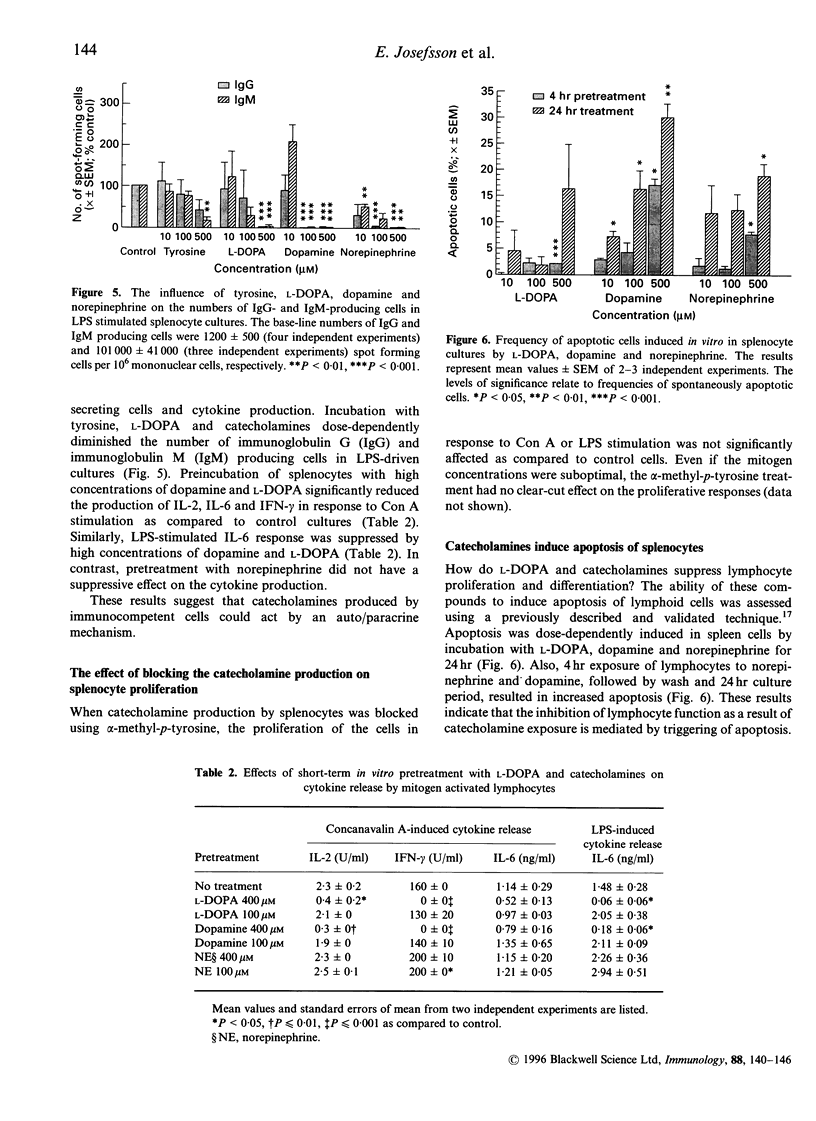
The immune and the nervous systems are anatomically closely related and interact with each other by molecules common to both systems, such as cytokines and neurotransmitters. The purpose of this study was to investigate the participation of catecholamines in the neuroimmunological network. The ability of immune cells to produce catecholamines was examined by a highly sensitive capillary electrophoresis assay, which permits detection of easily oxidized catecholamines in the zeptomole (10(-21)) range. In addition, the effects of catecholamines on in vitro proliferation, differentiation and apoptosis of lymphocytes were assessed. Mouse spleen cells and macrophages contained on average 7 x 10(-17) and 2 x 10(-17) mole dopamine per cell, respectively. In the former cell population also norepinephrine was found. Several mouse B- and T-cell hybridomas were also shown to contain endogenously produced dopamine in levels ranging from 7 x 10(-20) to 2 x 10(-18) mole dopamine per cell. In addition, one of the T-cell hybridomas proved to synthesize norepinephrine. The dopamine production of lymphocytes was blocked by the tyrosine hydroxylase inhibitor alpha-methyl-p-tyrosine, whereas incubation with the precursor L-DOPA increased the dopamine content. Incubation with L-DOPA, dopamine and norepinephrine dose-dependently suppressed mitogen induced proliferation and differentiation of mouse lymphocytes. Even short-time pretreatment of lymphocytes with L-DOPA and dopamine strongly suppressed lymphocyte proliferation and cytokine production. Incubation of lymphoid cells with L-DOPA, dopamine and norepinephrine dose-dependently induced apoptosis which, at least partly, explains the suppressive effects of catecholamines on lymphocyte function. Our results demonstrate that catecholamines: (i) are actively produced by lymphocytes and (ii) have the capacity to act as auto- and/or paracrine regulators of lymphocyte activity through induction of apoptosis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentley A. J., Zorgani A. A., Blackwell C. C., Weir D. M., Busuttil A. Bacterial toxins and sudden unexpected death in a young child. Forensic Sci Int. 1997 Aug 4;88(2):141–146. doi: 10.1016/s0379-0738(97)00067-4. [DOI] [PubMed] [Google Scholar]
- Bentley A. J., Zorgani A. A., Blackwell C. C., Weir D. M., Busuttil A. Bacterial toxins and sudden unexpected death in a young child. Forensic Sci Int. 1997 Aug 4;88(2):141–146. doi: 10.1016/s0379-0738(97)00067-4. [DOI] [PubMed] [Google Scholar]
- Bergquist J., Tarkowski A., Ekman R., Ewing A. Discovery of endogenous catecholamines in lymphocytes and evidence for catecholamine regulation of lymphocyte function via an autocrine loop. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12912–12916. doi: 10.1073/pnas.91.26.12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blalock J. E. A molecular basis for bidirectional communication between the immune and neuroendocrine systems. Physiol Rev. 1989 Jan;69(1):1–32. doi: 10.1152/physrev.1989.69.1.1. [DOI] [PubMed] [Google Scholar]
- Bremell T., Abdelnour A., Tarkowski A. Histopathological and serological progression of experimental Staphylococcus aureus arthritis. Infect Immun. 1992 Jul;60(7):2976–2985. doi: 10.1128/iai.60.7.2976-2985.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerkinsky C. C., Nilsson L. A., Nygren H., Ouchterlony O., Tarkowski A. A solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells. J Immunol Methods. 1983 Dec 16;65(1-2):109–121. doi: 10.1016/0022-1759(83)90308-3. [DOI] [PubMed] [Google Scholar]
- Dahlström A., Häggendal J., Hökfelt T. The noradrenaline content of the varicosities of sympathetic adrenergic nerve terminals in the rat. Acta Physiol Scand. 1966 Jul-Aug;67(3):289–294. doi: 10.1111/j.1748-1716.1966.tb03314.x. [DOI] [PubMed] [Google Scholar]
- Dahlström A., Häggendal J. Some quantitative studies on the noradrenaline content in the cell bodies and terminals of a sympathetic adrenergic neuron system. Acta Physiol Scand. 1966 Jul-Aug;67(3):271–277. doi: 10.1111/j.1748-1716.1966.tb03312.x. [DOI] [PubMed] [Google Scholar]
- Felsner P., Hofer D., Rinner I., Mangge H., Gruber M., Korsatko W., Schauenstein K. Continuous in vivo treatment with catecholamines suppresses in vitro reactivity of rat peripheral blood T-lymphocytes via alpha-mediated mechanisms. J Neuroimmunol. 1992 Mar;37(1-2):47–57. doi: 10.1016/0165-5728(92)90154-d. [DOI] [PubMed] [Google Scholar]
- Felten D. L., Felten S. Y., Bellinger D. L., Carlson S. L., Ackerman K. D., Madden K. S., Olschowki J. A., Livnat S. Noradrenergic sympathetic neural interactions with the immune system: structure and function. Immunol Rev. 1987 Dec;100:225–260. doi: 10.1111/j.1600-065x.1987.tb00534.x. [DOI] [PubMed] [Google Scholar]
- Grahnén A., Eckernäs S. A., Collin C., Ling-Andersson A., Tiger G., Nilsson M. Comparative multiple-dose pharmacokinetics of controlled-release levodopa products. Eur Neurol. 1992;32(6):343–348. doi: 10.1159/000116858. [DOI] [PubMed] [Google Scholar]
- Josefsson E., Månsson J. E., Blennow K., Tarkowski A. Immunomodulating and anti-inflammatory properties of the sympatholytic compound 6-hydroxydopamine. J Neuroimmunol. 1994 Dec;55(2):161–169. doi: 10.1016/0165-5728(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Konttinen Y. T., Kemppinen P., Segerberg M., Hukkanen M., Rees R., Santavirta S., Sorsa T., Pertovaara A., Polak J. M. Peripheral and spinal neural mechanisms in arthritis, with particular reference to treatment of inflammation and pain. Arthritis Rheum. 1994 Jul;37(7):965–982. doi: 10.1002/art.1780370701. [DOI] [PubMed] [Google Scholar]
- Levine J. D., Dardick S. J., Roizen M. F., Helms C., Basbaum A. I. Contribution of sensory afferents and sympathetic efferents to joint injury in experimental arthritis. J Neurosci. 1986 Dec;6(12):3423–3429. doi: 10.1523/JNEUROSCI.06-12-03423.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J. D., Fye K., Heller P., Basbaum A. I., Whiting-O'Keefe Q. Clinical response to regional intravenous guanethidine in patients with rheumatoid arthritis. J Rheumatol. 1986 Dec;13(6):1040–1043. [PubMed] [Google Scholar]
- Mapp P. I., Kidd B. L., Gibson S. J., Terry J. M., Revell P. A., Ibrahim N. B., Blake D. R., Polak J. M. Substance P-, calcitonin gene-related peptide- and C-flanking peptide of neuropeptide Y-immunoreactive fibres are present in normal synovium but depleted in patients with rheumatoid arthritis. Neuroscience. 1990;37(1):143–153. doi: 10.1016/0306-4522(90)90199-e. [DOI] [PubMed] [Google Scholar]
- Nicoletti I., Migliorati G., Pagliacci M. C., Grignani F., Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991 Jun 3;139(2):271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- Santambrogio L., Benedetti M., Chao M. V., Muzaffar R., Kulig K., Gabellini N., Hochwald G. Nerve growth factor production by lymphocytes. J Immunol. 1994 Nov 15;153(10):4488–4495. [PubMed] [Google Scholar]
- Santambrogio L., Lipartiti M., Bruni A., Dal Toso R. Dopamine receptors on human T- and B-lymphocytes. J Neuroimmunol. 1993 Jun;45(1-2):113–119. doi: 10.1016/0165-5728(93)90170-4. [DOI] [PubMed] [Google Scholar]
- Slominski A., Goodman-Snitkoff G. G. Dopa inhibits induced proliferative activity of murine and human lymphocytes. Anticancer Res. 1992 May-Jun;12(3):753–756. [PubMed] [Google Scholar]
- Tarkowski E., Naver H., Wallin B. G., Blomstrand C., Tarkowski A. Lateralization of T-lymphocyte responses in patients with stroke. Effect of sympathetic dysfunction? Stroke. 1995 Jan;26(1):57–62. doi: 10.1161/01.str.26.1.57. [DOI] [PubMed] [Google Scholar]
- Williams L. T., Snyderman R., Lefkowitz R. J. Identification of beta-adrenergic receptors in human lymphocytes by (-) (3H) alprenolol binding. J Clin Invest. 1976 Jan;57(1):149–155. doi: 10.1172/JCI108254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin D., Kondo S., Takeuchi J., Morimura T. Induction of apoptosis in murine ACTH-secreting pituitary adenoma cells by bromocriptine. FEBS Lett. 1994 Feb 14;339(1-2):73–75. doi: 10.1016/0014-5793(94)80387-0. [DOI] [PubMed] [Google Scholar]
- Zhao Y. X., Abdelnour A., Holmdahl R., Tarkowski A. Mice with the xid B cell defect are less susceptible to developing Staphylococcus aureus-induced arthritis. J Immunol. 1995 Aug 15;155(4):2067–2076. [PubMed] [Google Scholar]
- van der Poll T., Jansen J., Endert E., Sauerwein H. P., van Deventer S. J. Noradrenaline inhibits lipopolysaccharide-induced tumor necrosis factor and interleukin 6 production in human whole blood. Infect Immun. 1994 May;62(5):2046–2050. doi: 10.1128/iai.62.5.2046-2050.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]