Abstract
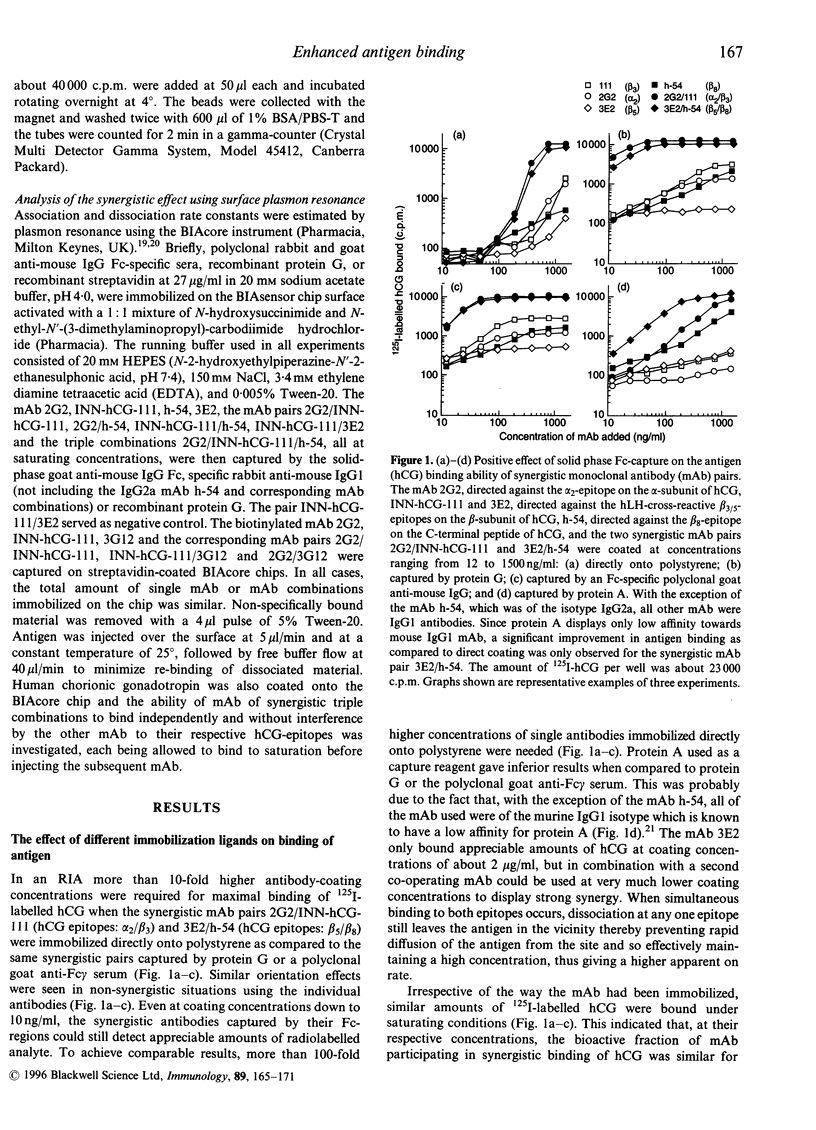
The effects of orientating pairs of synergistic monoclonal antibodies (mAb) on binding of human chorionic gonadotropin (hCG) was studied by radioimmunoassay (RIA), enzyme-linked immunosorbent assay (ELISA) and surface plasmon resonance (SPR). Antibody synergy towards hCG required two functionally intact antibodies located adjacent to each other and with different epitope specificities. We investigated whether immobilization procedures avoiding protein denaturation, increasing proper orientation and promoting higher molecular flexibility of the synergistic mAb resulted in significantly enhanced antigen, binding. Synergistic mAb pairs captured through their Fc-region by protein G or a polyclonal serum against the Fc-part of mouse IgG could be used at 10-fold lower coating concentrations to achieve maximal binding of the analyte as compared with the same mAb pairs coated directly onto polystyrene. The synergistic effect observed with protein A used as capture varied greatly with the subclasses of the two synergistic antibodies employed. Scatchard analysis revealed that the number of functionally synergistic antibody sites participating in the binding of hCG for one mAb pair was about 10 times higher for the protein G-captured as compared with the directly coated synergistic pair. Biotinylated synergistic mAb pairs, coated directly or captured by streptavidin, did not display any enhanced antigen binding when tested in SPR or ELISA. With SPR, synergy was only observed when the synergistic mAb had been captured through their Fc-region. Using protein G or a polyclonal rabbit anti-IgG1 serum as capture reagents in SPR, synergistic triple mAb combinations against hCG were demonstrated.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger P., Klieber R., Panmoung W., Madersbacher S., Wolf H., Wick G. Monoclonal antibodies against the free subunits of human chorionic gonadotrophin. J Endocrinol. 1990 May;125(2):301–309. doi: 10.1677/joe.0.1250301. [DOI] [PubMed] [Google Scholar]
- Berger P., Panmoung W., Khaschabi D., Mayregger B., Wick G. Antigenic features of human follicle stimulating hormone delineated by monoclonal antibodies and construction of an immunoradiomometric assay. Endocrinology. 1988 Nov;123(5):2351–2359. doi: 10.1210/endo-123-5-2351. [DOI] [PubMed] [Google Scholar]
- Cheong H. S., Chang J. S., Park J. M., Byun S. M. Affinity enhancement of bispecific antibody against two different epitopes in the same antigen. Biochem Biophys Res Commun. 1990 Dec 31;173(3):795–800. doi: 10.1016/s0006-291x(05)80857-5. [DOI] [PubMed] [Google Scholar]
- Dierks S. E., Butler J. E., Richerson H. B. Altered recognition of surface-adsorbed compared to antigen-bound antibodies in the ELISA. Mol Immunol. 1986 Apr;23(4):403–411. doi: 10.1016/0161-5890(86)90138-0. [DOI] [PubMed] [Google Scholar]
- Dudley R. A., Edwards P., Ekins R. P., Finney D. J., McKenzie I. G., Raab G. M., Rodbard D., Rodgers R. P. Guidelines for immunoassay data processing. Clin Chem. 1985 Aug;31(8):1264–1271. [PubMed] [Google Scholar]
- Ehrlich P. H., Moyle W. R., Moustafa Z. A., Canfield R. E. Mixing two monoclonal antibodies yields enhanced affinity for antigen. J Immunol. 1982 Jun;128(6):2709–2713. [PubMed] [Google Scholar]
- Elsner H. I., Mouritsen S. Use of psoralens for covalent immobilization of biomolecules in solid phase assays. Bioconjug Chem. 1994 Sep-Oct;5(5):463–467. doi: 10.1021/bc00029a014. [DOI] [PubMed] [Google Scholar]
- Karlsson R., Michaelsson A., Mattsson L. Kinetic analysis of monoclonal antibody-antigen interactions with a new biosensor based analytical system. J Immunol Methods. 1991 Dec 15;145(1-2):229–240. doi: 10.1016/0022-1759(91)90331-9. [DOI] [PubMed] [Google Scholar]
- Kofler R., Berger P., Wick G. Monoclonal antibodies against human chorionic gonadotropin (hCG): I. production, specificity, and intramolecular binding sites. Am J Reprod Immunol. 1982 Aug;2(4):212–216. doi: 10.1111/j.1600-0897.1982.tb00168.x. [DOI] [PubMed] [Google Scholar]
- Kurki P., Virtanen I. The detection of human antibodies against cytoskeletal components. J Immunol Methods. 1984 Mar 16;67(2):209–223. doi: 10.1016/0022-1759(84)90462-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lapthorn A. J., Harris D. C., Littlejohn A., Lustbader J. W., Canfield R. E., Machin K. J., Morgan F. J., Isaacs N. W. Crystal structure of human chorionic gonadotropin. Nature. 1994 Jun 9;369(6480):455–461. doi: 10.1038/369455a0. [DOI] [PubMed] [Google Scholar]
- Munson P. J. LIGAND: a computerized analysis of ligand binding data. Methods Enzymol. 1983;92:543–576. doi: 10.1016/0076-6879(83)92044-x. [DOI] [PubMed] [Google Scholar]
- Olson W. C., Spitznagel T. M., Yarmush M. L. Dissociation kinetics of antigen-antibody interactions: studies on a panel of anti-albumin monoclonal antibodies. Mol Immunol. 1989 Feb;26(2):129–136. doi: 10.1016/0161-5890(89)90094-1. [DOI] [PubMed] [Google Scholar]
- Panayotou G., Gish G., End P., Truong O., Gout I., Dhand R., Fry M. J., Hiles I., Pawson T., Waterfield M. D. Interactions between SH2 domains and tyrosine-phosphorylated platelet-derived growth factor beta-receptor sequences: analysis of kinetic parameters by a novel biosensor-based approach. Mol Cell Biol. 1993 Jun;13(6):3567–3576. doi: 10.1128/mcb.13.6.3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz S., Berger P., Wick G. The antigenic surface of human chorionic gonadotropin as mapped by murine monoclonal antibodies. Endocrinology. 1986 Jan;118(1):189–197. doi: 10.1210/endo-118-1-189. [DOI] [PubMed] [Google Scholar]
- Spitznagel T. M., Clark D. S. Surface-density and orientation effects on immobilized antibodies and antibody fragments. Biotechnology (N Y) 1993 Jul;11(7):825–829. doi: 10.1038/nbt0793-825. [DOI] [PubMed] [Google Scholar]
- Steward M. W., Lew A. M. The importance of antibody affinity in the performance of immunoassays for antibody. J Immunol Methods. 1985 Apr 22;78(2):173–190. doi: 10.1016/0022-1759(85)90074-2. [DOI] [PubMed] [Google Scholar]
- Wood W. G., Gadow A. Immobilisation of antibodies and antigens on macro solid phases--a comparison between adsorptive and covalent binding. A critical study of macro solid phases for use in immunoassay systems. Part I. J Clin Chem Clin Biochem. 1983 Dec;21(12):789–797. doi: 10.1515/cclm.1983.21.12.789. [DOI] [PubMed] [Google Scholar]
- van Erp R., Linders Y. E., van Sommeren A. P., Gribnau T. C. Characterization of monoclonal antibodies physically adsorbed onto polystyrene latex particles. J Immunol Methods. 1992 Aug 10;152(2):191–199. doi: 10.1016/0022-1759(92)90140-o. [DOI] [PubMed] [Google Scholar]




