Abstract
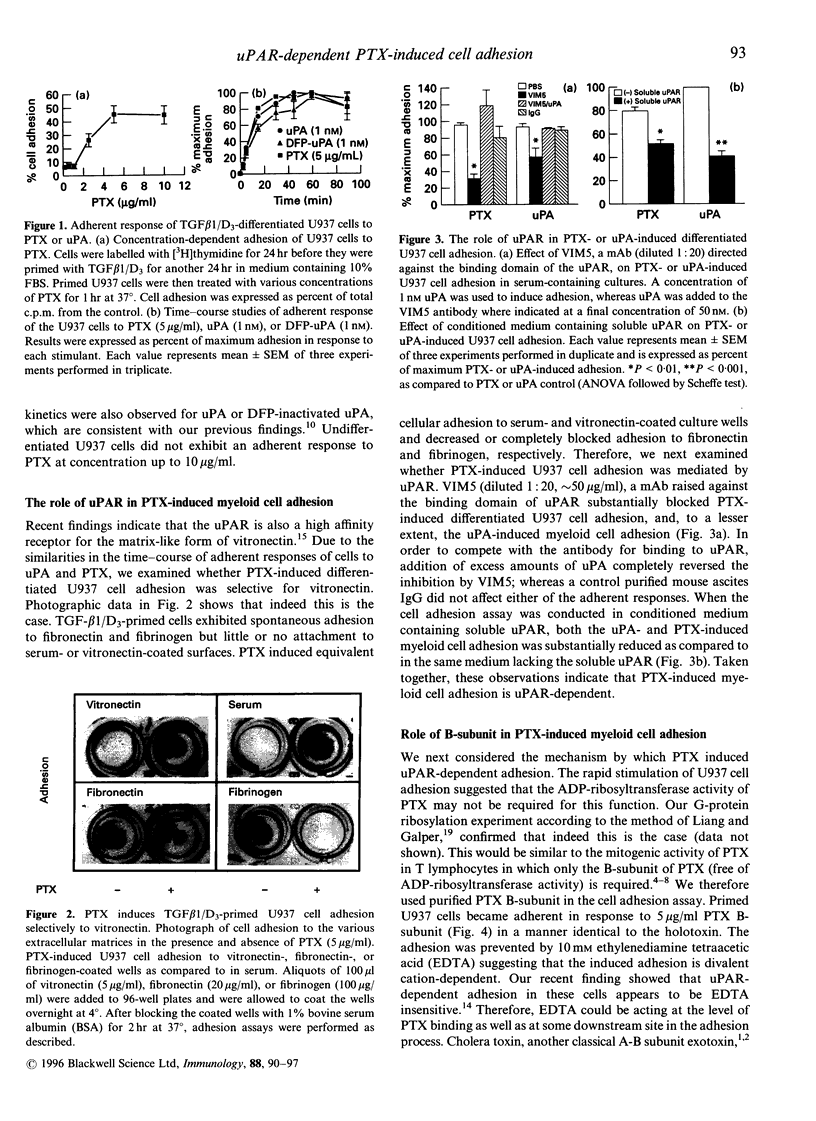
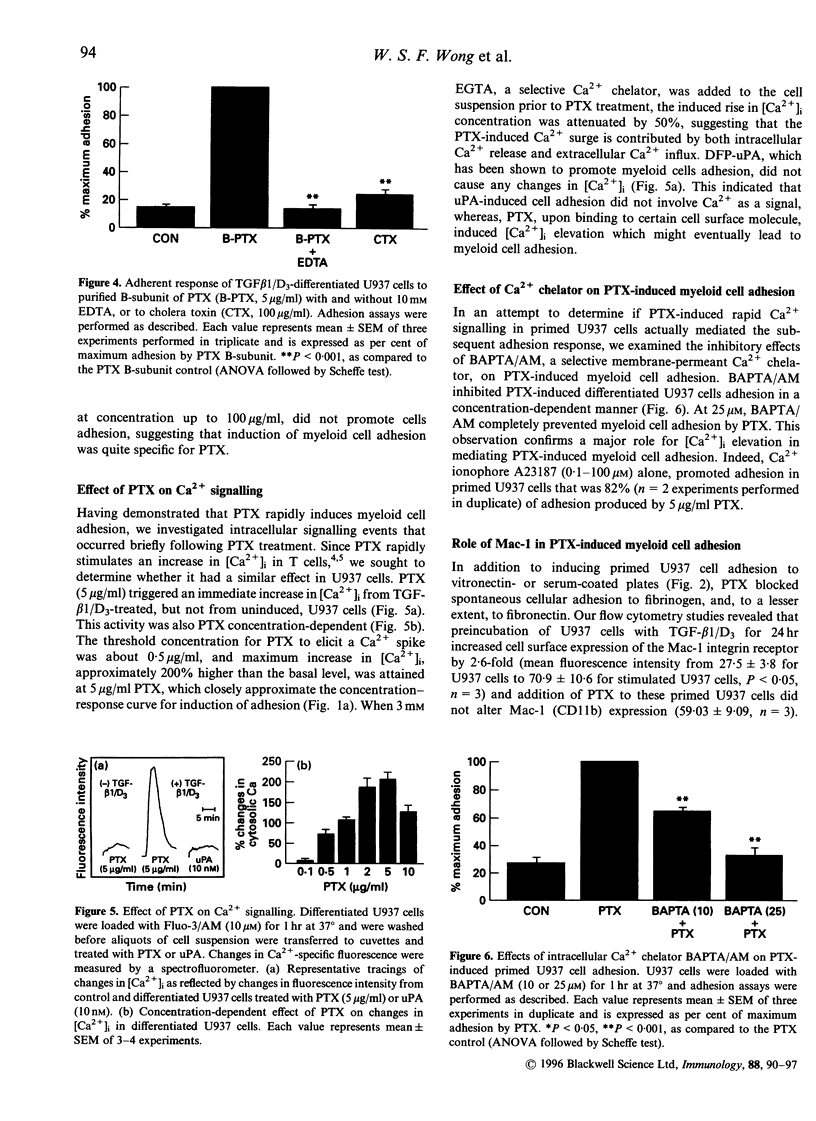
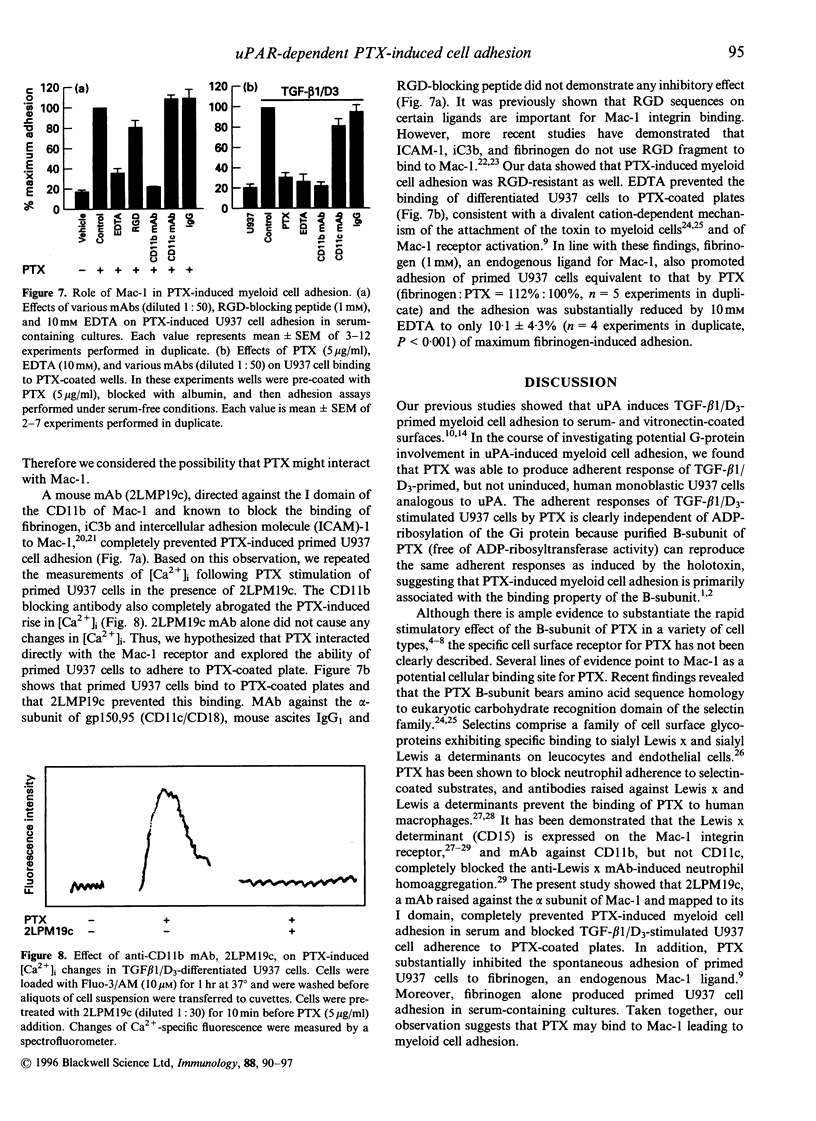
Stimulation of monoblastic U937 cells with transforming growth factor beta 1 and 1,25-(OH)2 vitamin D3 (TGF-beta 1/D3) upregulates urokinase receptor (uPAR) and confers urokinase-dependent adhesiveness to the cells for serum- or vitronectin-coated surfaces. Recent studies show that uPAR itself is a high-affinity adhesion receptor for vitronectin and that urokinase (uPA) is an activator of this adhesive function. In the course of exploring possible G-protein involvement in this adhesion it was observed that TGF-beta 1/D3-primed U937 cells became adhesive to vitronectin in an uPAR-dependent manner when exposed to pertussis toxin (PTX). The adherent response is concentration- and time-dependent, and was not due to the ADP-ribosyltransferase activity of the toxin because the purified B-subunit of PTX was equally effective. Although promoting adhesion to serum- or vitronectin-coated surfaces, PTX blocked spontaneous cell adhesion to fibrinogen, an endogenous ligand for the Mac-1 receptor (CD11b/CD18). Flow cytometry study showed that expression of the alpha-subunit of Mac-1 (CD11b) on primed cells was increased by nearly threefold. Monoclonal antibody to CD11b abolished the PTX-induced cell adhesion and the binding of the primed cells to PTX-coated plates. Activation of Mac-1 receptor by its endogenous ligand fibrinogen induced cell adherent response similar to PTX. PTX, but not uPA, triggered a rapid rise in [Ca2+]i in primed U937 cells, and PTX-induced adhesion was significantly attenuated by 1,2-bis-(2-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid/acetoxy-methyl ester (BAPTA/AM), a selective membrane-permeant [Ca2+]i chelator. PTX-induced cell adhesion was also prevented by antibodies to uPAR and by conditioned medium containing soluble uPAR. Together these data indicate that PTX B-subunit may bind to Mac-1 integrin, which leads to a rapid rise in [Ca2+]i and subsequent activation of uPAR for adherence to vitronectin, suggesting a functional link between Mac-1 and activation of uPAR important to cellular trafficking and host defence in response to Bordetella pertussis infection.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altieri D. C., Plescia J., Plow E. F. The structural motif glycine 190-valine 202 of the fibrinogen gamma chain interacts with CD11b/CD18 integrin (alpha M beta 2, Mac-1) and promotes leukocyte adhesion. J Biol Chem. 1993 Jan 25;268(3):1847–1853. [PubMed] [Google Scholar]
- Altieri D. C., Stamnes S. J., Gahmberg C. G. Regulated Ca2+ signalling through leukocyte CD11b/CD18 integrin. Biochem J. 1992 Dec 1;288(Pt 2):465–473. doi: 10.1042/bj2880465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banga H. S., Walker R. K., Winberry L. K., Rittenhouse S. E. Pertussis toxin can activate human platelets. Comparative effects of holotoxin and its ADP-ribosylating S1 subunit. J Biol Chem. 1987 Nov 5;262(31):14871–14874. [PubMed] [Google Scholar]
- Bradford P. G., Jin Y., Hui P. 1,25-dihydroxyvitamin D3 enhances the transcription and expression of the inositol trisphosphate receptor gene in HL-60 cells. Mol Pharmacol. 1993 Aug;44(2):292–297. [PubMed] [Google Scholar]
- Cao D., Mizukami I. F., Garni-Wagner B. A., Kindzelskii A. L., Todd R. F., 3rd, Boxer L. A., Petty H. R. Human urokinase-type plasminogen activator primes neutrophils for superoxide anion release. Possible roles of complement receptor type 3 and calcium. J Immunol. 1995 Feb 15;154(4):1817–1829. [PubMed] [Google Scholar]
- Diamond M. S., Garcia-Aguilar J., Bickford J. K., Corbi A. L., Springer T. A. The I domain is a major recognition site on the leukocyte integrin Mac-1 (CD11b/CD18) for four distinct adhesion ligands. J Cell Biol. 1993 Feb;120(4):1031–1043. doi: 10.1083/jcb.120.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond M. S., Springer T. A. A subpopulation of Mac-1 (CD11b/CD18) molecules mediates neutrophil adhesion to ICAM-1 and fibrinogen. J Cell Biol. 1993 Jan;120(2):545–556. doi: 10.1083/jcb.120.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierschik P. ADP-ribosylation of signal-transducing guanine nucleotide-binding proteins by pertussis toxin. Curr Top Microbiol Immunol. 1992;175:69–96. doi: 10.1007/978-3-642-76966-5_4. [DOI] [PubMed] [Google Scholar]
- Harris P., Ralph P. Human leukemic models of myelomonocytic development: a review of the HL-60 and U937 cell lines. J Leukoc Biol. 1985 Apr;37(4):407–422. doi: 10.1002/jlb.37.4.407. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Ignotz R. A., Heino J., Massagué J. Regulation of cell adhesion receptors by transforming growth factor-beta. Regulation of vitronectin receptor and LFA-1. J Biol Chem. 1989 Jan 5;264(1):389–392. [PubMed] [Google Scholar]
- Kao J. P., Harootunian A. T., Tsien R. Y. Photochemically generated cytosolic calcium pulses and their detection by fluo-3. J Biol Chem. 1989 May 15;264(14):8179–8184. [PubMed] [Google Scholar]
- Lasky L. A. Selectins: interpreters of cell-specific carbohydrate information during inflammation. Science. 1992 Nov 6;258(5084):964–969. doi: 10.1126/science.1439808. [DOI] [PubMed] [Google Scholar]
- Liang B. T., Galper J. B. Differential sensitivity of alpha o and alpha i to ADP-ribosylation by pertussis toxin in the intact cultured embryonic chick ventricular myocyte. Relationship to the role of G proteins in the coupling of muscarinic cholinergic receptors to inhibition of adenylate cyclase activity. Biochem Pharmacol. 1988 Dec 1;37(23):4549–4555. doi: 10.1016/0006-2952(88)90671-5. [DOI] [PubMed] [Google Scholar]
- Morikawa M., Harada N., Soma G., Yoshida T. Transforming growth factor-beta 1 modulates the effect of 1 alpha, 25-dihydroxyvitamin D3 on leukemic cells. In Vitro Cell Dev Biol. 1990 Jul;26(7):682–690. doi: 10.1007/BF02624424. [DOI] [PubMed] [Google Scholar]
- Ng-Sikorski J., Andersson R., Patarroyo M., Andersson T. Calcium signaling capacity of the CD11b/CD18 integrin on human neutrophils. Exp Cell Res. 1991 Aug;195(2):504–508. doi: 10.1016/0014-4827(91)90402-g. [DOI] [PubMed] [Google Scholar]
- Nicosia A., Perugini M., Franzini C., Casagli M. C., Borri M. G., Antoni G., Almoni M., Neri P., Ratti G., Rappuoli R. Cloning and sequencing of the pertussis toxin genes: operon structure and gene duplication. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4631–4635. doi: 10.1073/pnas.83.13.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao N. K., Shi G. P., Chapman H. A. Urokinase receptor is a multifunctional protein: influence of receptor occupancy on macrophage gene expression. J Clin Invest. 1995 Jul;96(1):465–474. doi: 10.1172/JCI118057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosoff P. M., Mohan C. Unidirectional, heterologous desensitization of the pertussis toxin receptor by the CD3/TCR complex. J Immunol. 1992 Nov 15;149(10):3191–3199. [PubMed] [Google Scholar]
- Rosoff P. M., Walker R., Winberry L. Pertussis toxin triggers rapid second messenger production in human T lymphocytes. J Immunol. 1987 Oct 1;139(7):2419–2423. [PubMed] [Google Scholar]
- Rozdzinski E., Burnette W. N., Jones T., Mar V., Tuomanen E. Prokaryotic peptides that block leukocyte adherence to selectins. J Exp Med. 1993 Sep 1;178(3):917–924. doi: 10.1084/jem.178.3.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saukkonen K., Burnette W. N., Mar V. L., Masure H. R., Tuomanen E. I. Pertussis toxin has eukaryotic-like carbohydrate recognition domains. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):118–122. doi: 10.1073/pnas.89.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skubitz K. M., Snook R. W., 2nd Monoclonal antibodies that recognize lacto-N-fucopentaose III (CD15) react with the adhesion-promoting glycoprotein family (LFA-1/HMac-1/gp 150,95) and CR1 on human neutrophils. J Immunol. 1987 Sep 1;139(5):1631–1639. [PubMed] [Google Scholar]
- Smith J. W., Vestal D. J., Irwin S. V., Burke T. A., Cheresh D. A. Purification and functional characterization of integrin alpha v beta 5. An adhesion receptor for vitronectin. J Biol Chem. 1990 Jul 5;265(19):11008–11013. [PubMed] [Google Scholar]
- Stocks S. C., Albrechtsen M., Kerr M. A. Expression of the CD15 differentiation antigen (3-fucosyl-N-acetyl-lactosamine, LeX) on putative neutrophil adhesion molecules CR3 and NCA-160. Biochem J. 1990 Jun 1;268(2):275–280. doi: 10.1042/bj2680275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strnad C. F., Carchman R. A. Human T lymphocyte mitogenesis in response to the B oligomer of pertussis toxin is associated with an early elevation in cytosolic calcium concentrations. FEBS Lett. 1987 Dec 10;225(1-2):16–20. doi: 10.1016/0014-5793(87)81123-7. [DOI] [PubMed] [Google Scholar]
- Stöckl J., Majdic O., Rosenkranz A., Fiebiger E., Kniep B., Stockinger H., Knapp W. Monoclonal antibodies to the carbohydrate structure Lewis(x) stimulate the adhesive activity of leukocyte integrin CD11b/CD18 (CR3, Mac-1, alpha m beta 2) on human granulocytes. J Leukoc Biol. 1993 May;53(5):541–549. doi: 10.1002/jlb.53.5.541. [DOI] [PubMed] [Google Scholar]
- Tamura M., Nogimori K., Murai S., Yajima M., Ito K., Katada T., Ui M., Ishii S. Subunit structure of islet-activating protein, pertussis toxin, in conformity with the A-B model. Biochemistry. 1982 Oct 26;21(22):5516–5522. doi: 10.1021/bi00265a021. [DOI] [PubMed] [Google Scholar]
- Taniguchi-Sidle A., Isenman D. E. Mutagenesis of the Arg-Gly-Asp triplet in human complement component C3 does not abolish binding of iC3b to the leukocyte integrin complement receptor type III (CR3, CD11b/CD18). J Biol Chem. 1992 Jan 5;267(1):635–643. [PubMed] [Google Scholar]
- Waltz D. A., Chapman H. A. Reversible cellular adhesion to vitronectin linked to urokinase receptor occupancy. J Biol Chem. 1994 May 20;269(20):14746–14750. [PubMed] [Google Scholar]
- Wei Y., Waltz D. A., Rao N., Drummond R. J., Rosenberg S., Chapman H. A. Identification of the urokinase receptor as an adhesion receptor for vitronectin. J Biol Chem. 1994 Dec 23;269(51):32380–32388. [PubMed] [Google Scholar]
- Xue W., Kindzelskii A. L., Todd R. F., 3rd, Petty H. R. Physical association of complement receptor type 3 and urokinase-type plasminogen activator receptor in neutrophil membranes. J Immunol. 1994 May 1;152(9):4630–4640. [PubMed] [Google Scholar]
- Yatohgo T., Izumi M., Kashiwagi H., Hayashi M. Novel purification of vitronectin from human plasma by heparin affinity chromatography. Cell Struct Funct. 1988 Aug;13(4):281–292. doi: 10.1247/csf.13.281. [DOI] [PubMed] [Google Scholar]
- Zhang X. M., Berland R., Rosoff P. M. Differential regulation of accessory mitogenic signaling receptors by the T cell antigen receptor. Mol Immunol. 1995 Apr;32(5):323–332. doi: 10.1016/0161-5890(94)00160-3. [DOI] [PubMed] [Google Scholar]




