Abstract
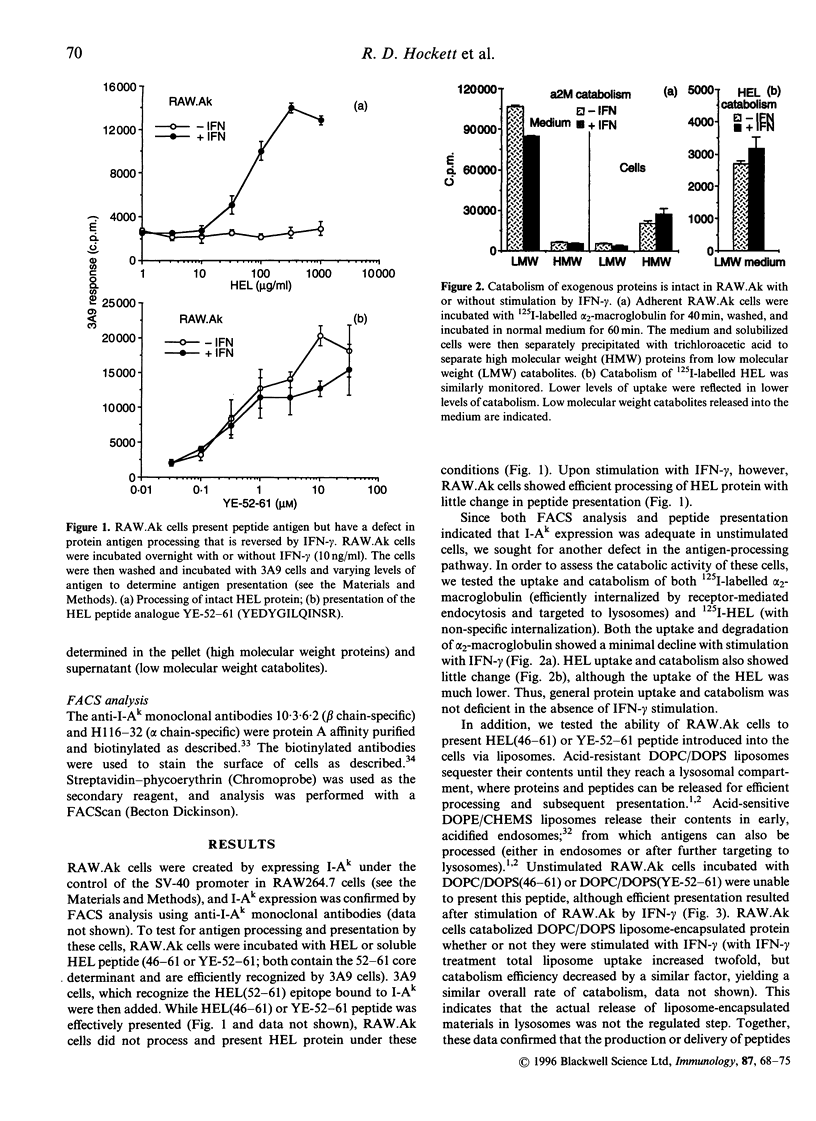
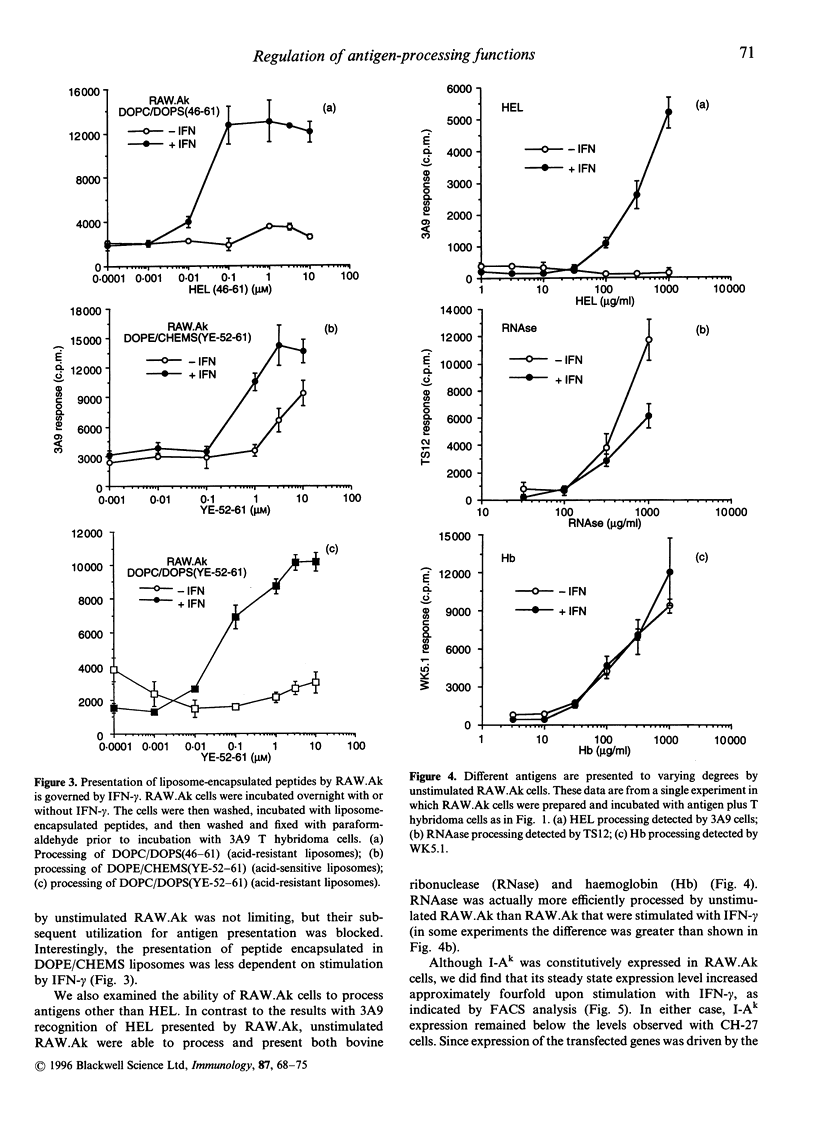
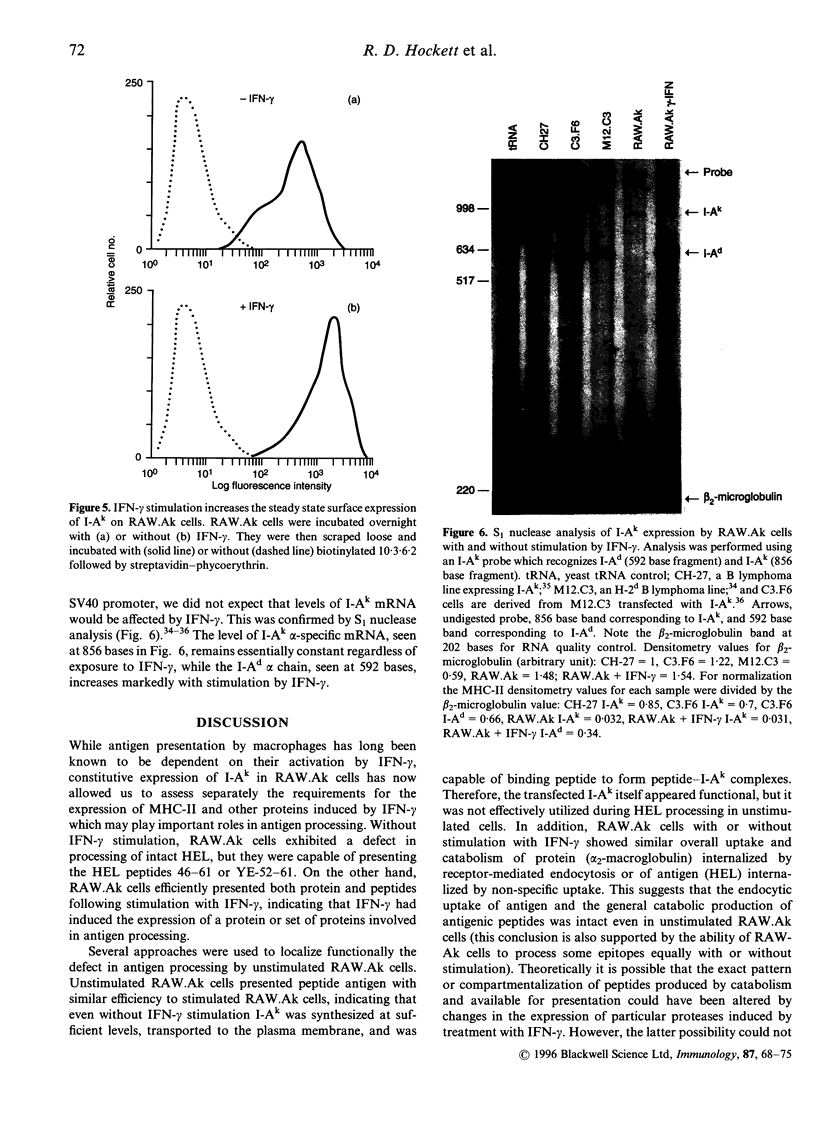
RAW264.7 cells were transfected to express constitutively the murine class II major histocompatibility complex (MHC-II) molecule, I-Ak. The resulting RAW.Ak cells presented HEL(46-61) peptide to 3A9 T hybridoma cells, but they were unable to process and present HEL protein in their resting state. However, IFN-gamma stimulation induced the ability of RAW.Ak to process and present HEL protein, with little effect on their ability to present HEL(46-61) peptide. Antigen catabolism showed little change with IFN-gamma stimulation, suggesting that the production of peptides was not the regulated step in the processing pathway. Furthermore, HEL(46-61) peptide delivered directly into lysosomes by acid-resistant liposomes was also presented only upon IFN-gamma stimulation, while the presentation of peptides delivered into endosomes by acid-sensitive liposomes showed a lesser dependence on IFN-gamma stimulation. Thus, IFN-gamma regulated the ability of peptides delivered into certain lysosomal compartments to meet with MHC-II molecules and form peptide-MHC complexes, or to transport subsequently to the plasma membrane. Two other antigens, ribonuclease A and haemoglobin, were processed by RAW.Ak cells without IFN-gamma stimulation, suggesting that these antigens could be processed by different mechanisms, perhaps in earlier endocytic compartments. Thus, different antigens may be processed in distinct endocytic compartments, and an IFN-gamma-regulated mechanism controls the rescue of peptides from lysosomal compartments for presentation at the plasma membrane.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen P. M., Matsueda G. R., Adams S., Freeman J., Roof R. W., Lambert L., Unanue E. R. Enhanced immunogenicity of a T cell immunogenic peptide by modifications of its N and C termini. Int Immunol. 1989;1(2):141–150. doi: 10.1093/intimm/1.2.141. [DOI] [PubMed] [Google Scholar]
- Allen P. M., Strydom D. J., Unanue E. R. Processing of lysozyme by macrophages: identification of the determinant recognized by two T-cell hybridomas. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2489–2493. doi: 10.1073/pnas.81.8.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amigorena S., Drake J. R., Webster P., Mellman I. Transient accumulation of new class II MHC molecules in a novel endocytic compartment in B lymphocytes. Nature. 1994 May 12;369(6476):113–120. doi: 10.1038/369113a0. [DOI] [PubMed] [Google Scholar]
- Bakke O., Dobberstein B. MHC class II-associated invariant chain contains a sorting signal for endosomal compartments. Cell. 1990 Nov 16;63(4):707–716. doi: 10.1016/0092-8674(90)90137-4. [DOI] [PubMed] [Google Scholar]
- Brooks A. G., Campbell P. L., Reynolds P., Gautam A. M., McCluskey J. Antigen presentation and assembly by mouse I-Ak class II molecules in human APC containing deleted or mutated HLA DM genes. J Immunol. 1994 Dec 15;153(12):5382–5392. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Collins D. S., Findlay K., Harding C. V. Processing of exogenous liposome-encapsulated antigens in vivo generates class I MHC-restricted T cell responses. J Immunol. 1992 Jun 1;148(11):3336–3341. [PubMed] [Google Scholar]
- Collins D. S., Unanue E. R., Harding C. V. Reduction of disulfide bonds within lysosomes is a key step in antigen processing. J Immunol. 1991 Dec 15;147(12):4054–4059. [PubMed] [Google Scholar]
- Donermeyer D. L., Allen P. M. Binding to Ia protects an immunogenic peptide from proteolytic degradation. J Immunol. 1989 Feb 15;142(4):1063–1068. [PubMed] [Google Scholar]
- Finn F. M., Titus G., Hofmann K. Ligands for insulin receptor isolation. Biochemistry. 1984 Jun 5;23(12):2554–2558. doi: 10.1021/bi00307a003. [DOI] [PubMed] [Google Scholar]
- Fling S. P., Arp B., Pious D. HLA-DMA and -DMB genes are both required for MHC class II/peptide complex formation in antigen-presenting cells. Nature. 1994 Apr 7;368(6471):554–558. doi: 10.1038/368554a0. [DOI] [PubMed] [Google Scholar]
- Germain R. N., Hendrix L. R. MHC class II structure, occupancy and surface expression determined by post-endoplasmic reticulum antigen binding. Nature. 1991 Sep 12;353(6340):134–139. doi: 10.1038/353134a0. [DOI] [PubMed] [Google Scholar]
- Glimcher L. H., McKean D. J., Choi E., Seidman J. G. Complex regulation of class II gene expression: analysis with class II mutant cell lines. J Immunol. 1985 Nov;135(5):3542–3550. [PubMed] [Google Scholar]
- Griffith I. J., Ghogawala Z., Nabavi N., Golan D. E., Myer A., McKean D. J., Glimcher L. H. Cytoplasmic domain affects membrane expression and function of an Ia molecule. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4847–4851. doi: 10.1073/pnas.85.13.4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding C. V., Collins D. S., Kanagawa O., Unanue E. R. Liposome-encapsulated antigens engender lysosomal processing for class II MHC presentation and cytosolic processing for class I presentation. J Immunol. 1991 Nov 1;147(9):2860–2863. [PubMed] [Google Scholar]
- Harding C. V., Collins D. S., Slot J. W., Geuze H. J., Unanue E. R. Liposome-encapsulated antigens are processed in lysosomes, recycled, and presented to T cells. Cell. 1991 Jan 25;64(2):393–401. doi: 10.1016/0092-8674(91)90647-h. [DOI] [PubMed] [Google Scholar]
- Harding C. V., Geuze H. J. Class II MHC molecules are present in macrophage lysosomes and phagolysosomes that function in the phagocytic processing of Listeria monocytogenes for presentation to T cells. J Cell Biol. 1992 Nov;119(3):531–542. doi: 10.1083/jcb.119.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding C. V., Geuze H. J. Immunogenic peptides bind to class II MHC molecules in an early lysosomal compartment. J Immunol. 1993 Oct 15;151(8):3988–3998. [PubMed] [Google Scholar]
- Harding C. V., Roof R. W., Allen P. M., Unanue E. R. Effects of pH and polysaccharides on peptide binding to class II major histocompatibility complex molecules. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2740–2744. doi: 10.1073/pnas.88.7.2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding C. V., Roof R. W., Unanue E. R. Turnover of Ia-peptide complexes is facilitated in viable antigen-presenting cells: biosynthetic turnover of Ia vs. peptide exchange. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4230–4234. doi: 10.1073/pnas.86.11.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughton G., Arnold L. W., Bishop G. A., Mercolino T. J. The CH series of murine B cell lymphomas: neoplastic analogues of Ly-1+ normal B cells. Immunol Rev. 1986 Oct;93:35–51. doi: 10.1111/j.1600-065x.1986.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Karlsson L., Péléraux A., Lindstedt R., Liljedahl M., Peterson P. A. Reconstitution of an operational MHC class II compartment in nonantigen-presenting cells. Science. 1994 Dec 2;266(5190):1569–1573. doi: 10.1126/science.7985028. [DOI] [PubMed] [Google Scholar]
- Lamb C. A., Yewdell J. W., Bennink J. R., Cresswell P. Invariant chain targets HLA class II molecules to acidic endosomes containing internalized influenza virus. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):5998–6002. doi: 10.1073/pnas.88.14.5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz R. G., Allen P. M. Direct evidence for functional self-protein/Ia-molecule complexes in vivo. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5220–5223. doi: 10.1073/pnas.85.14.5220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz R. G., Tyler A. N., Allen P. M. T cell recognition of bovine ribonuclease. Self/non-self discrimination at the level of binding to the I-Ak molecule. J Immunol. 1988 Dec 15;141(12):4124–4128. [PubMed] [Google Scholar]
- Lotteau V., Teyton L., Peleraux A., Nilsson T., Karlsson L., Schmid S. L., Quaranta V., Peterson P. A. Intracellular transport of class II MHC molecules directed by invariant chain. Nature. 1990 Dec 13;348(6302):600–605. doi: 10.1038/348600a0. [DOI] [PubMed] [Google Scholar]
- Miller J., Germain R. N. Efficient cell surface expression of class II MHC molecules in the absence of associated invariant chain. J Exp Med. 1986 Nov 1;164(5):1478–1489. doi: 10.1084/jem.164.5.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momburg F., Fuchs S., Drexler J., Busch R., Post M., Hämmerling G. J., Adorini L. Epitope-specific enhancement of antigen presentation by invariant chain. J Exp Med. 1993 Oct 1;178(4):1453–1458. doi: 10.1084/jem.178.4.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris P., Shaman J., Attaya M., Amaya M., Goodman S., Bergman C., Monaco J. J., Mellins E. An essential role for HLA-DM in antigen presentation by class II major histocompatibility molecules. Nature. 1994 Apr 7;368(6471):551–554. doi: 10.1038/368551a0. [DOI] [PubMed] [Google Scholar]
- Nabavi N., Ghogawala Z., Myer A., Griffith I. J., Wade W. F., Chen Z. Z., McKean D. J., Glimcher L. H. Antigen presentation abrogated in cells expressing truncated Ia molecules. J Immunol. 1989 Mar 1;142(5):1444–1447. [PubMed] [Google Scholar]
- Nadimi F., Moreno J., Momburg F., Heuser A., Fuchs S., Adorini L., Hämmerling G. J. Antigen presentation of hen egg-white lysozyme but not of ribonuclease A is augmented by the major histocompatibility complex class II-associated invariant chain. Eur J Immunol. 1991 May;21(5):1255–1263. doi: 10.1002/eji.1830210524. [DOI] [PubMed] [Google Scholar]
- Oi V. T., Jones P. P., Goding J. W., Herzenberg L. A., Herzenberg L. A. Properties of monoclonal antibodies to mouse Ig allotypes, H-2, and Ia antigens. Curr Top Microbiol Immunol. 1978;81:115–120. doi: 10.1007/978-3-642-67448-8_18. [DOI] [PubMed] [Google Scholar]
- Parnes J. R., Seidman J. G. Structure of wild-type and mutant mouse beta 2-microglobulin genes. Cell. 1982 Jun;29(2):661–669. doi: 10.1016/0092-8674(82)90182-9. [DOI] [PubMed] [Google Scholar]
- Peters P. J., Neefjes J. J., Oorschot V., Ploegh H. L., Geuze H. J. Segregation of MHC class II molecules from MHC class I molecules in the Golgi complex for transport to lysosomal compartments. Nature. 1991 Feb 21;349(6311):669–676. doi: 10.1038/349669a0. [DOI] [PubMed] [Google Scholar]
- Peterson M., Miller J. Antigen presentation enhanced by the alternatively spliced invariant chain gene product p41. Nature. 1992 Jun 18;357(6379):596–598. doi: 10.1038/357596a0. [DOI] [PubMed] [Google Scholar]
- Qiu Y., Xu X., Wandinger-Ness A., Dalke D. P., Pierce S. K. Separation of subcellular compartments containing distinct functional forms of MHC class II. J Cell Biol. 1994 May;125(3):595–605. doi: 10.1083/jcb.125.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschke W. C., Baird S., Ralph P., Nakoinz I. Functional macrophage cell lines transformed by Abelson leukemia virus. Cell. 1978 Sep;15(1):261–267. doi: 10.1016/0092-8674(78)90101-0. [DOI] [PubMed] [Google Scholar]
- Romagnoli P., Layet C., Yewdell J., Bakke O., Germain R. N. Relationship between invariant chain expression and major histocompatibility complex class II transport into early and late endocytic compartments. J Exp Med. 1993 Mar 1;177(3):583–596. doi: 10.1084/jem.177.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson F., Kleijmeer M. J., Kelly A., Verwoerd D., Tulp A., Neefjes J. J., Geuze H. J., Trowsdale J. Accumulation of HLA-DM, a regulator of antigen presentation, in MHC class II compartments. Science. 1994 Dec 2;266(5190):1566–1569. doi: 10.1126/science.7985027. [DOI] [PubMed] [Google Scholar]
- Stebbins C. C., Loss G. E., Jr, Elias C. G., Chervonsky A., Sant A. J. The requirement for DM in class II-restricted antigen presentation and SDS-stable dimer formation is allele and species dependent. J Exp Med. 1995 Jan 1;181(1):223–234. doi: 10.1084/jem.181.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger B., Pessara U., Lin R. H., Habicht J., Grez M., Koch N. A role of Ia-associated invariant chains in antigen processing and presentation. Cell. 1989 Feb 24;56(4):683–689. doi: 10.1016/0092-8674(89)90590-4. [DOI] [PubMed] [Google Scholar]
- Tulp A., Verwoerd D., Dobberstein B., Ploegh H. L., Pieters J. Isolation and characterization of the intracellular MHC class II compartment. Nature. 1994 May 12;369(6476):120–126. doi: 10.1038/369120a0. [DOI] [PubMed] [Google Scholar]
- Ulrich M. J., Ley T. J. Function of normal and mutated gamma-globin gene promoters in electroporated K562 erythroleukemia cells. Blood. 1990 Feb 15;75(4):990–999. [PubMed] [Google Scholar]
- West M. A., Lucocq J. M., Watts C. Antigen processing and class II MHC peptide-loading compartments in human B-lymphoblastoid cells. Nature. 1994 May 12;369(6476):147–151. doi: 10.1038/369147a0. [DOI] [PubMed] [Google Scholar]