Abstract
The Hsp90 protein encoded by the Hsp83 gene is required for the development of many traits in Drosophila. Hsp83 is also thought to play a role in the expression of phenotypic and genetic variability for subsequent selection and evolutionary change. Here we examine the impact of different E(sev) and Su(Raf) Hsp83 mutants on means and phenotypic variances of invariant and variable bristle traits. One of the mutants influenced the normally invariant thoracic bristle number, while none affected invariant scutellar bristle number. E(sev) alleles consistently influenced variable bristle traits while there were fewer effects of the Su(Raf) alleles. For the variable traits, none of the Hsp83 alleles had any effect on phenotypic variance, environmental variance, or developmental stability of the bristle traits. When alleles were combined in trans-heterozygotes, there were both cumulative and complementary effects on thoracic and variable bristle trait numbers, depending on the allelic combination. Overall, the results suggest that Hsp83 mutants do not have detectable effects on the phenotypic or environmental variance of bristle traits and that complementation of E(sev) and Su(Raf) Hsp83 mutants can extend to thoracic bristles as well as previously reported effects on viability. Some allelic combinations lead to more severe effects on variable bristle trait means than do single Hsp83 mutations.
THE Hsp90 chaperone protein is required for the development of many different morphological structures in Drosophila melanogaster, such as bristles, eyes, and wings (Rutherford and Lindquist 1998). Apart from being of interest because of its central role in development, Hsp90 has also been investigated because of its potential role in facilitating evolutionary change. In particular, Rutherford and Lindquist (1998) used both Hsp90 inhibitors and mutations in the Hsp83 gene, which encodes the Hsp90 protein in Drosophila, to demonstrate that lowered levels of Hsp90 can lead to the expression of abnormal phenotypes. Moreover, these phenotypes could then be selected to increase in frequency. This “evolutionary capacitor” model involves two components: the increased expression of phenotypic variation and a subsequent genetic change based on the utilization of this variation.
In conjunction with its proposed evolutionary capacitance, Hsp83 has also been regarded as a candidate gene for “canalization.” Waddington was the first to use this term, defining it as the ability of an organism to produce a consistent phenotype despite variation in the genotype or environment (Waddington 1957). He later widened this definition to include the ability of developmental networks to endure genetic or environmental disruptions. Schmalhausen (1949) also independently considered the concept of canalization, using the term “autonomization” instead of canalization, although he and Waddington were essentially describing the same process.
Rutherford and Lindquist's (1998) hypothesis that Hsp90 acts as an important evolutionary capacitor and canalization gene has recently been questioned. Bergman and Siegal (2003) simulated a complex gene network model and used yeast expression data to show that almost any knockout mutation in a gene can result in the expression of previously hidden variation. They suggested that Hsp90 is just one of a large class of evolutionary capacitors. Hermisson and Wagner (2004) argued that hidden genetic variation is a by-product of complex genetic networks and that hidden genetic variation could exist for any trait. They argued that no specific molecular mechanism was necessarily needed for the release of hidden genetic variance. There is also limited evidence that Hsp90 influences canalization and the expression of phenotypic variation: the Hsp90 inhibitor geldanamycin had no impact on variance in noncanalized bristle traits, and there was also no impact on the developmental instability of these traits as measured by fluctuating asymmetry (Milton et al. 2003).
Hsp90's chaperoning activity is known to particularly target signal transduction proteins. This role in the maintenance of signaling networks was confirmed by the identification of Hsp83 mutations in D. melanogaster during screens for suppressors of Raf and enhancers of sevenless (Simon et al. 1991; Cutforth and Rubin 1994; Dickson et al. 1996; van der Straten et al. 1997). Both of these genes are the central components of signaling pathways. The Raf signaling cascade allows the nuclear translocation of mitogen-activated protein kinase to take place (van der Straten et al. 1997). The sevenless signaling cascade enables differentiation of the R7 photoreceptor neuron to proceed in the developing eye of D. melanogaster (Cutforth and Rubin 1994).
Five Hsp83 mutations were identified as Enhancers of sevenless [E(sev)] alleles (Simon et al. 1991; Cutforth and Rubin 1994), while two Hsp83 mutations were identified as Suppressors of Raf [Su(Raf)] alleles (van der Straten et al. 1997). While each of these Hsp83 mutations is homozygous lethal, several are viable as trans-heterozygotes; that is, they are able to complement one another for viability (van der Straten et al. 1997). Generally, those Hsp83 alleles isolated as E(sev) mutations complement those isolated as Su(Raf) mutations. It is still unclear why complementation occurs. Two explanations have been proposed in the literature on the basis of the understanding of the structure of the Hsp90 protein, which contains three highly conserved domains that are found in all Hsp90 family members and that are connected by a “charged linker” of variable length and composition (Scheibel and Buchner 1998; Pearl and Prodromou 2000). The ∼25-kDa N-terminal domain contains the binding site for ATP or geldanamycin, an ansamycin antibiotic that specifically inhibits Hsp90 (Young et al. 2001). The ∼33-kDa middle segment of Hsp90 contains a binding site for the protein kinase PKB/Akt (Sato et al. 2000). The ∼22-kDa C-terminal domain is involved in dimerization, which is essential for the in vivo function of Hsp90 (reviewed in Minami et al. 2001). Thus, the first explanation is that these complementation patterns may result from Hsp90 proteins acting as dimers. A dimer containing proteins with different defects may still be able to perform the normal role of Hsp90 (van der Straten et al. 1997; Yue et al. 1999). Alternatively, complementation of the different Hsp83 alleles may be reliant on the nature of a mutation, that is, whether the mutation is antimorphic or hypomorphic (van der Straten et al. 1997). For example, none of the antimorphic Su(Raf) alleles map to the C-terminal region of Hsp90, which is necessary for dimerization of the protein. All but one of the hypomorphic E(sev) alleles map to this region (Minami et al. 1994; van der Straten et al. 1997).
Yue et al. (1999) noted no abnormalities in the bristles of these trans-heterozygotes, although they did not examine phenotypic variation in detail or measure the variance of traits. However, Rutherford and Lindquist (1998) detected abnormalities in bristle traits, as well as in eye and leg defects in a Hsp839J1/Hsp83e1D trans-heterozygote. Other Hsp90 mutant strains have also been shown to influence the means of bristle traits (Milton et al. 2003). Considering that the Hsp83 trans-heterozygotes contain two defective copies of Hsp83 (compared to the Hsp83/+ flies previously analyzed, which possessed one defective and one wild-type copy of Hsp83), large influences on bristle traits might be expected in trans-heterozygotes, depending on the pattern of complementation of the mutants.
In this study we test one prediction of the evolutionary capacitor hypothesis, namely that Hsp90 increases some components of phenotypic variance in bristle traits, using the suppressors of Raf and enhancers of sevenless Hsp83 mutants. Two canalized (“invariant”) bristle traits and four variable traits were considered to test if the mutants influenced the bristle traits (cf. Milton et al. 2003). As well as bristle variances, the impact on bristle abnormalities of invariant bristle traits was also considered. The phenotypic variances were separated into within-strain and developmental instability components. We also test the hypothesis that complementation occurs among some Hsp83 mutants when in trans-heterozygote form. The impact of mutant trans-heterozygotes of Hsp83 on bristle traits was analyzed to determine whether flies carrying two defective, but complementary, copies of Hsp83 exhibited complementary or cumulative effects on bristle trait variation. If there are complementary effects, then trans-heterozygotes should exhibit lower levels of bristle variation than single heterozygotes. Alternatively, if there is a cumulative effect, higher levels of phenotypic variation should be observed in trans-heterozygotes compared with single heterozygotes.
MATERIALS AND METHODS
Trait measurements:
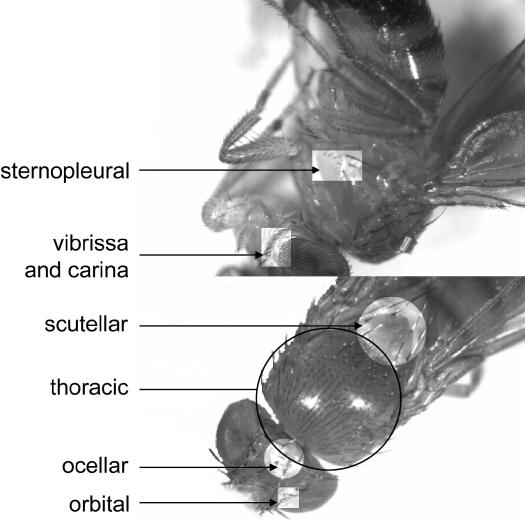
The number of scutellar, thoracic, sternopleural, orbital, ocellar, and vibrissa and carina bristles were counted on the left and right sides of each of 50 flies. Two of these traits, the scutellar and thoracic bristles, are regarded as largely invariant or canalized between two thresholds (Rendel 1967) because almost all individuals show the same number of bristles while a few rare individuals show higher or lower scores. The remaining four characters were variable traits. These six bristle traits are shown in Figure 1.
Figure 1.
The six bristle characters analyzed for trait variation. The position of the invariant traits [thoracic (circled) and scutellar bristles] and the four variable traits (sternopleural, ocellar, orbital, and vibrissa and carina bristles) are marked.
Strains:
The strains used in this study are shown in Table 1. Hsp8313F3 and Hsp8319F2 are independent isolates of the same amino acid replacement. The strains were kindly provided by Suzanne Rutherford (w1118, w1118sevd2, Hsp839J1, Hsp8313F3, Hsp8319F2, Hsp83e1D, and Hsp83e3A) and the Bloomington Stock Center (Hsp83e6A and Hsp83e6D). The Hsp83 alleles were initially isolated by Simon et al. (1991), Cutforth and Rubin (1994), Dickson et al. (1996), and van der Straten et al. (1997). Hsp839J1 is viable with all of the alleles except Hsp8313F3 and Hsp8319F2. Hsp8313F3 and Hsp8319F2 complement Hsp83e1D, Hsp83e3A, and Hsp83e6A but are lethal over Hsp83e6D. Marker strains w1118 and w1118sevd2 were used as the controls. These were the respective genetic backgrounds used in the initial screens in which the Su(Raf) and E(sev) alleles were recovered. Therefore, the genetic variation contained in the Hsp83 alleles originated from these backgrounds. Previous results (Milton et al. 2003) indicated that genetic background plays a significant role in bristle variation when a mutation is present in Hsp83.
TABLE 1.
D. melanogaster strains used in this study and their viability with other mutants when in trans-heterozygote form
| Category | Strain | Description | Viability in trans-heterozygotes |
|---|---|---|---|
| Screening stocks | w1118 | w1118 used in original screen | — |
| w1118, sevd2 | sevd2 used in original screen | — | |
| Hsp83 strains | w; Hsp839J1/TM3, Tb | E377K | Hsp83e1D, Hsp83e3A, Hsp83e6A, Hsp83e6D |
| w; Hsp8313F3/TM6B, Tb | R48C | Hsp83e1D, Hsp83e3A | |
| w; Hsp8319F2/TM6B, Tb | R48C | Hsp83e1D, Hsp83e3A | |
| w; Hsp83e1D/TM6B, Tb | S38L | Hsp839J1, Hsp8313F3 Hsp8319F2 | |
| Hsp83e3A/TM6B, Tb | S574C | Hsp839J1, Hsp8313F3 Hsp8319F2 | |
| Hsp83e6A/TM6B, Tb | S592F | Hsp839J1 | |
| Hsp83e6D/TM6B, Tb | E317K | Hsp839J1 |
Crosses:
To obtain Hsp83 trans-heterozygotes (flies carrying two different Hsp83 alleles), crosses were performed between the lines in Table 1 that complement each other to form viable trans-heterozygotes. Virgin females were crossed to males in population cages at 25° and allowed to lay on standard Drosophila medium plates spread with yeast paste. To control for density and vial effects, first instar larvae were collected from the plates and transferred to vials containing standard medium at a constant density of 50 larvae per vial. Five vials were used per genotype. The balancer marker Tubby can be scored in the larvae, so larvae that did not carry the balancer could be selected. The strains were kept at a constant temperature of 25°. From each vial, 10 females (50 total) were selected at random and the six bristle characters were scored.
To obtain Hsp83 heterozygotes (intermediates) for comparisons with the trans-heterozygotes and controls, the Hsp83 alleles were crossed back into the genetic background in which they were initially isolated. Therefore, Su(Raf) alleles (Hsp839J1, Hsp8313F3, and Hsp8319F2) were crossed into w1118 and E(sev) alleles were crossed into w1118sevd2. Crosses were performed in population cages as described above. Hsp83/+ larvae from each cross were placed at constant temperature and density as before, and 10 female F1 Hsp83 heterozygotes were selected from each vial and scored for bristle characters as described previously. A total of 50 females were scored for each genotype.
The two control lines (w1118 and w1118sevd2) were placed in population cages and larvae were collected and seeded at a constant density as described above. Ten females from each vial were selected and scored for bristle characters as described above. Fifty females were scored for each control strain.
Data analysis:
A different approach was used in the analysis of the invariant and variable traits. For the invariant traits, to examine effects of the genetic background and the Hsp83 allele on invariable bristle numbers, each genotype was divided into two groups: the number of flies that exhibited the canalized phenotype and the number of flies that had more or less bristles than the canalized phenotype. The canalized wild-type phenotype for thoracic bristles is 11 bristles on either side of the thorax. To determine the effect of the genetic background (w1118/w1118sevd2) on the invariable bristle traits, chi-square contingency tests were performed comparing the number of flies in these two classes for w1118 and w1118sevd2. Chi-square contingency tests were also performed to determine the effect of each of the seven Hsp83 alleles on invariable bristle number, with the single Hsp83 heterozygotes compared to the genetic background in which the Hsp83 allele was recovered. Because low numbers of individuals in cells meant that probabilities based on the chi-square distribution were not valid, significance levels were determined by permutation using SPSS version 11.5. In all analyses, probability values were corrected for multiple comparisons with the Dunn-Sidak method due to the number of traits compared or number of comparisons made with the same background strain (Sokal and Rohlf 1995).
To compare trans-heterozygotes to the mutant strains for the invariant thoracic bristle trait, contingency tests were used. If complementation occurred, bristle abnormalities were expected to decline relative to either of the parental strains. However, under combinatory effects, bristle numbers were expected to increase in frequency relative to the parentals. Because the two genetic backgrounds from which the mutants were derived did not influence thoracic bristle abnormalities, background effects were ignored when making these comparisons. Similar procedures were used for the scutellar bristle system, but these turned out to exhibit a low level of variability and were not further considered.
For the variable traits, the effect of genetic background (w1118/w1118sevd2) on variable trait means was examined using one-way ANOVAs. To test for effects of individual Hsp83 alleles, one-way ANOVAs were performed comparing each Hsp83 allele to the genetic background in which it was recovered. Variances of the single Hsp83 heterozygotes and the two w backgrounds were analyzed following the method outlined by Pertoldi et al. (2001). The different components of phenotypic variance (VP) for the single Hsp83 heterozygotes and the two w backgrounds were separated. Developmental instability (DI) was defined as the variance of the difference between bristle counts on the left and right sides of the fly and the measured variation within an individual. We also estimated variation among individuals from a particular mutant/background. This variation is likely to be mainly due to environmental variance (Ve) but also includes a component of genetic variance due to minor genetic differences within the mutant strains. We therefore refer to this as the variance within strains (VWS). Confidence intervals for these parameters were estimated by bootstrapping, while permutation was used to compare each mutant to its w background for each of the four variable traits, using the PopTools add-in for Microsoft Excel.
The effects of interactions in the trans-heterozygotes on variable trait means was complicated by the fact that there were differences due to background between the strains from which the mutants had been derived (see below). This meant that background as well as the effect of the mutants had to be taken into account. When the trans-heterozygotes differ from the parental strains by having higher or lower trait values than those of either parent, this might indicate more severe effects of Hsp83 on the trait than those exerted by either mutant in the heterozygous state (i.e., a lack of complementation), at least if changes are in the same direction as any differences between the parental and mutant strains. Any tendency of trans-heterozygotes to show changes in means that made trait values more similar to those of the parental strains might indicate complementation, but it was not possible to interpret results in terms of complementation because of the large differences among the parental strains. MANOVAs and ANOVAs were carried out to compare trans-heterozygotes to the midvalue of two heterozygous mutant strains. Differences between the trans-heterozygotes and the parental strains were also assessed to test for evidence that trans-heterozygotes exhibited extreme phenotypes that could arise from combinations of the mutant alleles.
RESULTS
Genetic background and single Hsp83 heterozygotes
Invariant traits:
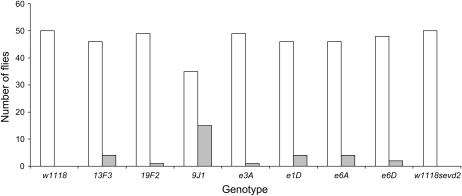
To determine what effect the various Hsp83 alleles have on invariant traits (traits in which little variation from the wild-type phenotype is observed), the thoracic and scutellar bristles were analyzed. The number of flies with canalized/noncanalized thoracic bristle phenotypes are shown in Figure 2. Results of chi-square tests comparing thoracic bristle phenotypes of the two different w backgrounds, and each Hsp83 allele and its particular w background, are shown in Table 2. Genetic background had no effect on the number of thoracic bristles, as all 50 flies of both w1118 and w1118sevd2 exhibited the canalized thoracic phenotype. Of the seven Hsp83 alleles examined, only Hsp839J1 exhibited a significantly different thoracic bristle phenotype. Of the 50 Hsp839J1 flies, 15 exhibited a changed thoracic bristle phenotype, 12 had an extra bristle, and 3 were missing bristles. The Hsp839J1 allele affects three thoracic bristles: the aDC, pDC, and H bristles.
Figure 2.
Number of flies with canalized and noncanalized thoracic phenotypes for genetic background (w1118/w1118sevd2) and single Hsp83 heterozygotes. The canalized phenotype (□) for the thoracic bristles is 11 bristles on either side of the thorax, with the noncanalized phenotype ( ) having either less than or more than 11 bristles on either side of the thorax. A total of 50 flies were scored for each genotype.
) having either less than or more than 11 bristles on either side of the thorax. A total of 50 flies were scored for each genotype.
TABLE 2.
Contingency tests on effect of Hsp83 allele and w background on canalized vs. noncanalized thoracic bristle numbers
| Allele | Chi-square (d.f. = 1) | P |
|---|---|---|
| Hsp8313F3 | 4.17 | 0.117 |
| Hsp8319F2 | 1.01 | 1 |
| Hsp839J1 | 17.65 | <0.001a |
| Hsp83e3A | 1.01 | 1 |
| Hsp83e1D | 4.17 | 0.117 |
| Hsp83e6A | 4.17 | 0.117 |
| Hsp83e6D | 2.04 | 0.495 |
| w background | 0 | — |
Mutants were compared to their originating background strain. Probabilities are based on permutation tests.
Significant after correcting probabilities for multiple comparisons made with the same background strain.
For the scutellar bristles, very few individuals (3%) exhibited an aberrant phenotype and there was no evidence of differences between the Hsp83 alleles and the w backgrounds. Therefore, scutellar bristle data were not analyzed further.
Variable traits:
To investigate the effect of Hsp83 alleles on variable traits (traits in which there is no stereotyped or canalized phenotype), the sternopleural, orbital, ocellar, and vibrissa and carina bristles were analyzed. ANOVAs indicated that genetic background (w1118/w1118sevd2) had a highly significant effect (P < 0.001) on the means of all four variable bristle traits (results not shown). A MANOVA on all four variable bristle traits also indicated a significant effect of genetic background (Wilks λ = 0.362, F = 41.93, d.f. = 4, 95, P < 0.001). Results of MANOVAs for each of the seven Hsp83 alleles compared to their originating w background are shown in Table 3. These indicated that the Hsp83 alleles had varying effects on bristle trait means, with the E(sev) alleles having significant effects on variable bristle trait means compared to the w1118sevd2 background, while there was no significant difference between the Su(Raf) alleles and the w1118 background.
TABLE 3.
MANOVAs comparing effects of Hsp83 alleles on variable bristle trait means relative to the w background in which they were recovered
| Allele | Wilks λ | F (d.f. = 4, 95) | P |
|---|---|---|---|
| Hsp8313F3 | 0.909 | 2.37 | 0.058 |
| Hsp8319F2 | 0.914 | 2.24 | 0.070 |
| Hsp839J1 | 0.978 | 0.54 | 0.710 |
| Hsp83e3A | 0.358 | 42.59 | <0.001a |
| Hsp83e1D | 0.556 | 18.96 | <0.001a |
| Hsp83e6A | 0.285 | 59.64 | <0.001a |
| Hsp83e6D | 0.407 | 34.54 | <0.001a |
Significant after correcting probabilities for the number of comparisons made with the same background strain.
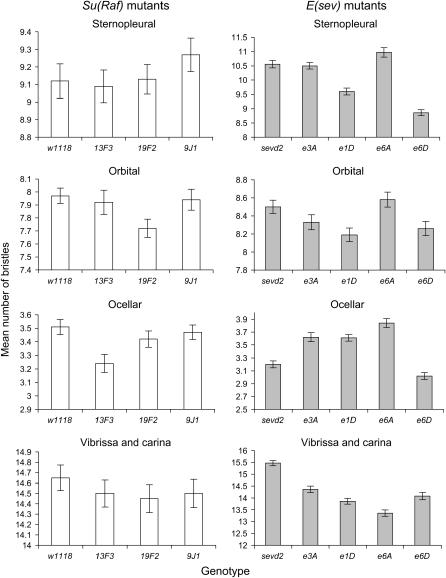
Means are shown in Figure 3. Most of the significant differences in trait mean were exhibited by the E(sev) alleles. ANOVAs indicated that all four E(sev) alleles had a significant effect on vibrissa and carina means (Hsp83e3A: F = 37.86, d.f. = 1, 98, P < 0.001; Hsp83e1D: F = 98.12, d.f. = 1, 98, P < 0.001; Hsp83e6A: F = 147.95, d.f. = 1, 98, P < 0.001; Hsp83e6D: F = 52.37, d.f. = 1, 98, P < 0.001). In each case bristle numbers decreased in the mutants (Figure 3). The E(sev) alleles, with the exception of Hsp83e6D, also had a significant effect on ocellar bristle trait mean (Hsp83e3A: F = 22.42, d.f. = 1, 98, P < 0.001; Hsp83e1D: F = 30.18, d.f. = 1, 98, P < 0.001; Hsp83e6A: F = 55.08, d.f. = 1, 98, P < 0.001; Hsp83e6D: F = 5.68, d.f. = 1, 98, P = 0.019). This involved an increase in bristle number (Figure 3). Hsp83e6D and Hsp83e1D also caused a significant decrease in sternopleural bristle trait when compared to the w1118sevd2 background (Hsp83e6D: F = 104.37, d.f. = 1, 98, P < 0.001; Hsp83e1D: F = 29.01, d.f. = 1, 98, P < 0.001). The only Su(Raf) allele to exhibit a difference in variable bristle trait mean compared to the w1118 background was Hsp8313F3, which had a significant effect on ocellar bristle trait mean compared to the w1118 background (F = 9.73, d.f. = 1, 98, P = 0.002) involving a decrease in bristle number.
Figure 3.
Means for variable bristle traits for single Hsp83 heterozygotes (± standard error). The two different genetic backgrounds are indicated by open and shaded bars.
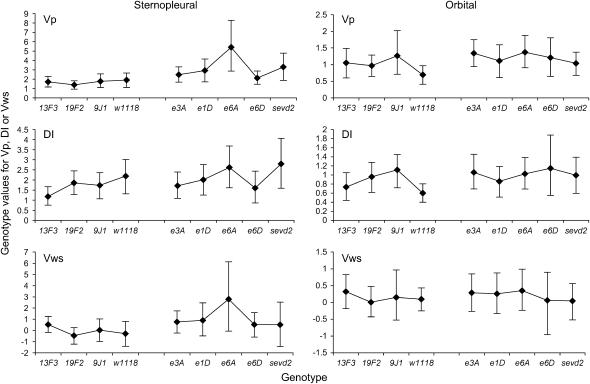
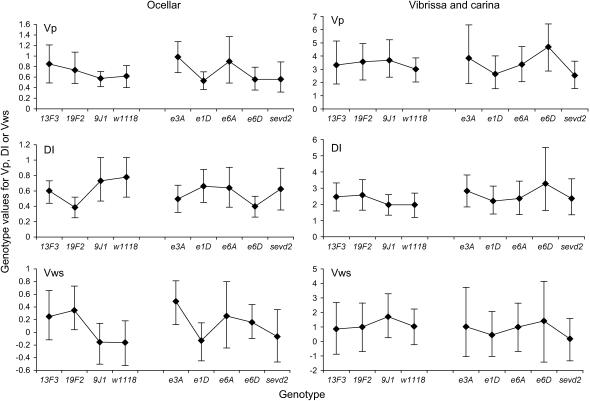
While the Hsp83 mutants influenced bristle trait means, there were no consistent effects on trait variability. Figure 4 shows the VP, VWS and DI values and the 95% confidence limits for each variable trait. Generally, the confidence intervals overlapped and there were few significant differences between the mutants and their background stocks when compared by permutation. Overall only 6 comparisons were significant—in each case at the 5% level and not the 1% level—which is similar to chance expectations, given that there were 96 comparisons. Moreover, in cases where differences between background strains and mutants were significant, the mutants did not necessarily have higher levels of VP, VWS, or DI than the background strains (Figure 4). Therefore, the results indicate that the Hsp83 mutants do not lead to detectable changes in the phenotypic variance or environmental variance of these bristle traits. Furthermore, they do not increase developmental instability.
Figure 4.
Comparison of the phenotypic variance (VP), variance within strains (VWS), and DI values for each single Hsp83 heterozygote to its originating w background for each variable trait (±95% confidence limits).
Hsp83 trans-heterozygotes
Invariant traits:
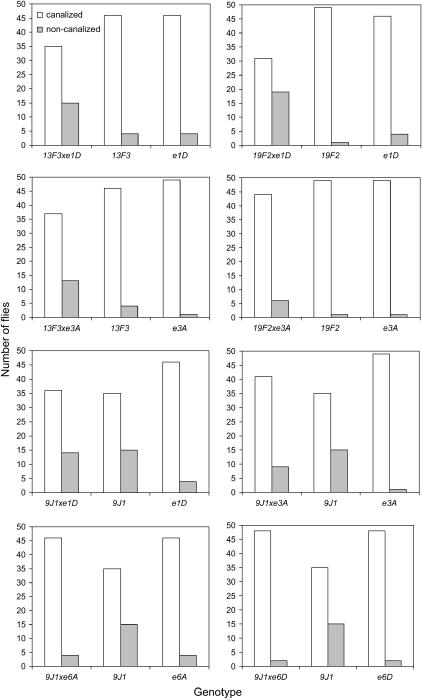
The number of flies from the trans-heterozygote and single heterozygote categories that had abnormal or canalized numbers of thoracic bristles are shown in Figure 5. As there was no difference among the genetic backgrounds for this trait, differences between the trans-heterozygotes and heterozygous mutant strains were interpreted in terms of the effects of the combined mutants on bristle abnormalities. Trans-heterozygotes had an increase in the number of abnormal bristles compared to parental heterozygotes in four cases (Figure 5: Hsp8313F3 × Hsp83e1D, Hsp8319F2 × Hsp83e1D, Hsp8313F3 × Hsp83e3A, and Hsp8319F2 × Hsp83e3A). The difference between the trans-heterozygote and either parental strain was significant in three of the four cases by permutation tests on contingency tables (the comparison involving the Hsp8319F2 × Hsp83e3A cross was nonsignificant). Some combinations of alleles therefore seem to lead to cumulative effects on abnormal bristle frequency rather than to complementation.
Figure 5.
Number of flies with canalized and noncanalized thoracic phenotypes for Hsp83 trans-heterozygotes and single Hsp83 heterozygotes. The canalized phenotype for the thoracic bristles is 11 bristles on either side of the thorax, with the noncanalized phenotype having either less than or more than 11 bristles on either side of the thorax. Fifty flies were scored for each genotype.
For the remaining crosses involving the Hsp839J1 mutant that markedly increased the incidence of abnormality, there was no change in the incidence of abnormalities in one cross (Hsp839J1 × Hsp83e1D) or a decrease in the incidence of abnormalities when compared to Hsp839J1. The decrease was nonsignificant in one cross (Hsp839J1 × Hsp83e3A). However, it was significant in two other cases (Hsp839J1 × Hsp83e6A and Hsp839J1 × Hsp83e6D) when the incidence of abnormalities was similar to that observed in the other mutant strain involved in the cross (Figure 5). Therefore, there seems to have been complementation reducing the incidence of abnormality toward that of the wild-type background strain. When combined with the E(sev) alleles, the same three thoracic bristles affected by the single Hsp839J1 heterozygote were usually affected, as well as two additional bristles, aPA and pSA.
Variable traits:
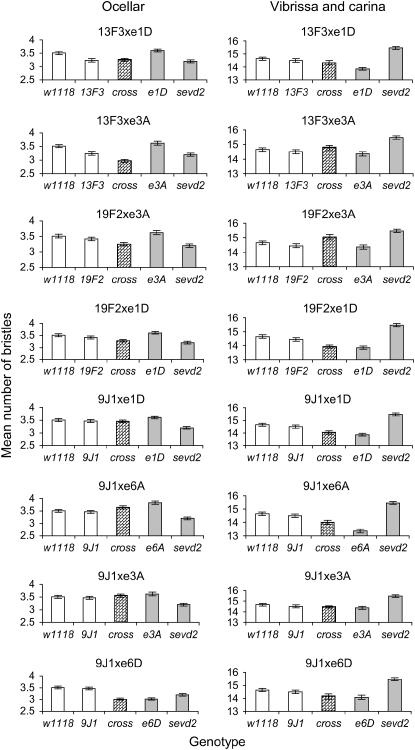
As the differences in the genetic background of the Su(Raf) and E(sev) alleles resulted in significant changes in variable bristle trait means, data from the trans-heterozygotes need to be interpreted cautiously and compared to parental as well as the mutant heterozygote strains. MANOVAs were initially undertaken to test if trans-heterozygotes differed from the mean value of the two mutant heterozygotes. In all cases, MANOVAs indicated significant (P < 0.001) deviations from expectations (analyses not shown). Planned contrasts in ANOVAs were then undertaken to examine patterns for bristle traits (Table 4). In addition, mean values for the trans-heterozygote crosses as well as the mutant heterozygotes and original parental strains were plotted (Figure 6) to interpret patterns and assess potential effects of background as well as mutant combinations on the bristle scores.
TABLE 4.
Planned contrasts comparing the mean of the trans- heterozygotes to the midvalue of the single heterozygotes for the variable bristle traits
| Trait | T (d.f. = 147) | P |
|---|---|---|
| Hsp8313F3/Hsp83e1D vs. Hsp8313F3/+ and Hsp83e1D/+ | ||
| Sternopleural | 2.02 | 0.045 |
| Orbital | 3.09 | 0.002a |
| Ocellar | −2.29 | 0.023 |
| Vibrissa and carina | 0.86 | 0.389 |
| Hsp8313F3/Hsp83e3A vs. Hsp8313F3/+ and Hsp83e3A/+ | ||
| Sternopleural | 1.97 | 0.051 |
| Orbital | 1.04 | 0.301 |
| Ocellar | −5.92 | <0.001a |
| Vibrissa and carina | 2.11 | 0.036 |
| Hsp8319F2/Hsp83e1D vs. Hsp8319F2/+ and Hsp83e1D/+ | ||
| Sternopleural | 0.11 | 0.909 |
| Orbital | −0.16 | 0.872 |
| Ocellar | −3.40 | 0.001a |
| Vibrissa and carina | −1.43 | 0.155 |
| Hsp8319F2/Hsp83e3A vs. Hsp8319F2/+ and Hsp83e3A/+ | ||
| Sternopleural | 3.23 | 0.002a |
| Orbital | −0.48 | 0.631 |
| Ocellar | −3.60 | <0.001a |
| Vibrissa and carina | 3.39 | 0.001a |
| Hsp839J1/Hsp83e1D vs. Hsp839J1/+ and Hsp83e1D/+ | ||
| Sternopleural | 1.25 | 0.215 |
| Orbital | 3.41 | 0.001a |
| Ocellar | −1.21 | 0.227 |
| Vibrissa and carina | −0.82 | 0.412 |
| Hsp839J1/Hsp83e3A vs. Hsp839J1/+ and Hsp83e3A/+ | ||
| Sternopleural | 0.97 | 0.334 |
| Orbital | 1.32 | 0.190 |
| Ocellar | 0.19 | 0.846 |
| Vibrissa and carina | 0.06 | 0.951 |
| Hsp839J1/Hsp83e6A vs. Hsp839J1/+ and Hsp83e6A/+ | ||
| Sternopleural | 2.78 | 0.006 |
| Orbital | 5.25 | <0.00a |
| Ocellar | −0.07 | 0.947 |
| Vibrissa and carina | 0.41 | 0.680 |
| Hsp839J1/Hsp83e6D vs. Hsp839J1/+ and Hsp83e6D/+ | ||
| Sternopleural | 2.52 | 0.013 |
| Orbital | 3.35 | 0.001a |
| Ocellar | −3.78 | <0.001a |
| Vibrissa and carina | −0.73 | 0.467 |
Significant after correcting probabilities for the number of traits being compared.
Figure 6.
Means of Hsp83 trans-heterozygotes compared to single Hsp83 heterozygotes and background values (± standard error). The two different genetic backgrounds are indicated by open and shaded bars, while the trans-heterozygote cross is indicated by a patterned bar.
For sternopleural bristle number, the trans-heterozygote means were similar to one of the mutant heterozygotes or were intermediate between the mutant heterozygotes (Figure 6). Differences from the midmutant value were significant in two cases (Hsp8319F2 × Hsp83e3A and Hsp839J1 × Hsp83e6A) when the trans-heterozygote was more similar to the mutant with higher bristle values but nevertheless still intermediate between the two values (Figure 6). The results suggest that the defective Hsp83 in the trans-heterozygotes never influenced bristle number sufficiently to extend outside the effects of the mutants expressed as heterozygotes or outside values of the parental strains.
For orbital bristle number, the trans-heterozygotes differed significantly from the mean values of the mutant heterozygotes in four cases (Table 4). In each case, the trans-heterozygote had bristle numbers that were greater than those of both the mutants, and in one case (Hsp839J1 × Hsp83e6A) the value of both parents was also exceeded (Figure 6). These results suggest more severe effects associated with the combination of mutants. In the three cases where bristle numbers in the trans-heterozygote reverted to parental numbers, there may have been compensation where the combination of the two different alleles complements each other's defects in the Hsp90 protein.
Ocellar bristle means in the trans-heterozygotes form the midmutant value in four crosses (Table 4). The trans-heterozygote mean was lower than that of both mutant heterozygotes in three crosses (Hsp8313F3 × Hsp83e3A, Hsp8319F2 × Hsp83e3A, and Hsp839J1 × Hsp83e6D) and was similar to the lower mutant heterozygote in one of the other crosses (Hsp8313F3 × Hsp83e1D) as evident in Figure 6. In one case (Hsp8313F3 × Hsp83e3A) the trans-heterozygote mean fell outside that of the parentals, suggesting that additional effects result from combinations of the two mutants.
Finally, for the vibrissa and carina bristles, the trans-heterozygote differed from the mean of the mutant heterozygotes in only one case (Table 4). This involved an increase in mean bristle number in the Hsp8319F2 × Hsp83e3A cross, but not outside the mean of both the parental strains (Figure 6).
DISCUSSION
Hsp83 alleles and genetic background affected bristle trait means but not variances or developmental instability:
Consistent with our previously reported results (Milton et al. 2003), the seven Hsp83 alleles examined in this study have an influence on bristle numbers, whether as single heterozygotes or as trans-heterozygotes. These effects had not been detected in previous studies (Rutherford and Lindquist 1998; Yue et al. 1999) in which Hsp83 trans-heterozygotes were analyzed. However, the significant changes in bristle means observed in this study are not as wide ranging or across the board as seen for the alleles analyzed previously. The majority of changes are decreases in bristle number, which is consistent with previous results. However, unlike the previous results, there is an increase in ocellar bristle numbers in all of the E(sev) alleles except for Hsp83e6D. In the single heterozygotes, the orbital bristles appeared to be the best buffered against changes in trait mean, as no significant differences were detected.
When in single heterozygote form, the E(sev) allelic class tended to influence variable traits more than the Su(Raf) allelic class did. Nine differences were detected for the E(sev) heterozygotes, while only one change in mean was observed for the Su(Raf) heterozygotes. The ocellar and vibrissa and carina traits were particularly affected by the E(sev) alleles. It seems unlikely that these different effects of the two allelic types result from the locations of the amino acid replacements within Hsp83, as the E(sev) mutations are located throughout the three conserved regions of the gene.
While the Hsp83 alleles had an effect on bristle numbers, they generally had no effect on the VP, VWS, and DI values for any of the four variable traits. Therefore, one component of the evolutionary capacitance hypothesis of Hsp90—the increased expression of phenotypic variability—was not supported. In previous research on bristle trait variability, Hsp83 mutants were shown to influence the response to selection for various eye and wing deformities, due to the fact that inhibited Hsp90 levels increase variability in canalized traits, including some bristle traits, in specific genetic backgrounds (Rutherford and Lindquist 1998). In contrast, the present results suggest that the expression of variation in bristle traits that are variable in populations is unaffected by variation in Hsp90 levels. Moreover, variation within an individual, as measured by DI, was also not affected. The DI result is consistent with Milton et al. (2003) who found that bristle DI, as measured by fluctuating asymmetry, was not affected by the Hsp90 inhibitor geldanamycin or by a different set of mutants that decreased Hsp90 activity. Moreover, Milton et al. (2003) also showed that geldanamycin did not influence phenotypic variability in bristle traits.
Rutherford and Lindquist (1998) have suggested that Hsp90 may act as a capacitor for evolutionary change. They showed that Hsp90 mutant strains led to the expression of morphological abnormalities in several traits and that those new variants could be selected for several generations and expressed without the mutant background. They argued that a similar process could occur under stress if levels of Hsp90 were suppressed and that Hsp90 could therefore facilitate some types of evolutionary change. The expression of variation in thoracic bristle numbers in some of the Hsp90 mutants is consistent with the notion that Hsp90 expression levels could have some effect on the evolution of morphological change. In contrast, the absence of Hsp90 mutant effects on the variance of the other bristle traits suggests that the Hsp90 capacitor model does not apply to these traits.
However, our experiments have limitations for testing the capacitor hypothesis. One issue is whether small effects on trait variance could have been detected due to Hsp90-associated abnormalities. Power tests indicate that we would have detected a difference in variance only between strains of 30% or more. Thus abnormalities at a low frequency may not have been detected, given our sample sizes. Another issue is that the lines were not ideal for testing for specific effects of Hsp90 alleles. Given that cryptic genetic variation may be released whenever genetic or environmental change occurs (Hermisson and Wagner 2004), there is the potential for strain differences other than those associated with Hsp90 to obscure effects of this gene on trait variability. Ideally, strains should have the same background and differ only for Hsp90 alleles. Finally, we did not specifically test for changes in genetic variance (VG). While DI reflected variation within individuals, genetic variation may have contributed to differences among individuals within strains. To examine VG specifically, the effects of the same allele across different backgrounds should have been examined.
Hsp839J1 results in decanalization of thoracic bristle phenotype:
The allele Hsp839J1 leads to a marked change in the thoracic bristle phenotype. Essentially, the presence of Hsp839J1 appears to result in decanalization of the processes underlying thoracic bristle development. The majority of flies with a noncanalized thoracic phenotype had an extra bristle. The remaining six Hsp83 alleles did not have a significant impact on thoracic bristle numbers.
On its own, the Hsp839J1 allele affects two main regions of the thorax. The aDC and pDC bristles are situated toward the middle of the thorax, while the H bristles are located on the very top or shoulder region of the thorax. Why the Hsp839J1 allele has an effect on these regions only is unclear.
Cumulative or complementary effects of Hsp83 trans-heterozygotes?
By examining trans-heterozygotes, it can be determined whether combining two different Hsp83 alleles results in cumulative or complementary effects on bristle trait means. Effects in the trans-heterozygotes were dependent on which alleles were present. The Su(Raf) alleles in particular behaved differently when in trans-heterozygote form. Specifically, Hsp8313F3 and Hsp8319F2 exhibited cumulative effects while Hsp839J1 exhibited complementary effects.
Why do these alleles, all isolated as Su(Raf) alleles, behave in such different ways when in trans-heterozygote form? The difference may relate partly to the fact that Hsp839J1 has a much stronger effect than either Hsp8313F3 or Hsp8319F2. Hsp839J1 on its own altered the canalization of the thoracic bristles, whereas the other mutants did not have this effect. Perhaps there is a limit to the incidence of abnormal bristle phenotypes that has been reached in the case of Hsp839J1 heterozygotes, which might explain why trans-heterozygotes with Hsp83e1D and Hsp839J1 heterozygotes had a similar incidence of abnormalities. However, there are new phenotypic changes in the trans-heterozygotes that are not evident in the single-mutant heterozygotes. When the Hsp8313F3 and Hsp8319F2 alleles are combined with Hsp83e3A, only the aDC and H bristles are affected. Changes in these bristles are also observed when Hsp8313F3 and Hsp8319F2 are as single heterozygotes. But when the Hsp8313F3 and Hsp8319F2 alleles are combined with Hsp83e1D, a wider range of thoracic bristles are affected, with pDC, aSA, aPA, and aNP also affected. These results suggest that Hsp83 allele-specific effects are dependent on trans-heterozygote combinations. The different locations of the point mutations may be another reason for the different trans-heterozygote behavior, with Hsp8313F3 and Hsp8319F2 located in the N-terminal domain and Hsp839J1 located in the middle region of the protein.
However, despite Hsp8313F3 and Hsp8319F2 being independent isolates of the same allele, some differences are observed in the interactions that include Hsp8313F3 and Hsp8319F2. For example, when Hsp8313F3 is combined with Hsp83e1D, the orbital bristles are significantly affected; when Hsp8319F2 is combined with Hsp83e1D, the orbital bristle mean does not change while the ocellar bristle mean is significantly different. This may be due to slight variation in the original backgrounds of these alleles.
The thoracic data also suggest that there is compensation in combinations of the Hsp839J1 allele with Hsp83e6A and Hsp83e6D. As seen in Figure 5, the highly significant effect of the Hsp839J1 allele on the thoracic bristle phenotype disappears in the trans-heterozygotes; the phenotype reverts to the thoracic phenotype of the Hsp83e6A and Hsp83e6D single heterozygotes. Therefore, there is an underlying mechanism that prevents the Hsp839J1 allele from exerting its usual effects on the thoracic bristles. Given their location, it is not clear why there is compensation with these alleles and not the other E(sev) alleles.
The interpretation of changes in the thoracic bristle phenotypes of the trans-heterozygotes is relatively simple due to the lack of differences in the two w backgrounds for the thoracic bristles. In comparison, the analysis of the four variable bristle traits was complicated by highly significant differences between these two w backgrounds. Despite this, the results suggest that some combinations of the Hsp83 alleles have more severe effects on the variable traits than single mutants do. This applied particularly to the ocellar and orbital bristles where the trans-heterozygote means fell outside those for the single-mutant heterozygote and parental means. Cumulative effects were specific to particular types of bristles, suggesting that specific types of Hsp90 impairment influenced the traits differently.
Conclusions:
The results of this study support previous findings indicating that impaired Hsp90 has an impact on bristle numbers in D. melanogaster, but not on the expression of variability among or within individuals as predicted by the evolutionary capacitor hypothesis. In these Hsp83 mutants there is a tendency toward a reduction of bristle numbers, with the exception of the ocellar bristles, which increase for most of the E(sev) alleles. The different alleles of Hsp83 have diverse effects on bristle numbers, which may be the result of the point mutations being located in different regions of the protein. Viable combinations of the Hsp83 alleles also have an impact upon bristle numbers, but these effects are allele and trait specific. Decanalization of the thoracic bristles is observed for Hsp839J1 but not for the other alleles, and this decanalization can be complemented by some E(sev) alleles. The absence of mutant effects on bristle trait variance within strains and developmental instability in the noncanalized bristle traits suggests that Hsp90 is unlikely to act as a capacitor for the evolution of these traits.
Acknowledgments
We thank Suzanne Rutherford for provision of some strains. This research was supported by the Australian Research Council via their Special Research Centre scheme and by a Federation Fellowship to A.A.H.
References
- Bergman, A., and M. L. Siegal, 2003. Evolutionary capacitance as a general feature of complex gene networks. Nature 424: 549–552. [DOI] [PubMed] [Google Scholar]
- Cutforth, T., and G. M. Rubin, 1994. Mutations in Hsp83 and cdc37 impair signaling by the sevenless receptor tyrosine kinase in Drosophila. Cell 77: 1027–1036. [DOI] [PubMed] [Google Scholar]
- Dickson, B. J., A. van der Straten, M. Dominguez and E. Hafen, 1996. Mutations modulating Raf signaling in Drosophila eye development. Genetics 142: 163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermisson, J., and G. P. Wagner, 2004. The population genetic theory of hidden variation and genetic robustness. Genetics 168: 2271–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton, C. C., B. Huynh, P. Batterham, S. L. Rutherford and A. A. Hoffmann, 2003. Quantitative trait symmetry independent of Hsp90 buffering: distinct modes of genetic canalization and developmental stability. Proc. Natl. Acad. Sci. USA 100: 13396–13401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami, M., M. Nakamura, Y. Emori and Y. Minami, 2001. Both the N- and C-terminal chaperone sites of Hsp90 participate in protein refolding. Eur. J. Biochem. 268: 2520–2524. [DOI] [PubMed] [Google Scholar]
- Minami, Y., Y. Kimura, H. Kawasaki, K. Suzuki and I. Yahara, 1994. The carboxy-terminal region of mammalian HSP90 is required for its dimerization and function in vivo. Mol. Cell. Biol. 14: 1459–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl, L. H., and C. Prodromou, 2000. Structure and in vivo function of Hsp90. Curr. Opin. Struct. Biol. 10: 46–51. [DOI] [PubMed] [Google Scholar]
- Pertoldi, C., T. N. Kristensen and V. Loeschcke, 2001. A new method for estimating environmental variability for clonal organisms, and the use of fluctuating asymmetry as an indicator of developmental instability. J. Theor. Biol. 210: 407–410. [DOI] [PubMed] [Google Scholar]
- Rendel, J. M., 1967. Canalisation and Gene Control. Logos Press, London.
- Rutherford, S. L., and S. Lindquist, 1998. Hsp90 as a capacitor for morphological evolution. Nature 396: 336–342. [DOI] [PubMed] [Google Scholar]
- Sato, S., N. Fujita and T. Tsuruo, 2000. Modulation of Akt kinase activity by binding to Hsp90. Proc. Natl. Acad. Sci. USA 97: 10832–10837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibel, T., and J. Buchner, 1998. The Hsp90 complex—a super-chaperone machine as a novel drug target. Biochem. Pharmacol. 56: 675–682. [DOI] [PubMed] [Google Scholar]
- Schmalhausen, I. I., 1949. Factors of Evolution. Blakiston, Philadephia.
- Simon, M. A., D. D. Bowtell, G. S. Dodson, T. R. Laverty and G. M. Rubin, 1991. Ras1 and a putative guanine nucleotide exchange factor perform crucial steps in signaling by the sevenless protein tyrosine kinase. Cell 67: 701–716. [DOI] [PubMed] [Google Scholar]
- Sokal, R. R., and F. J. Rohlf, 1995. Biometry: The Principles and Practice of Statistics in Biological Research. W. H. Freeman, New York.
- van der Straten, A., C. Rommel, B. Dickson and E. Hafen, 1997. The heat shock protein 83 (Hsp83) is required for Raf-mediated signalling in Drosophila. EMBO J. 16: 1961–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington, C. H., 1957. The Strategy of the Genes: A Discussion of Some Aspects of Theoretical Biology. Macmillan, New York.
- Young, J. C., I. Moarefi and F. U. Hartl, 2001. Hsp90: a specialized but essential protein-folding tool. J. Cell Biol. 154: 267–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue, L., T. L. Karr, D. F. Nathan, H. Swift, S. Srinivasan et al., 1999. Genetic analysis of viable Hsp90 alleles reveals a critical role in Drosophila spermatogenesis. Genetics 151: 1065–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]