Abstract
Unlike lymphocytes from adults, lymphocytes from cord blood of neonates cannot synthesize immunoglobulin G (IgG) in response to pokeweed mitogen (PWM). By using this mitogen in concert with interferon-gamma (IFN-gamma), interleukin-2 (IL-2), or interleukin-6 (IL-6), we studied the induction of IgG subclass molecules in lymphocytes of human neonates. IFN-gamma induced a limited, but substantial, enhancement of IgG2 production by neonatal lymphocytes. IL-2 dose dependently increased the production of each neonatal IgG subclass, whereas IL-6 did not. However, in adult lymphocytes, and under specific conditions, IL-6 or IL-2 each increased the production of all four IgG subclasses. Early in the culture IFN-gamma synergized with IL-2 during the latter or whole culture period to enhance cord blood IgG2 levels. This finding contrasted with the adult IgG2 synthesis synergistically up-regulated by IFN-gamma and IL-6. IL-2 caused a graded increase in immunoglobulin production in neonatal lymphocytes with IgG3 being the highest and IgG2 the lowest, thus corresponding to the differential increase of serum levels of IgG3/IgG1 and IgG4/IgG2 early in childhood. Results suggest that IL-2, but not IL-6, is critical to the development of human IgG subclass production.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amoroso K., Lipsky P. E. Frequency of human B cells that differentiate in response to anti-CD3-activated T cells. J Immunol. 1990 Nov 15;145(10):3155–3161. [PubMed] [Google Scholar]
- Andersson B., Skoglund A. C., Rönnholm M., Lindsten T., Lamon E. W., Collisson E. W., Walia A. S. Functional aspects of IgM and IgG Fc receptors on murine T lymphocytes. Immunol Rev. 1981;56:5–50. doi: 10.1111/j.1600-065x.1981.tb01046.x. [DOI] [PubMed] [Google Scholar]
- Barrett D. J., Ayoub E. M. IgG2 subclass restriction of antibody to pneumococcal polysaccharides. Clin Exp Immunol. 1986 Jan;63(1):127–134. [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Calvert J. E., Johnstone R., Duggan-Keen M. F., Bird P. Immunoglobulin G subclasses secreted by human B cells in vitro in response to interleukin-2 and polyclonal activators. Immunology. 1990 Jun;70(2):162–167. [PMC free article] [PubMed] [Google Scholar]
- Clement L. T., Vink P. E., Bradley G. E. Novel immunoregulatory functions of phenotypically distinct subpopulations of CD4+ cells in the human neonate. J Immunol. 1990 Jul 1;145(1):102–108. [PubMed] [Google Scholar]
- Durandy A., Fischer A., Griscelli C. Active suppression of B lymphocyte maturation by two different newborn T lymphocyte subsets. J Immunol. 1979 Dec;123(6):2644–2650. [PubMed] [Google Scholar]
- Ehlers S., Smith K. A. Differentiation of T cell lymphokine gene expression: the in vitro acquisition of T cell memory. J Exp Med. 1991 Jan 1;173(1):25–36. doi: 10.1084/jem.173.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freijd A., Hammarström L., Persson M. A., Smith C. I. Plasma anti-pneumococcal antibody activity of the IgG class and subclasses in otitis prone children. Clin Exp Immunol. 1984 May;56(2):233–238. [PMC free article] [PubMed] [Google Scholar]
- GREY H. M., KUNKEL H. G. H CHAIN SUBGROUPS OF MYELOMA PROTEINS AND NORMAL 7S GAMMA-GLOBULIN. J Exp Med. 1964 Aug 1;120:253–266. doi: 10.1084/jem.120.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski T. F., Schell S. R., Nau G., Fitch F. W. Regulation of T-cell activation: differences among T-cell subsets. Immunol Rev. 1989 Oct;111:79–110. doi: 10.1111/j.1600-065x.1989.tb00543.x. [DOI] [PubMed] [Google Scholar]
- Gathings W. E., Kubagawa H., Cooper M. D. A distinctive pattern of B cell immaturity in perinatal humans. Immunol Rev. 1981;57:107–126. doi: 10.1111/j.1600-065x.1981.tb00444.x. [DOI] [PubMed] [Google Scholar]
- Hayward A. R., Lawton A. R. Induction of plasma cell differentiation of human fetal lymphocytes: evidence for functional immaturity of T and B cells. J Immunol. 1977 Oct;119(4):1213–1217. [PubMed] [Google Scholar]
- Itoh K., Inoue T., Ito K., Hirohata S. The interplay of interleukin-10 (IL-10) and interleukin-2 (IL-2) in humoral immune responses: IL-10 synergizes with IL-2 to enhance responses of human B lymphocytes in a mechanism which is different from upregulation of CD25 expression. Cell Immunol. 1994 Sep;157(2):478–488. doi: 10.1006/cimm.1994.1243. [DOI] [PubMed] [Google Scholar]
- Jelinek D. F., Splawski J. B., Lipsky P. E. The roles of interleukin 2 and interferon-gamma in human B cell activation, growth and differentiation. Eur J Immunol. 1986 Aug;16(8):925–932. doi: 10.1002/eji.1830160809. [DOI] [PubMed] [Google Scholar]
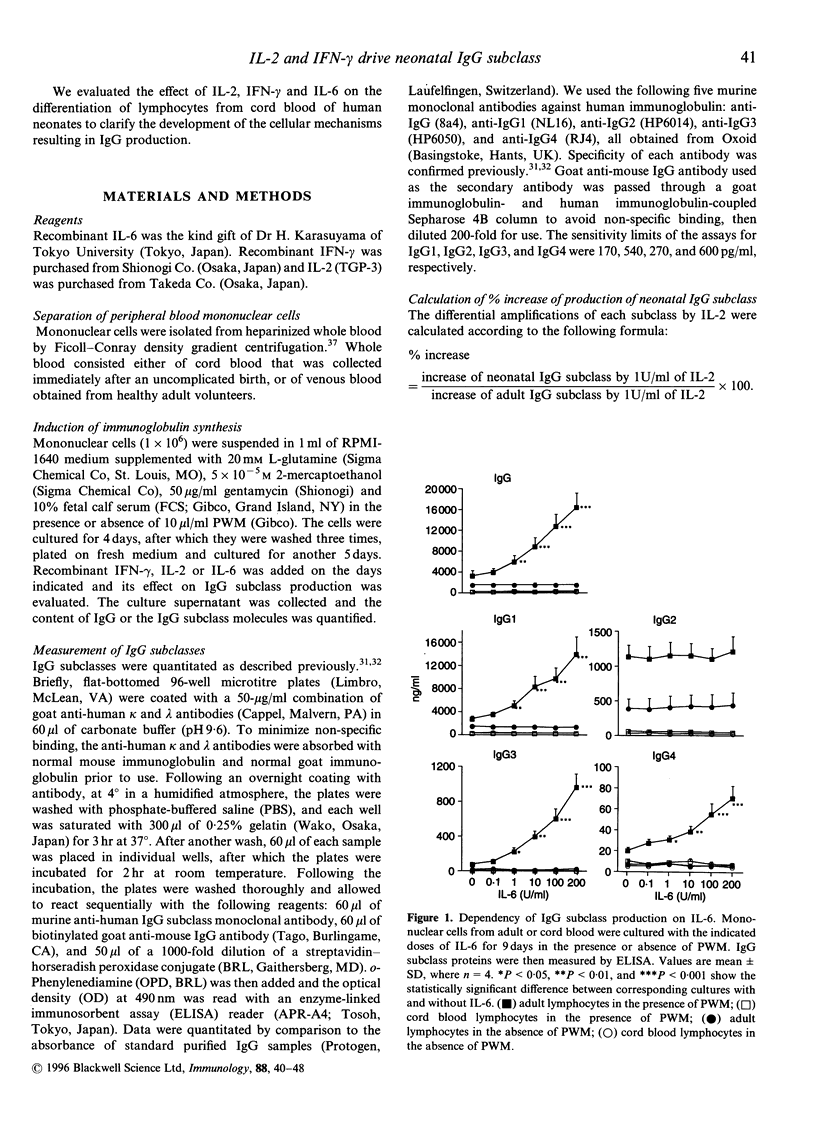
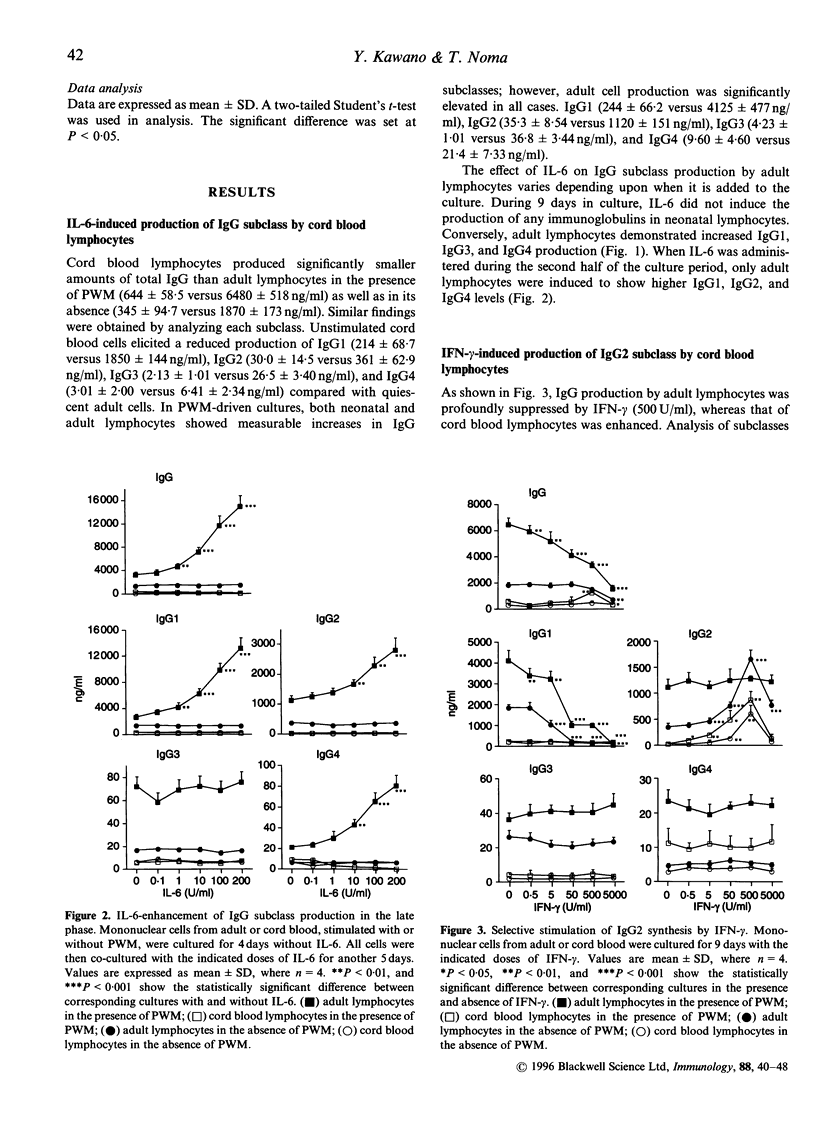
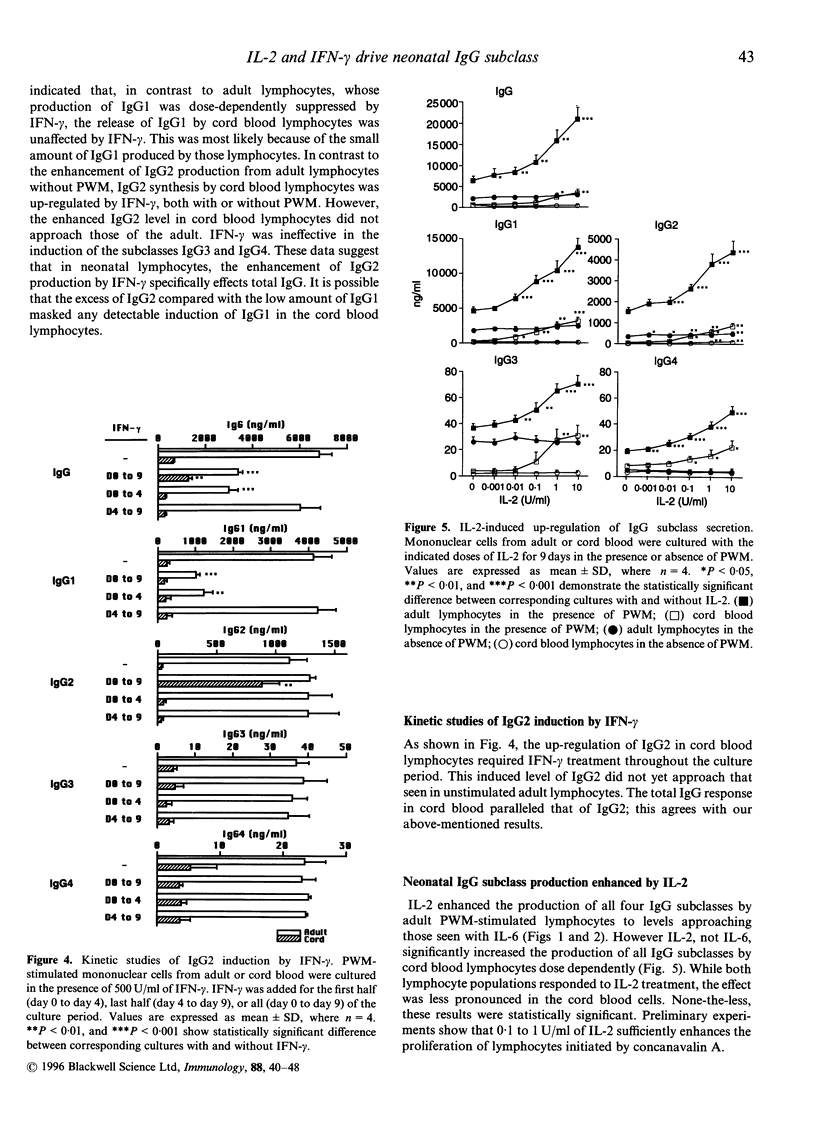
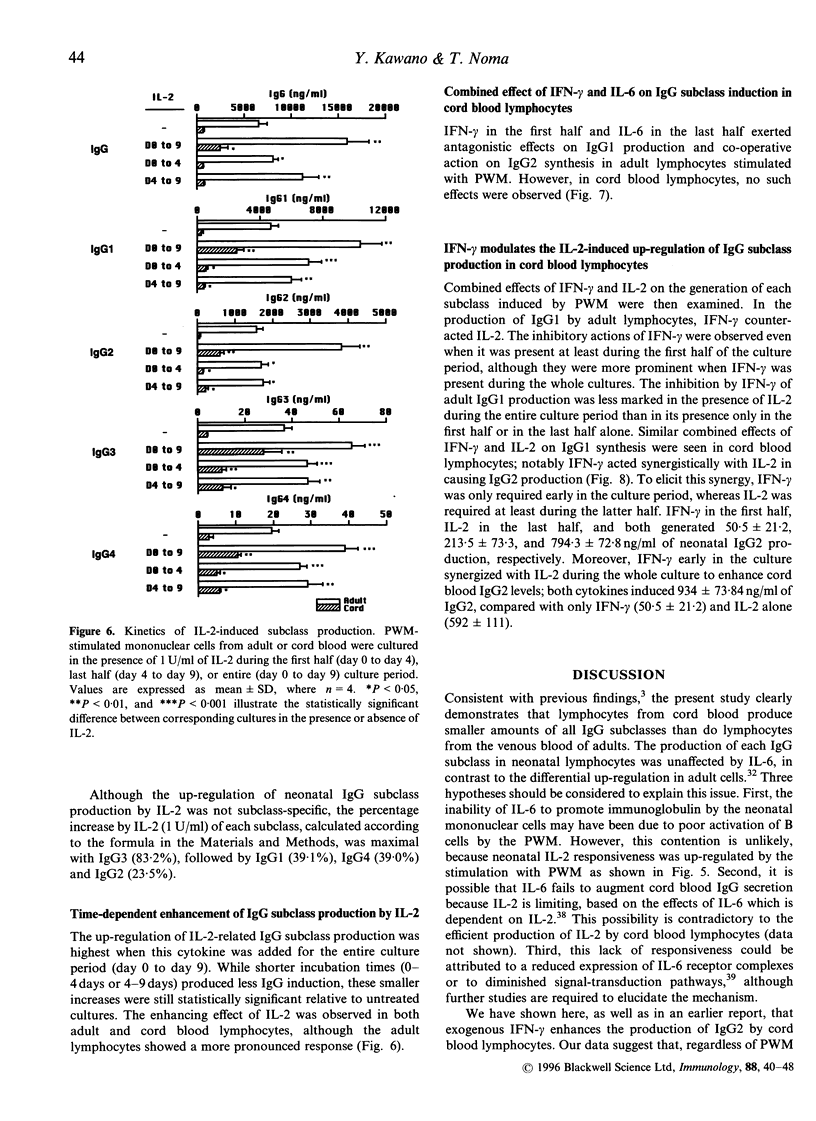
- Kawano Y., Noma T., Kou K., Yoshizawa I., Yata J. Regulation of human IgG subclass production by cytokines: human IgG subclass production enhanced differentially by interleukin-6. Immunology. 1995 Feb;84(2):278–284. [PMC free article] [PubMed] [Google Scholar]
- Kawano Y., Noma T., Yata J. Regulation of human IgG subclass production by cytokines. IFN-gamma and IL-6 act antagonistically in the induction of human IgG1 but additively in the induction of IgG2. J Immunol. 1994 Dec 1;153(11):4948–4958. [PubMed] [Google Scholar]
- Kishimoto T., Taga T., Akira S. Cytokine signal transduction. Cell. 1994 Jan 28;76(2):253–262. doi: 10.1016/0092-8674(94)90333-6. [DOI] [PubMed] [Google Scholar]
- Lewis D. B., Larsen A., Wilson C. B. Reduced interferon-gamma mRNA levels in human neonates. Evidence for an intrinsic T cell deficiency independent of other genes involved in T cell activation. J Exp Med. 1986 Apr 1;163(4):1018–1023. doi: 10.1084/jem.163.4.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D. B., Yu C. C., Meyer J., English B. K., Kahn S. J., Wilson C. B. Cellular and molecular mechanisms for reduced interleukin 4 and interferon-gamma production by neonatal T cells. J Clin Invest. 1991 Jan;87(1):194–202. doi: 10.1172/JCI114970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren M., Persson U., Larsson P., Magnusson C., Smith C. I., Hammarström L., Severinson E. Interleukin 4 induces synthesis of IgE and IgG4 in human B cells. Eur J Immunol. 1989 Jul;19(7):1311–1315. doi: 10.1002/eji.1830190724. [DOI] [PubMed] [Google Scholar]
- Lê thi Bich-Thuy, Fauci A. S. Direct effect of interleukin 2 on the differentiation of human B cells which have not been preactivated in vitro. Eur J Immunol. 1985 Nov;15(11):1075–1079. doi: 10.1002/eji.1830151102. [DOI] [PubMed] [Google Scholar]
- Mayumi M., Kuritani T., Kubagawa H., Cooper M. D. IgG subclass expression by human B lymphocytes and plasma cells: B lymphocytes precommitted to IgG subclass can be preferentially induced by polyclonal mitogens with T cell help. J Immunol. 1983 Feb;130(2):671–677. [PubMed] [Google Scholar]
- Miller K. M., Pittard W. B., Sorensen R. U. Cord blood B cell differentiation. Synergistic effect of pokeweed mitogen and Staphylococcus aureus on in vitro differentiation of B cells from human neonates. Clin Exp Immunol. 1984 May;56(2):415–424. [PMC free article] [PubMed] [Google Scholar]
- Miyawaki T., Seki H., Kubo M., Taniguchi N. Suppressor activity of T lymphocytes from infants assessed by co-culture with unfractionated adult lymphocytes in the pokeweed mitogen system. J Immunol. 1979 Sep;123(3):1092–1096. [PubMed] [Google Scholar]
- Morell A., Skvaril F., Hitzig W. H., Barandun S. IgG subclasses: development of the serum concentrations in "normal" infants and children. J Pediatr. 1972 Jun;80(6):960–964. doi: 10.1016/s0022-3476(72)80007-6. [DOI] [PubMed] [Google Scholar]
- Morell A., Van Loghem E., Nef M., Theilkaes L., Skvaril F. Determination of IgG subclasses and Gm allotypes in culture supernatants of pokeweed mitogen-stimulated human blood lymphocytes. J Immunol. 1981 Sep;127(3):1099–1102. [PubMed] [Google Scholar]
- Morito T., Bankhurst A. D., Williams R. C., Jr Studies of human cord blood and adult lymphocyte interactions with in vitro immunoglobulin production. J Clin Invest. 1979 Oct;64(4):990–995. doi: 10.1172/JCI109565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natvig J. B., Førre O. The initial recognition of IgG subclasses. Monogr Allergy. 1986;19:1–6. [PubMed] [Google Scholar]
- Nonoyama S., Farrington M. L., Ochs H. D. Effect of IL-2 on immunoglobulin production by anti-CD40-activated human B cells: synergistic effect with IL-10 and antagonistic effect with IL-4. Clin Immunol Immunopathol. 1994 Sep;72(3):373–379. doi: 10.1006/clin.1994.1155. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Tishon A., Moretta L. Active thymus derived suppressor lymphocytes in human cord blood. Nature. 1977 Sep 22;269(5626):333–335. doi: 10.1038/269333a0. [DOI] [PubMed] [Google Scholar]
- Oxelius V. A. IgG subclass levels in infancy and childhood. Acta Paediatr Scand. 1979 Jan;68(1):23–27. doi: 10.1111/j.1651-2227.1979.tb04424.x. [DOI] [PubMed] [Google Scholar]
- Pabst H. F., Kreth H. W. Ontogeny of the immune response as a basis of childhood disease. J Pediatr. 1980 Oct;97(4):519–534. doi: 10.1016/s0022-3476(80)80003-5. [DOI] [PubMed] [Google Scholar]
- Punnonen J. The role of interleukin 2 in the regulation of proliferation and IgM synthesis of human newborn mononuclear cells. Clin Exp Immunol. 1989 Mar;75(3):421–426. [PMC free article] [PubMed] [Google Scholar]
- Ralph P., Jeong G., Welte K., Mertelsmann R., Rabin H., Henderson L. E., Souza L. M., Boone T. C., Robb R. J. Stimulation of immunoglobulin secretion in human B lymphocytes as a direct effect of high concentrations of IL 2. J Immunol. 1984 Nov;133(5):2442–2445. [PubMed] [Google Scholar]
- Romagnani S., Del Prete G., Giudizi M. G., Biagiotti R., Almerigogna F., Tiri A., Alessi A., Mazzetti M., Ricci M. Direct induction of human B-cell differentiation by recombinant interleukin-2. Immunology. 1986 May;58(1):31–35. [PMC free article] [PubMed] [Google Scholar]
- Ruuskanen O., Pittard W. B., 3rd, Miller K., Pierce G., Sorensen R. U., Polmar S. H. Staphylococcus aureus Cowan I-induced immunoglobulin production in human cord blood lymphocytes. J Immunol. 1980 Jul;125(1):411–413. [PubMed] [Google Scholar]
- Scott M. G., Nahm M. H. Mitogen-induced human IgG subclass expression. J Immunol. 1984 Nov;133(5):2454–2460. [PubMed] [Google Scholar]
- Seppälä I. J., Routonen N., Sarnesto A., Mattila P. A., Mäkelä O. The percentages of six immunoglobulin isotypes in human antibodies to tetanus toxoid: standardization of isotype-specific second antibodies in solid-phase assay. Eur J Immunol. 1984 Sep;14(9):868–875. doi: 10.1002/eji.1830140918. [DOI] [PubMed] [Google Scholar]
- Shackelford P. G., Nelson S. J., Palma A. T., Nahm M. H. Human antibodies to group A streptococcal carbohydrate. Ontogeny, subclass restriction, and clonal diversity. J Immunol. 1988 May 1;140(9):3200–3205. [PubMed] [Google Scholar]
- Splawski J. B., Lipsky P. E. Cytokine regulation of immunoglobulin secretion by neonatal lymphocytes. J Clin Invest. 1991 Sep;88(3):967–977. doi: 10.1172/JCI115400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Splawski J. B., McAnally L. M., Lipsky P. E. IL-2 dependence of the promotion of human B cell differentiation by IL-6 (BSF-2). J Immunol. 1990 Jan 15;144(2):562–569. [PubMed] [Google Scholar]
- Teranishi T., Hirano T., Lin B. H., Onoue K. Demonstration of the involvement of interleukin 2 in the differentiation of Staphylococcus aureus Cowan I-stimulated B cells. J Immunol. 1984 Dec;133(6):3062–3067. [PubMed] [Google Scholar]
- Tosato G., Magrath I. T., Koski I. R., Dooley N. J., Blaese R. M. B cell differentiation and immunoregulatory T cell function in human cord blood lymphocytes. J Clin Invest. 1980 Aug;66(2):383–388. doi: 10.1172/JCI109867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucci A., Mouzaki A., James H., Bonnefoy J. Y., Zubler R. H. Are cord blood B cells functionally mature? Clin Exp Immunol. 1991 Jun;84(3):389–394. [PMC free article] [PubMed] [Google Scholar]
- Walker L., Johnson G. D., MacLennan I. C. The IgG subclass responses of human lymphocytes to B-cell activators. Immunology. 1983 Oct;50(2):269–272. [PMC free article] [PubMed] [Google Scholar]
- Wu L. Y., Blanco A., Cooper M. D., Lawton A. R. Ontogeny of B-lymphocyte differentiation induced by pokeweed mitogen. Clin Immunol Immunopathol. 1976 Mar;5(2):208–217. doi: 10.1016/0090-1229(76)90026-x. [DOI] [PubMed] [Google Scholar]
- Xia X., Lee H. K., Clark S. C., Choi Y. S. Recombinant interleukin (IL) 2-induced human B cell differentiation is mediated by autocrine IL6. Eur J Immunol. 1989 Dec;19(12):2275–2281. doi: 10.1002/eji.1830191215. [DOI] [PubMed] [Google Scholar]
- Yount W. J., Dorner M. M., Kunkel H. G., Kabat E. A. Studies on human antibodies. VI. Selective variations in subgroup composition and genetic markers. J Exp Med. 1968 Mar 1;127(3):633–646. doi: 10.1084/jem.127.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]



