Abstract
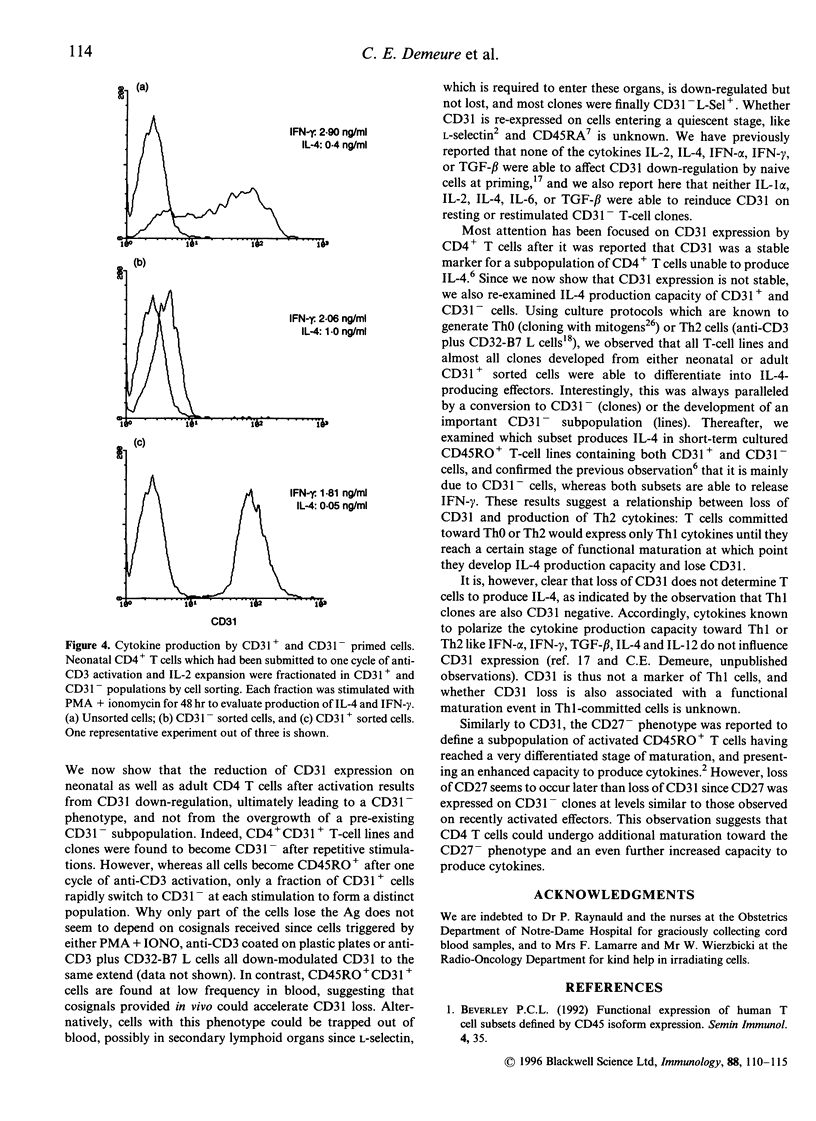
The CD31 antigen (PECAM-1) has been reported to be a stable marker for a human CD4 T-cell subpopulation unable to produce interleukin-4 (IL-4). We show here that CD31 expression is not stable inasmuch as CD4 T-cell lines and clones derived from cell-sorted neonatal CD31+ cells lose CD31 upon repetitive cycles of stimulation and IL-2 expansion. Moreover, various cytokines (IL-1 alpha, IL-4, IL-6, transforming growth factor-beta) fail to reinduce CD31 on CD31- clones. Whereas all CD31+ CD4 T cells rapidly express high levels of the CD45RO antigen and down-regulate the L-selectin antigen after priming, CD31 disappears more slowly because only part of the cells lose CD31 expression upon each cycle of stimulation. Loss of CD31 reflects a functional maturation of CD45RO+ cells since, in a system which favours the development of Th2 effectors, IL-4 is produced by CD31- but not CD31+ effector T cells, whereas interferon-gamma is produced by both types of cells. However, CD31 is not a Th1 marker since it is not expressed on several Th1 antigen-specific clones. We conclude that CD31 is a maturation marker expressed on the great majority of naive CD45RO- CD4 T cells and on a subset of CD45RO+ CD4 T cells that are at an intermediate stage of maturation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashman L. K., Aylett G. W. Expression of CD31 epitopes on human lymphocytes: CD31 monoclonal antibodies differentiate between naive (CD45RA+) and memory (CD45RA-) CD4-positive T cells. Tissue Antigens. 1991 Nov;38(5):208–212. doi: 10.1111/j.1399-0039.1991.tb01899.x. [DOI] [PubMed] [Google Scholar]
- Berman M. E., Muller W. A. Ligation of platelet/endothelial cell adhesion molecule 1 (PECAM-1/CD31) on monocytes and neutrophils increases binding capacity of leukocyte CR3 (CD11b/CD18). J Immunol. 1995 Jan 1;154(1):299–307. [PubMed] [Google Scholar]
- Beverley P. C. Functional analysis of human T cell subsets defined by CD45 isoform expression. Semin Immunol. 1992 Feb;4(1):35–41. [PubMed] [Google Scholar]
- Brod S. A., Rudd C. E., Purvee M., Hafler D. A. Lymphokine regulation of CD45R expression on human T cell clones. J Exp Med. 1989 Dec 1;170(6):2147–2152. doi: 10.1084/jem.170.6.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisser H. M., Newman P. J., Albelda S. M. Molecular and functional aspects of PECAM-1/CD31. Immunol Today. 1994 Oct;15(10):490–495. doi: 10.1016/0167-5699(94)90195-3. [DOI] [PubMed] [Google Scholar]
- Del Prete G., De Carli M., Almerigogna F., Daniel C. K., D'Elios M. M., Zancuoghi G., Vinante F., Pizzolo G., Romagnani S. Preferential expression of CD30 by human CD4+ T cells producing Th2-type cytokines. FASEB J. 1995 Jan;9(1):81–86. [PubMed] [Google Scholar]
- Demeure C. E., Wu C. Y., Shu U., Schneider P. V., Heusser C., Yssel H., Delespesse G. In vitro maturation of human neonatal CD4 T lymphocytes. II. Cytokines present at priming modulate the development of lymphokine production. J Immunol. 1994 May 15;152(10):4775–4782. [PubMed] [Google Scholar]
- Demeure C. E., Yang L. P., Byun D. G., Ishihara H., Vezzio N., Delespesse G. Human naive CD4 T cells produce interleukin-4 at priming and acquire a Th2 phenotype upon repetitive stimulations in neutral conditions. Eur J Immunol. 1995 Sep;25(9):2722–2725. doi: 10.1002/eji.1830250950. [DOI] [PubMed] [Google Scholar]
- Gallatin M., St John T. P., Siegelman M., Reichert R., Butcher E. C., Weissman I. L. Lymphocyte homing receptors. Cell. 1986 Mar 14;44(5):673–680. doi: 10.1016/0092-8674(86)90832-9. [DOI] [PubMed] [Google Scholar]
- Hintzen R. Q., de Jong R., Lens S. M., van Lier R. A. CD27: marker and mediator of T-cell activation? Immunol Today. 1994 Jul;15(7):307–311. doi: 10.1016/0167-5699(94)90077-9. [DOI] [PubMed] [Google Scholar]
- Morimoto C., Schlossman S. F. P. Rambotti Lecture. Human naive and memory T cells revisited: new markers (CD31 and CD27) that help define CD4+ T cell subsets. Clin Exp Rheumatol. 1993 May-Jun;11(3):241–247. [PubMed] [Google Scholar]
- Muller W. A., Weigl S. A., Deng X., Phillips D. M. PECAM-1 is required for transendothelial migration of leukocytes. J Exp Med. 1993 Aug 1;178(2):449–460. doi: 10.1084/jem.178.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paliard X., de Waal Malefijt R., Yssel H., Blanchard D., Chrétien I., Abrams J., de Vries J., Spits H. Simultaneous production of IL-2, IL-4, and IFN-gamma by activated human CD4+ and CD8+ T cell clones. J Immunol. 1988 Aug 1;141(3):849–855. [PubMed] [Google Scholar]
- Piali L., Albelda S. M., Baldwin H. S., Hammel P., Gisler R. H., Imhof B. A. Murine platelet endothelial cell adhesion molecule (PECAM-1)/CD31 modulates beta 2 integrins on lymphokine-activated killer cells. Eur J Immunol. 1993 Oct;23(10):2464–2471. doi: 10.1002/eji.1830231013. [DOI] [PubMed] [Google Scholar]
- Roth M. D. Interleukin 2 induces the expression of CD45RO and the memory phenotype by CD45RA+ peripheral blood lymphocytes. J Exp Med. 1994 Mar 1;179(3):857–864. doi: 10.1084/jem.179.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu U., Kiniwa M., Wu C. Y., Maliszewski C., Vezzio N., Hakimi J., Gately M., Delespesse G. Activated T cells induce interleukin-12 production by monocytes via CD40-CD40 ligand interaction. Eur J Immunol. 1995 Apr;25(4):1125–1128. doi: 10.1002/eji.1830250442. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994 Jan 28;76(2):301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Swain S. L., Huston G., Tonkonogy S., Weinberg A. Transforming growth factor-beta and IL-4 cause helper T cell precursors to develop into distinct effector helper cells that differ in lymphokine secretion pattern and cell surface phenotype. J Immunol. 1991 Nov 1;147(9):2991–3000. [PubMed] [Google Scholar]
- Tanaka Y., Albelda S. M., Horgan K. J., van Seventer G. A., Shimizu Y., Newman W., Hallam J., Newman P. J., Buck C. A., Shaw S. CD31 expressed on distinctive T cell subsets is a preferential amplifier of beta 1 integrin-mediated adhesion. J Exp Med. 1992 Jul 1;176(1):245–253. doi: 10.1084/jem.176.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torimoto Y., Rothstein D. M., Dang N. H., Schlossman S. F., Morimoto C. CD31, a novel cell surface marker for CD4 cells of suppressor lineage, unaltered by state of activation. J Immunol. 1992 Jan 15;148(2):388–396. [PubMed] [Google Scholar]
- Wu C. Y., Demeure C. E., Gately M., Podlaski F., Yssel H., Kiniwa M., Delespesse G. In vitro maturation of human neonatal CD4 T lymphocytes. I. Induction of IL-4-producing cells after long-term culture in the presence of IL-4 plus either IL-2 or IL-12. J Immunol. 1994 Feb 1;152(3):1141–1153. [PubMed] [Google Scholar]
- Yang L. P., Byun D. G., Demeure C. E., Vezzio N., Delespesse G. Default development of cloned human naive CD4 T cells into interleukin-4- and interleukin-5- producing effector cells. Eur J Immunol. 1995 Dec;25(12):3517–3520. doi: 10.1002/eji.1830251247. [DOI] [PubMed] [Google Scholar]
- Zehnder J. L., Hirai K., Shatsky M., McGregor J. L., Levitt L. J., Leung L. L. The cell adhesion molecule CD31 is phosphorylated after cell activation. Down-regulation of CD31 in activated T lymphocytes. J Biol Chem. 1992 Mar 15;267(8):5243–5249. [PubMed] [Google Scholar]
- Zehnder J. L., Shatsky M., Leung L. L., Butcher E. C., McGregor J. L., Levitt L. J. Involvement of CD31 in lymphocyte-mediated immune responses: importance of the membrane-proximal immunoglobulin domain and identification of an inhibiting CD31 peptide. Blood. 1995 Mar 1;85(5):1282–1288. [PubMed] [Google Scholar]




