Abstract
The role of heterotrimeric G-proteins in cAMP-dependent germination of conidia was investigated in the filamentous ascomycete Aspergillus nidulans. We demonstrate that the Gα-subunit GanB mediates a rapid and transient activation of cAMP synthesis in response to glucose during the early period of germination. Moreover, deletion of individual G-protein subunits resulted in defective trehalose mobilization and altered germination kinetics, indicating that GanB(α)-SfaD(β)-GpgA(γ) constitutes a functional heterotrimer and controls cAMP/PKA signaling in response to glucose as well as conidial germination. Further genetic analyses suggest that GanB plays a primary role in cAMP/PKA signaling, whereas the SfaD-GpgA (Gβγ) heterodimer is crucial for proper activation of GanB signaling sensitized by glucose. In addition, the RGS protein RgsA is also involved in regulation of the cAMP/PKA pathway and germination via attenuation of GanB signaling. Genetic epistatic analyses led us to conclude that all controls exerted by GanB(α)-SfaD(β)-GpgA(γ) on conidial germination are mediated through the cAMP/PKA pathway. Furthermore, GanB may function in sensing various carbon sources and subsequent activation of downstream signaling for germination.
SURVIVAL of fungi is ensured by the production and dissemination of specialized structures, spores, that are long-lived and highly resistant to environmental stress. Spore germination represents a critical stage in the life cycle of fungi and constitutes a prerequisite to colonization in a new environment. The germination process can be divided into three steps: (1) an activation step triggered by environmental cues that leads the resting spore to germination, (2) an isotropic growth phase representing the first morphological event referred to as swelling and characterized by metabolic changes such as resumption of protein synthesis, and (3) a polarized growth phase (reviewed in d'Enfert 1997).
Molecular genetic studies focused on the mechanisms regulating the successive steps of spore germination have led to the identification of key components specifically required for distinct stages (reviewed in d'Enfert 1997; Wendland 2001). The primary requirement for initiation of germination and completion of the subsequent steps is the sensing of external signals. Early studies demonstrated that germination of ascospores in Saccharomyces cerevisiae is most efficient in the presence of a readily fermentable carbon source, suggesting that initiation of germination is regulated by nutrient availability (Savarese 1974; Tingle et al. 1974). In this regard, glucose has been shown to be necessary and sufficient to induce activation of ascospore germination (Herman and Rine 1997). In S. cerevisiae, glucose sensing is mediated by the G-protein-coupled receptor (GPCR) Gpr1p that in turn activates the heterotrimeric G-protein α-subunit encoded by the GPA2 gene (Lorenz and Heitman 1997; Xue et al. 1998; Kraakman et al. 1999). The Gpr1p-Gpa2p system mediates glucose-dependent activation of the cAMP-dependent protein kinase (PKA) pathway that is associated with mobilization of trehalose, decreased stress resistance, and expression of ribosomal protein (rp) genes (Kraakman et al. 1999). In S. cerevisiae, adenylate cyclase activity is also regulated by the small GTPase Ras2 that responds to intracellular acidification during transition to growth on glucose (Colombo et al. 1998). The nutrient-sensing GPCR-G-protein-cAMP-PKA pathway is conserved in Schizosaccharomyces pombe, where it appears to be crucial for efficient ascospore germination (Welton and Hoffman 2000; Hatanaka and Shimoda 2001).
To date, no similar nutrient-sensing pathway regulating sexual or asexual spore germination has been identified in filamentous fungi. Little is known about the molecular mechanisms controlling spore germination of filamentous fungi. Several physiological changes are associated with germination, such as trehalose degradation, decreased stress resistance, and stimulation of rp gene expression, suggesting similarities with molecular mechanisms involved in growth resumption in yeasts (d'Enfert 1997). The cAMP/PKA signal transduction cascade and heterotrimeric G-proteins have attracted growing interest in recent years, which has led to extensive information on molecular signals involved in fungal morphogenesis and virulence (reviewed in Lengeler et al. 2000). Only a few of these studies specifically investigated the spore germination process. The first unambiguous study focused on the involvement of G-proteins in spore germination was described in the dimorphic fungus Penicillium marneffei (Zuber et al. 2003). The characterization of three Gα-subunits revealed that one of these, GasC, is crucial for efficient germination (Zuber et al. 2002, 2003). The gasC deletion mutant is severely delayed in germination while a dominant-activating mutation in gasC triggers precocious germination. Surprisingly, this gain-of-function mutant strain is unable to germinate in the absence of any carbon source, suggesting that GasC does not mediate carbon source sensing during germination. In Aspergillus nidulans, the biological processes regulated by GasC in P. marneffei, i.e., conidial germination, production of secondary metabolites, and conidiation, are regulated by the cAMP/PKA pathway (Shimizu and Keller 2001; Fillinger et al. 2002) and it has therefore been proposed that GasC signals through the cAMP/PKA pathway (Zuber et al. 2003).
Requirement of the cAMP/PKA pathway for conidial germination was proposed early on the basis of the observation that the trehalose pool in spores is rapidly mobilized at the onset of germination. Indeed, this reaction is catalyzed by neutral trehalases that are potential targets of PKA (Thevelein 1984; d'Enfert et al. 1999). This model was demonstrated in A. nidulans with characterization of the genes encoding adenylate cyclase (cyaA) and PKA (pkaA) (Shimizu and Keller 2001; Fillinger et al. 2002). Deletion of cyaA causes severe defects in conidial germination, i.e., delayed germ tube formation (several hours) and a dramatic decrease in trehalose degradation. Inactivation of pkaA also leads to germination defects, indicating the involvement of the cAMP-PKA signaling pathway in activation of early events of conidial germination via carbon source sensing. Yet, germination defects of the pkaA mutant are less pronounced than those of the cyaA mutant, suggesting that cAMP might act positively not only on PkaA but also on other signaling components necessary for efficient germination. These may include additional catalytic subunits that have been revealed by sequencing of the A. nidulans genome. An additional transduction pathway is required for efficient germination in A. nidulans: Ras signaling has been proposed to control the switch from isotropic to polarized growth as overproduction of a dominant-activating form of RasA results in giant swollen conidia with multiple nuclei unable to produce a germ tube (Som and Kolaparthi 1994). Whereas in S. cerevisiae cAMP signaling is regulated in part by the small GTPases Ras1 and Ras2, in A. nidulans ras and cAMP signaling control the germination process in an independent manner since overexpression of the dominant-active form of RasA blocks germ tube formation even in the absence of a functional adenylate cyclase (Fillinger et al. 2002). Therefore, it has been proposed that activation of adenylate cyclase could be mediated by heterotrimeric G-proteins in response to glucose during early germination as described for fission and budding yeasts. Among the three Gα-subunits identified in A. nidulans, FadA, GanA, and GanB (Yu et al. 1996; Chang et al. 2004), on the basis of the level of sequence similarity with Gpa2 of S. cerevisiae, GanB appeared to be the most likely candidate.
Two recent studies focused on G-protein signaling components in A. nidulans have brought support to this model and established the involvement of the A. nidulans GanB and RgsA proteins in conidial germination (Chang et al. 2004; Han et al. 2004b). A. nidulans strains with ganB null or dominant inactivating mutations show delayed germination and decreased germination rates. In contrast, a dominant-activating form of GanB significantly accelerates germination rates and is able to induce germ tube emergence in the absence of any external carbon source. Interestingly, inactivation of the regulator of G-protein signaling (RGS) protein encoded by the rgsA gene results in phenotypes similar to those observed in a ganB gain-of-function mutant strain. Deletion of ganB fully suppresses alterations caused by deletion of rgsA, indicating that the primary role of RgsA is to downregulate GanB signaling (Han et al. 2004b). Yet, none of these studies have addressed the link between GanB signaling and the cAMP/PKA pathway.
In this study we investigated the role of heterotrimeric G-proteins in activation of the cAMP/PKA pathway at the onset of germination and shed light on the molecular mechanisms underlying the early events of conidial germination. Our findings reveal that the heterotrimeric G-protein GanB(α)-SfaD(β)-GpgA(γ) is activated by carbon source sensing and triggers a rapid and transient cAMP signal and subsequent stimulation of PKA activity critical for initiation of germination. In this model, GanB is the activating element while the primary function of the SfaD-GpgA heterodimer is to relocalize GanB to the plasma membrane and allow reactivation by carbon source sensing. Moreover, our observations provide evidence that RgsA inhibits cAMP-dependent events of spore germination through downregulation of GanB signaling.
MATERIALS AND METHODS
A. nidulans strains, growth conditions, and sexual crosses:
A. nidulans strains used in this study and parental strains used to generate various double mutants by genetic crosses are listed in Table 1. Growth conditions for A. nidulans were as described previously (d'Enfert and Fontaine 1997). Germinating conidiospores for trehalose and cAMP measurements were incubated at 37° in liquid minimal medium containing glucose (1%) at a final number of 2 × 107 conidia/ml with shaking (140 rpm). Trehalose in germinating conidia was determined as previously described (d'Enfert and Fontaine 1997). At least two independent experiments were performed. Conidiospore germination was monitored on coverslips in petri dishes essentially as previously described (Harris et al. 1994). Coverslips were placed on the bottom of the petri dish and gently overlaid with liquid minimal media containing 2 × 107 conidia/ml of relevant strains, in the presence or absence of carbon sources at the specified concentration. The conidia settled to the bottom of the petri dish and adhered tightly to the coverslips. Cultures were grown at 37° and coverslips were removed at different times and the percentage of germinated spores was recorded. The spores were considered germinated when presenting a protrusion corresponding to the emerging germ tube. Two sets of 100 spores were monitored at each time point and at least two independent experiments were performed.
TABLE 1.
A. nidulans strains used in this study
| Strain | Genotypea | Origin |
|---|---|---|
| RMdgA32 | yA2 pabaA1 argBΔ∷trpC+ganAΔ∷argB+trpC801 veA1 | Chang et al. (2004) |
| RMdgB03 | yA2 pabaA1 argBΔ∷trpC+ganBΔ∷argB+trpC801 veA1 | Chang et al. (2004) |
| RJY918.6 | biA1 argB2 methG1 fadAΔ∷argB+veA1 | Yu et al. (1996) |
| RMgBQL801 | yA2 pabaA1 argBΔ∷trpC+ganBQ208L∷argB+trpC801 veA1 | Chang et al. (2004) |
| RMgBCI1633 | yA2 pabaA1 argBΔ∷trpC+ganBG207R∷argB+trpC801 veA1 | Chang et al. (2004) |
| rSRB1.15 | biA1 sfaDΔ veA1 | Rosen et al. (1999) |
| rKH51.9 | yA2 pabaA1 rgsAΔ∷argB+veA1 | Han et al. (2004b) |
| rKH52.02 | yA2 pabaA1 rgsAΔ∷argB+ganBΔ∷argB veA1 | Han et al. (2004b) |
| rJAG 19.9 | yA2 pabaA1 gpgAΔ∷argB veA1 | Seo et al. (2005, this issue) |
| CEA178 | wA3 pyroA4 veA1 | Fillinger et al. (2002) |
| CEA179 | wA3 pyroA4 pyrG89 cyaA∷pyrG+veA1 | Fillinger et al. (2002) |
| CEA209 | yA2 pabaA1 veA1 | Fillinger et al. (2002) |
| CEA276 | wA3 pyroA4 ganAΔ∷argB+veA1 | This study (RMdgA32 × CEA178) |
| CEA278 | wA3 pyroA4 ganBΔ∷argB+veA1 | This study (RMdgB03 × CEA178) |
| CEA306 | wA3 pyroA4 fadAΔ∷argB+veA1 | This study (RJY918.6 × CEA178) |
| CEA308 | yA2 pabaA1 sfaDΔ∷argB+veA1 | This study (rSRB1.15 × CEA209) |
| CEA310 | wA3 pyroA4 cyaA∷pyrG+ganBΔ∷argB+veA1 | This study (CEA179 × RMdgB03) |
| CEA312 | wA3 pyroA4 rgsAΔ∷argB+veA1 | This study (rKH51.9 × CEA178) |
| CEA314 | wA3 pyroA4 rgsAΔ∷argB+cyaA∷pyrG+veA1 | This study (rKH51.9 × CEA179) |
| CEA316 | yA2 pabaA1 rgsAΔ∷argB+sfaDΔ∷argB+veA1 | This study (rKH51.9 × rSRB1.15) |
Genotypes at the yA, argB, pyrG, and trpC loci were not tested in wA3, argB+, pyrG+, and trpC+ obtained from genetic crosses.
Molecular biology:
Oligonucleotides used to identify genotypes of mutants obtained from genetic crosses are listed in Table 2. Genotypes of the rgsA, sfaD, ganA, ganB, and fadA loci were tested by PCR using the oligonucleotides OKH01 and OKH02, OKH17 and OKH18, ganAF1577 and ganAR2959, ganBF2181 and ganBR3620, and fadAF201 and fadAR2270, respectively. Sizes of the amplicons of the wild-type rgsA, sfaD, ganA, ganB, and fadA alleles are 2, 2.1, 1.4, 1.4, and 2 kb and of the null alleles are 2.4, 2.7, 3, 3, and 2.5 kb, respectively. Deletion at the cyaA locus was tested with the oligonucleotide pair zeoF11 and zeoR443, which can exclusively anneal with the zeocyn marker.
TABLE 2.
Oligonucleotides used in this study
| Oligonucleotide | Sequence |
|---|---|
| ganAF1577 | 5′-CTGAGTACATAGATTTCTGG-3′ |
| ganAR2959 | 5′-CAAAGACAGTCGTTCGATAG-3′ |
| ganBF2181 | 5′-GTTCCACCGGACGCTGTCGA-3′ |
| ganBR3620 | 5′-GGCTCAATGAGGCTAAGGCG-3′ |
| fadAF201 | 5′-GATCTTCTCCCTTCCCTTTC-3′ |
| fadAR2270 | 5′-GTATGAAAGTCTCAACGCCA-3′ |
| OKH01 | 5′-GAAAACCACCAACAGTGC-3′ |
| OKH02 | 5′-TTCTTTCCAGATGATCCG-3′ |
| OKH17 | 5′-TGGCTCTGAGTGGATTGC-3′ |
| OKH18 | 5′-TGAAGGCGAGTGGTATGG-3′ |
| zeoF11 | 5′-ATTCTCAGTCCTGCTCCTC-3′ |
| zeoR443 | 5′-TCATCGGCATAGTATATCG-3′ |
cAMP extraction and quantification:
Intracellular cAMP extractions from conidia were performed essentially as described by Rocha et al. (2001). Conidia were inoculated in minimal medium lacking carbon source at a final number of 2 × 107 conidia/ml for 20 min at 37°. After addition of glucose at 1% (time zero), aliquots of 600 μl of spore suspensions were transferred to 2-ml tubes containing 400 μl of acid-washed glass beads (Sigma, St. Louis) and 600 μl of 10% trichloroacetic acid. The tubes were mixed and immediately frozen in liquid nitrogen for 30 min. After thawing, the spores were broken in a Fast Prep (BIO 101, Vista, CA; 20 sec at speed 4.5) at 4° and centrifuged at 11,000 × g for 15 min. The supernatants were neutralized by washing five times with water-saturated ether and lyophilized. Extracts were then resuspended in 500 μl of assay buffer (0.05 m acetate buffer, pH 5.8, 0.02% bovine serum albumin). cAMP concentration was determined using the cAMP Biotrak enzyme immunoassay (EIA) system (Amersham, Arlington Heights, IL) according to the supplier's instructions. Samples were quantified in duplicate in three independent experiments. cAMP concentrations are presented in fmol/2 × 107 spores.
RESULTS
The Gα-protein GanB is a positive regulator of the cAMP/PKA pathway in response to glucose:
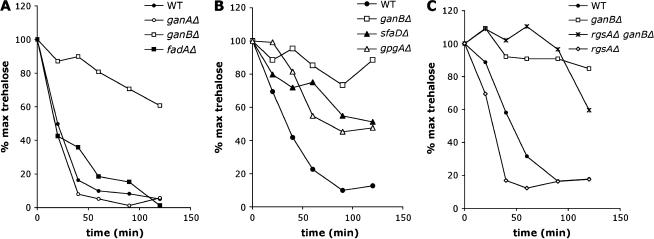
A previous study in our laboratory demonstrated that activation of the cAMP/PKA pathway by carbon source sensing is not mediated by the small GTPase RasA but possibly by heterotrimeric G-proteins in A. nidulans (Fillinger et al. 2002). We further investigated the role of individual Gα-subunits during the early stages of conidial germination in A. nidulans. Trehalose breakdown, which is a direct outcome of activation of the cAMP/PKA pathway during early germination but is not a prerequisite to germ tube outgrowth (d'Enfert et al. 1999), was monitored in A. nidulans mutant strains defective for one of the three A. nidulans Gα-subunits FadA, GanA, and GanB (Yu et al. 1996; Chang et al. 2004). Figure 1A shows that trehalose degradation was severely reduced in the ganB null mutant and remained unaffected in the fadA and ganA null mutants. In the wild-type strain, trehalose degradation occurred immediately after induction of germination by addition of glucose and trehalose levels were decreased to 10–20% at 90 min. In the ganBΔ mutant, the kinetics of degradation were altered greatly in that the pool was reduced only to 60% at 120 min after glucose addition.
Figure 1.
Roles of the G-protein subunits α, β, and γ in trehalose mobilization in response to glucose at the onset of germination. Kinetics of trehalose breakdown is shown in germinating conidia of A. nidulans strains (A) CEA178 (WT), CEA 276 (ganAΔ), CEA278 (ganBΔ), and CEA244 (fadAΔ); (B) CEA209 (WT), CEA 245 (ganBΔ), CEA309 (sfaDΔ), and rJAG19.9 (gpgAΔ); and (C) CEA209 (WT), CEA245 (ganBΔ), CEA293 (rgsAΔ), and CEA294 (rgsAΔganBΔ) inoculated in liquid minimal medium with glucose (1%) at 37°. Trehalose levels in each sample were normalized for the trehalose content in conidia (100%) of the corresponding strain. Results are representative of three (A and B) or two (C) independent experiments.
To evaluate more precisely the role of GanB in regulating the cAMP/PKA pathway in response to glucose, trehalose degradation was monitored in A. nidulans strains expressing the dominant-activating (GanBQ208L) and dominant-inactivating (GanBG207R) alleles of GanB. The kinetics of trehalose degradation in the ganBG207R mutant were similar to those observed in the ganB null mutant (data not shown). The conidia of the ganBQ208L mutant exhibited no trehalose mobilization in response to glucose (data not shown): this feature may be due to very low levels of trehalose measured in the resting conidia of the ganBQ208L mutant. Average trehalose levels in all strains studied varied between 0.8 and 1.4 pg/spore while it was only 0.1 pg/spore in ganBQ208L mutant conidia. This latter value was similar to that measured in wild-type germinating conidia upon completing degradation of the trehalose pool (0.12 ± 0.04 pg/spore). These data suggested that trehalose metabolism in the ganBQ208L conidia is imbalanced toward catabolism compared to that in the wild-type strain. This may result from an activation of the cAMP/PKA pathway in the mutant conidia regardless of the presence or absence of glucose, leading to constitutive degradation of trehalose by the neutral trehalase TreB and abnormally low levels of trehalose in the resting conidia.
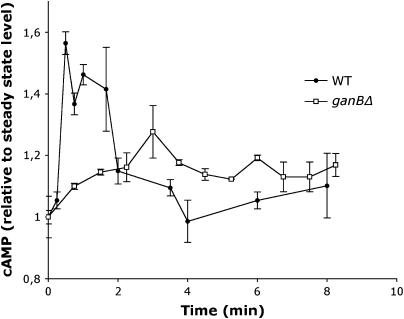
To explicitly demonstrate that GanB signaling regulates adenylate cyclase in response to glucose, we assessed cAMP levels in the resting and germinating spores of wild-type and ganBΔ strains. Results presented in Figure 2 show that addition of glucose to dormant spores of wild type induced a rapid and transient increase in cAMP levels that was fully abolished in the ganBΔ conidia. However, no significant difference in the steady-state levels of cAMP between the wild-type (154 ± 9 fmol/2 × 107 conidia) and ganBΔ (178 ± 16 fmol/2 × 107conidia) dormant conidia were observed. Furthermore, levels of cAMP were below detectable limits in the A. nidulans strain defective for adenylate cyclase (data not shown). Taken together, these results suggest that GanB is responsible for regulating cAMP synthesis in response to glucose at the onset of germination but is not involved in regulating intracellular cAMP basal levels.
Figure 2.
Intracellular cAMP levels in germinating conidia of wild-type and ganBΔ strains. Conidia were inoculated in liquid minimal medium at 37° at 2 × 107/ml. After addition of glucose (1%) aliquots of spore suspension were removed at the indicated times and intracellular cAMP levels were measured as indicated in materials and methods. Error bars indicate standard deviations of duplicate samples. Kinetics of cAMP levels are representative of three independent assays.
SfaD (Gβ) and GpgA (Gγ) regulate the cAMP/PKA pathway during the early phase of germination:
In budding and fission yeasts, the glucose/cAMP pathway is controlled by the Gα, Gpa2p, and seven-kelch domain proteins Gpb1/2p (Harashima and Heitman 2002) and a heterotrimeric G-protein Gpa2(α)-Gbp1(β)-γ (Lorenz and Heitman 1997; Landry and Hoffman 2001), respectively. We addressed whether the nutrient sensor GanB is a part of a heterotrimeric complex involved in activation of the cAMP/PKA pathway at the onset of germination. In A. nidulans, as in most fungi, one gene encoding a Gβ-subunit (Rosen et al. 1999) and one gene encoding a Gγ-subunit have been identified (Seo et al. 2005, accompanying article in this issue). Trehalose degradation was monitored upon germination of the sfaDΔ and gpgAΔ mutant spores. Results presented in Figure 1B show that trehalose degradation was impaired in both mutants although to a lesser extent than when ganB is inactivated, suggesting that SfaD and GpgA positively regulate the glucose/cAMP pathway at the onset of glucose-induced germination.
RgsA/GanB signaling regulates the cAMP/PKA pathway at the onset of germination:
Han et al. (2004b) have identified a new RGS protein (RgsA) in A. nidulans that negatively controls GanB signaling and is therefore involved in attenuating biological processes stimulated by GanB. We thereby postulated that RgsA also regulates the cAMP/PKA pathway in response to carbon source via downregulation of GanB signaling and investigated the ability of the rgsAΔ and rgsAΔ ganBΔ mutants to stimulate trehalose breakdown during early germination. Deletion of rgsA triggered accelerated trehalose degradation in response to glucose. In addition, this phenotype was suppressed by ganBΔ (Figure 1C), indicating that uncontrolled activation of GanB caused by rgsAΔ leads to overstimulation of the cAMP/PKA pathway in response to glucose.
To further understand functional interactions between the different modules of GanB signaling, we generated the double mutant rgsAΔ sfaDΔ and evaluated its ability to regulate the glucose-induced cAMP/PKA signaling pathway. Our results demonstrated that deletion of sfaD resulted in suppression of the effects caused by the rgsAΔ mutation (data not shown). The fact that the rgsAΔ sfaDΔ and sfaDΔ mutants exhibit similar patterns with respect to trehalose breakdown suggests that overstimulation of the cAMP/PKA pathway by GanB requires the Gβ-subunit, SfaD.
The heterotrimeric G-protein GanB(α)-SfaD(β)-GpgA(γ) is required for efficient conidial germination in A. nidulans:
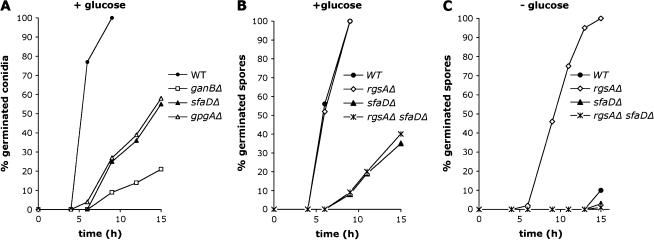
Two recent studies have revealed that (i) GanB positively regulates conidial germination via carbon source sensing (Chang et al. 2004) and (ii) RgsA is a negative regulator of germ tube formation through downregulation of GanB signaling (Han et al. 2004b). We further investigated the role of the Gβ- (SfaD) and Gγ- (GpgA) subunits in the regulation of conidial germination. We analyzed the kinetics of germ tube emergence in the sfaD and gpgA null mutants in comparison to that in wild-type and ganB null mutant strains and observed significant defects in germination rates in both sfaDΔ and gpgAΔ strains similar to (but less severe than) those observed in a ganBΔ strain (Figure 3A). These data clearly showed that the G-protein subunits Gα GanB, Gβ SfaD, and Gγ GpgA activate conidial germination. To define whether GanB(α) and SfaD(β) regulate germ tube emergence as components of a G-protein complex, we checked germination rates of the rgsAΔ sfaDΔ mutant conidia. Figure 3, B and C, shows that the rgsAΔ sfaDΔ mutant exhibited defects in conidial germination similar to those observed in the single sfaD mutant. Therefore, the sfaDΔ mutation suppressed germination in the absence of any external carbon source associated with the upregulation of GanB caused by the rgsAΔ mutation (Figure 3C). These data indicated that activation of GanB-dependent germination requires the Gβ subunit, as previously demonstrated for activation of the GanB-dependent cAMP/PKA pathway, suggesting that formation of the heterotrimeric αβγ is a prerequisite for activation of GanB signaling in response to glucose.
Figure 3.
The G-proteins GanB (α), SfaD (β), and GpgA (γ) constitute a genetically related complex required for efficient conidial germination. Kinetics of germ tube outgrowth in A. nidulans strains (A) CEA209 (WT), CEA245 (ganBΔ), CEA308 (sfaDΔ), and rJAG19.9 (gpgAΔ) and (B and C) CEA 209 (WT), CEA293 (rgsAΔ), CEA309 (sfaDΔ), and CEA316 (rgsAΔ sfaDΔ) inoculated in liquid minimal medium at 37° in the presence (A and B) or absence (C) of glucose (1%) are shown. The number of conidia showing a germ tube or a protrusion was recorded at different times in at least two microscopic fields and is presented as a percentage of the total number of conidia (100) in these fields. Results are representative of two independent experiments.
Deletion of cyaA suppresses germination phenotypes associated with upregulation of GanB signaling:
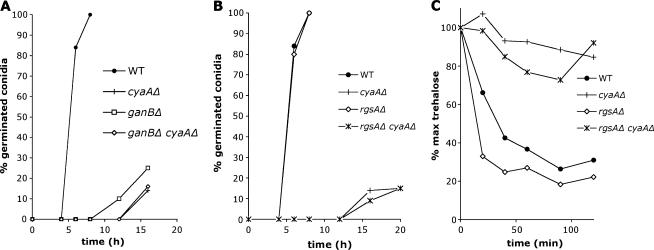
Our studies revealed that the G-protein GanB(α)-SfaD(β)-GpgA(γ) is required for both activation of the glucose-dependent cAMP/PKA pathway during the early phase of germination and efficient conidial germination. These findings, in conjunction with the requirement of the cAMP/PKA pathway for efficient germ tube formation (Fillinger et al. 2002), strongly suggest that the G-protein GanB(α)-SfaD(β)-GpgA(γ) activates conidial germination through the cAMP/PKA pathway in response to glucose. To investigate this hypothesis, we carried out genetic epistatic analyses between cyaA, rgsA, and ganB and found that cyaAΔ is epistasic to ganBΔ and rgsAΔ. These mutants did not display any morphological abnormalities during conidial germination. The only defects observed were delayed/precocious emergence of germ tubes and decreased/accelerated germination rate. Viability of the cyaAΔ and ganBΔ mutant conidia might be partially impaired as after 20 hr of incubation, some conidia remained ungerminated (Chang et al. 2004 and data not shown). The cyaAΔ ganBΔ mutant (Figure 4A and data not shown) as well as the cyaAΔ rgsAΔ mutant (Figure 4, B and C) displayed defects in conidial germination and in trehalose breakdown similar to those observed in the cyaAΔ mutant. Deletion of cyaA suppressed hypergermination phenotypes associated with uncontrolled activation of GanB caused by rgsAΔ, i.e., accelerated trehalose breakdown (Figure 4C) and ability to produce a germ tube in the absence of external carbon source (data not shown). Moreover, the cyaAΔ mutant exhibited delayed germination more severe than that exhibited by the ganBΔ mutant (Figure 4A). Possible interpretations for this result are described in the discussion.
Figure 4.
Deletion of cyaA suppresses hypergermination phenotypes caused by deletion of rgsA. Kinetics of (A and B) germ tube outgrowth and (C) trehalose breakdown in germinating conidia of A. nidulans strains (A–C) CEA178 (WT) and CEA179 (cyaAΔ), (A) CEA278 (ganBΔ) and CEA310 (ganBΔ cyaAΔ), and (B and C) CEA312 (rgsAΔ) and CEA314 (rgsAΔ cyaAΔ) inoculated in liquid minimal medium supplemented with glucose (1%) at 37° are shown. The number of conidia showing a germ tube or a protrusion was recorded at different times in at least two microscopic fields and is presented as a percentage of the total number of conidia (100) in these fields. Results of trehalose breakdown and germ tube outgrowth are representative of three independent experiments.
GanB signaling is involved in carbon source sensing:
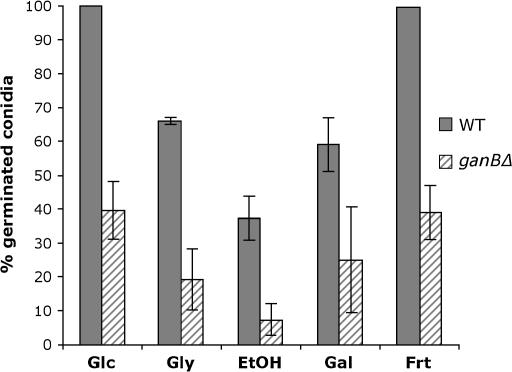
In A. nidulans, activation of the cAMP/PKA pathway at the onset of germination can be induced by various carbon sources such as fructose, ethanol, or acetate with specific kinetics of trehalose breakdown for each compound (Fillinger et al. 2002). Results presented above provide evidence that GanB signaling is required for induction of cAMP-dependent germination in response to glucose. To test whether GanB also mediates sensing of other carbon sources, germination of the ganBΔ conidia was monitored in the presence of various carbon sources. Results presented in Figure 5 show that germination was impaired in the ganB null mutant independent of carbon sources, suggesting that GanB signaling might mediate activation of the cAMP/PKA pathway in response to various carbon sources.
Figure 5.
GanB is required for conidial germination in response to various carbon sources. Conidia of A. nidulans strains CEA209 (WT) and CEA245 (ganBΔ) were germinated at 37° in liquid minimal medium with the indicated carbon sources (1%) and the number of conidia with a germ tube or a protrusion was recorded at 14 hr after addition of the carbon source. Results are representative of three independent experiments. Error bars indicate standard deviations of three independent experiments.
DISCUSSION
In A. nidulans, the cAMP/PKA pathway is activated during the early period of germination as demonstrated by rapid mobilization of trehalose occurring upon induction of germination by addition of a carbon source (d'Enfert and Fontaine 1997; Fillinger et al. 2002). In this study, we have demonstrated that adenylate cyclase is activated very early at the onset of germination: our findings reveal a rapid and transient increase in intracellular cAMP levels within a few minutes following addition of glucose to dormant conidia. This rapid and transient activation of cAMP signaling was described for the first time in S. cerevisiae upon glucose addition to glucose-deprived (derepressed) cells and referred to as “glucose-induced cAMP signaling” (Thevelein et al. 1987). A G-protein-coupled receptor-G-protein system (Gpr1p-Gpa2p) acts upstream of the cAMP signaling pathway and mediates glucose sensing in S. cerevisiae (Colombo et al. 1998; Xue et al. 1998; Kraakman et al. 1999). Our findings provide a series of evidence that in A. nidulans the Gα-subunit closely related to Gpa2p GanB mediates activation of the cAMP/PKA pathway in response to glucose. These observations suggest that this nutrient-sensing pathway has been conserved through evolution to regulate processes linked to growth resumption such as diauxic growth and spore germination. To date, no functional homolog of the nutrient sensor Gpr1p has been identified in A. nidulans or in any filamentous fungus. However, the recent identification of nine putative seven-transmembrane-spanning GPCRs in the genome of A. nidulans will open a new direction for the study of signal transduction mediated by G-proteins in filamentous fungi (Han et al. 2004a).
Our data provide evidence that SfaD (Gβ) and GpgA (Gγ) are upstream positive regulators of the cAMP/PKA pathway in response to glucose. These are also consistent with a model in which the Gα-subunit GanB constitutes the primary signaling element of the cascade while the SfaD(β)-GpgA(γ) dimer acts to reassociate with and relocalize GanB to its hypothetical cognate receptor and allow reactivation by carbon source sensing (Figure 6). Activation of G-proteins is classically based on dissociation of the heterotrimeric complex (αβγ) into two functional units, the βγ-dimer and the GTP-bound Gα-subunit. In filamentous fungi, the Gα-subunit acts usually as the primary signaling element (Lengeler et al. 2000) whereas the Gβγ-complex from yeasts frequently plays an active role in signaling cascades. For example, in the budding yeast, the Gβγ-dimer initiates the pheromone response pathway whereas the Gα-subunit, Gpa1, plays a negative role by repressing Gβγ (Whiteway et al. 1989). In the basidiomycetous yeast Cryptococcus neoformans, the Gbp1Gβ-subunit regulates mating via a MAP kinase cascade in parallel with the Gpa1Gα-subunit, which signals via a cAMP cascade (Wang et al. 2000). Another unusual feature specific to the budding and fission yeasts is the existence of a monomeric Gα-protein that functions without a genuine βγ-dimer (Lengeler et al. 2000). In S. pombe, the Gα-protein Gpa1 plays an active role in the pheromone-activated MAPK signaling pathway (Obara et al. 1991; Xu et al. 1994); Git5, the only Gβ-subunit present in S. pombe, is not coupled to Gpa1 (Landry et al. 2000). In S. cerevisiae, the glucose/cAMP signaling pathway is regulated by the monomeric Gα-protein Gpa2p and atypical proteins, namely Gpb1/2p and Gpg1p, that act as structural mimics of a βγ-dimer to prevent activation of adenylate cyclase by Gpa2p (Harashima and Heitman 2002). This unusual regulation, additionally with the absence of any homolog of Gpb1/2p or Gpg1p proteins in the genome of A. nidulans, might explain the inability of GanB to functionally complement the gpa2 null mutant of S. cerevisiae (our unpublished data). Although the modules of the G-protein/cAMP/PKA pathway are highly conserved, their contribution to signaling pathways appears to have diverged during evolution between filamentous fungi and yeasts: filamentous fungi share a “classical” mode of action similar to that observed in mammalian cells, whereas yeasts may have adopted novel strategies for signal transduction.
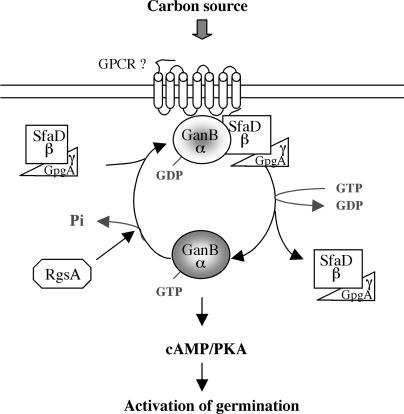
Figure 6.
Proposed model depicting the molecular mechanisms regulating the early events of germination by carbon source sensing in A. nidulans. Sensing of an external carbon source triggers activation of the heterotrimeric G-protein GanB(Gα)-SfaD(Gβ)-GpgA(γ), which in turn initiates the early events of conidial germination through activation the cAMP/PKA pathway. The Gα-subunit (GanB) appears to be the primary signaling element responsible for activation of the cAMP/PKA pathway. The Gβγ-dimer SfaD-GpgA is necessary for proper activation of GanB by carbon source sensing. RgsA is involved in downregulation of cAMP-PKA-dependent germination through inhibition of the GanB activity.
A recent study has reported the characterization of a novel RGS protein, called RgsA, that specifically downregulates the Gα-subunit GanB (Han et al. 2004b). RgsA is similar to ScRgs2 of S. cerevisiae, which is responsible for downregulation of the GanB-related Gα-subunit, Gpa2p (Versele et al. 1999). As described for deletion of RGS2, our results reveal that inactivation of rgsA leads to stimulation of the cAMP/PKA signaling pathway, evidenced by accelerated trehalose breakdown kinetics. Moreover, the double mutant rgsAΔ ganBΔ displays defects in trehalose breakdown similar to those displayed by the ganBΔ mutant. These results indicate that RgsA modulates the cAMP/PKA pathway through downregulation of GanB signaling at the onset of germination (Figure 6). Whereas RgsA appears to be differentially expressed during the developmental cycle of A. nidulans (Han et al. 2004b), the mechanisms underlying its regulation remain to be uncovered. However, an elevated level of transcriptional expression is reported for rgsA in ascospores of A. nidulans (Han et al. 2004b). This, in combination with the requirement of GanB for efficient ascospore and conidial germination (Chang et al. 2004), suggests that RgsA plays a crucial role in preventing germination under inappropriate environmental conditions via downregulation of GanB signaling in dormant spores. Furthermore, our epistatic analysis between the sfaD and rgsA genes demonstrates that deletion of sfaD suppresses germination defects associated with the upregulation of GanB, resulting from inactivation of rgsA. These data are in good agreement with our model (Figure 6) in which one of the functions of the βγ-dimer within the heterotrimeric G-protein GanB-SfaD-GpgA is to reassociate with and to redirect GanB to its cognate GPCR for reactivation by glucose.
A very recent report has revealed the requirement of the Gα-subunit GanB for efficient spore germination in response to glucose sensing (Chang et al. 2004). In our study, we provide evidence that GanB regulates conidial germination within the heterotrimeric G-protein GanB(α)-SfaD(β)-GpgA(γ) through activation of the cAMP/PKA pathway in response to glucose (Figure 6). Our observations revealed that the cyaAΔ mutant shows a germination defect more severe than that of the ganBΔ mutant. To explain these observations, one attractive hypothesis could be the existence of additional upstream regulators of the cAMP/PKA pathway. Two possible candidates are the Gα-subunits, FadA and GanA: although the fadAΔ and ganAΔ mutants showed no defect in activation of the cAMP/PKA pathway in response to glucose, functional redundancy between them cannot be excluded. In N. crassa, whereas inactivation of the Gα-subunit Gna-2 does not yield detectable phenotype, simultaneous inactivation of gna-2 and gna-1 resulted in synthetic defects, suggesting overlapping functions (Baasiri et al. 1997). A complementary hypothesis involves the maintenance of a basal level of cellular cAMP to ensure efficient germination. Previous studies in several plant pathogens revealed interconnections between cAMP signaling and MAPK pathways. In both Magnoporthe grisea and Ustilago maydis, cAMP appears to regulate positively the MAPK pathway involved in appressorium formation and pheromone response, respectively (Xu and Hamer 1996; Lee et al. 2003). In Sclerotinia sclerotiorum, cAMP inhibits the MAPK pathway responsible for sclerotial development (Chen and Dickman 2005). A previous study revealed that RasA from A. nidulans regulates conidial germination via an undefined signaling pathway in parallel to the cAMP/PKA pathway (Fillinger et al. 2002). Since signaling pathways often operate in interconnecting networks, it can be hypothesized that in A. nidulans, cAMP might act positively on Ras signaling, maybe through a MAPK pathway. Indeed, germination defects of the cyaAΔ mutant could be due to inactivation of both cAMP/PKA and Ras signaling pathways.
In A. nidulans, activation of the cAMP/PKA pathway at the onset of germination can be induced by various carbon sources such as fructose, ethanol, or acetate with specific kinetics of trehalose breakdown for each energy source (Fillinger et al. 2002). Our data suggest that, regardless of the carbon source, sensing and subsequent activation of the cAMP/PKA pathway is mediated by GanB signaling. These results are in contrast with those of S. cerevisiae in which Gpa2p is associated with a GPCR specifically sensitized by glucose and sucrose, Gpr1p (Kraakman et al. 1999; Lemaire et al. 2004). Our studies suggest that either the receptor associated with GanB is able to perceive many carbon sources or GanB can interact with various GPCRs. This last hypothesis is in good agreement with phylogenetic studies of nine putative GPCRs in the A. nidulans genome, where three of them appeared to share the highest similarity with the glucose sensor of S. cerevisiae, Gpr1p, suggesting potential functional redundancy of GPCRs (Han et al. 2004a). Interestingly, deletion of gprD triggers a delay in germ tube formation—∼3 hr—indicating that GprD is required for proper germination.
Acknowledgments
We are extremely grateful to Kwang-Yeop Jahng and Mi-Hee Chang for providing ganB and ganA mutant strains prior to publication. We thank Marie-Kim Chaveroche for precious technical help. This work was supported by the Institut Pasteur, the Institut National de la Recherche Agronomique (to C.d'E.), and the National Science Foundation (grant MCB-0421863 to J.-H.Y.). A.L. received a Ph.D. grant from the Ministère de la Recherche.
References
- Baasiri, R. A., X. Lu, P. S. Rowley, G. E. Turner and K. A. Borkovich, 1997. Overlapping functions for two G-protein α-subunits in Neurospora crassa. Genetics 147: 137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, M. H., K. S. Chae, D. M. Han and K. Y. Jahng, 2004. The GanB Gα-protein negatively regulates asexual sporulation and plays a positive role in conidial germination in Aspergillus nidulans. Genetics 167: 1305–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, C., and M. B. Dickman, 2005. cAMP blocks MAPK activation and sclerotial development via Rap-1 in a PKA-independent manner in Sclerotinia sclerotiorum. Mol. Microbiol. 55: 299–311. [DOI] [PubMed] [Google Scholar]
- Colombo, S., P. Ma, L. Cauwenberg, J. Winderickx, M. Crauwels et al., 1998. Involvement of distinct G-proteins, Gpa2 and Ras, in glucose- and intracellular acidification-induced cAMP signalling in the yeast Saccharomyces cerevisiae. EMBO J. 17: 3326–3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Enfert, C., 1997. Fungal spore germination: insights from the molecular genetics of Aspergillus nidulans and Neurospora crassa. Fungal Genet. Biol. 21: 163–172. [Google Scholar]
- d'Enfert, C., and T. Fontaine, 1997. Molecular characterization of the Aspergillus nidulans treA gene encoding an acid trehalase required for growth on trehalose. Mol. Microbiol. 24: 203–216. [DOI] [PubMed] [Google Scholar]
- d'Enfert, C., B. M. Bonini, P. D. Zapella, T. Fontaine, A. M. da Silva et al., 1999. Neutral trehalases catalyse intracellular trehalose breakdown in the filamentous fungi Aspergillus nidulans and Neurospora crassa. Mol. Microbiol. 32: 471–483. [DOI] [PubMed] [Google Scholar]
- Fillinger, S., M. K. Chaveroche, K. Shimizu, N. Keller and C. d'Enfert, 2002. cAMP and ras signalling independently control spore germination in the filamentous fungus Aspergillus nidulans. Mol. Microbiol. 44: 1001–1016. [DOI] [PubMed] [Google Scholar]
- Han, K. H., J. A. Seo and J. H. Yu, 2004. a A putative G protein-coupled receptor negatively controls sexual development in Aspergillus nidulans. Mol. Microbiol. 51: 1333–1345. [DOI] [PubMed] [Google Scholar]
- Han, K. H., J. A. Seo and J. H. Yu, 2004. b Regulators of G-protein signalling in Aspergillus nidulans: RgsA downregulates stress response and stimulates asexual sporulation through attenuation of GanB (Galpha) signalling. Mol. Microbiol. 53: 529–540. [DOI] [PubMed] [Google Scholar]
- Harashima, T., and J. Heitman, 2002. The Galpha protein Gpa2 controls yeast differentiation by interacting with kelch repeat proteins that mimic Gbeta subunits. Mol. Cell 10: 163–173. [DOI] [PubMed] [Google Scholar]
- Harris, S. D., J. L. Morrell and J. E. Hamer, 1994. Identification and characterization of Aspergillus nidulans mutants defective in cytokinesis. Genetics 136: 517–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanaka, M., and C. Shimoda, 2001. The cyclic AMP/PKA signal pathway is required for initiation of spore germination in Schizosaccharomyces pombe. Yeast 18: 207–217. [DOI] [PubMed] [Google Scholar]
- Herman, P. K., and J. Rine, 1997. Yeast spore germination: a requirement for Ras protein activity during re-entry into the cell cycle. EMBO J. 16: 6171–6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraakman, L., K. Lemaire, P. Ma, A. W. Teunissen, M. C. Donaton et al., 1999. A Saccharomyces cerevisiae G-protein coupled receptor, Gpr1, is specifically required for glucose activation of the cAMP pathway during the transition to growth on glucose. Mol. Microbiol. 32: 1002–1012. [DOI] [PubMed] [Google Scholar]
- Landry, S., and C. S. Hoffman, 2001. The git5 Gβ and git11 Gγ form an atypical Gβγ-dimer acting in the fission yeast glucose/cAMP pathway. Genetics 157: 1159–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry, S., M. T. Pettit, E. Apolinario and C. S. Hoffman, 2000. The fission yeast git5 gene encodes a Gβ-subunit required for glucose-triggered adenylate cyclase activation. Genetics 154: 1463–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, N., C. A. D'Souza and J. W. Kronstad, 2003. Of smuts, blasts, mildews, and blights: cAMP signaling in phytopathogenic fungi. Annu. Rev. Phytopathol. 41: 399–427. [DOI] [PubMed] [Google Scholar]
- Lemaire, K., S. Van de Velde, P. Van Dijck and J. M. Thevelein, 2004. Glucose and sucrose act as agonist and mannose as antagonist ligands of the G protein-coupled receptor Gpr1 in the yeast Saccharomyces cerevisiae. Mol. Cell 16: 293–299. [DOI] [PubMed] [Google Scholar]
- Lengeler, K. B., R. C. Davidson, C. D'Souza, T. Harashima, W. C. Shen et al., 2000. Signal transduction cascades regulating fungal development and virulence. Microbiol. Mol. Biol. Rev. 64: 746–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz, M. C., and J. Heitman, 1997. Yeast pseudohyphal growth is regulated by GPA2, a G protein alpha homolog. EMBO J. 16: 7008–7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obara, T., M. Nakafuku, M. Yamamoto and Y. Kaziro, 1991. Isolation and characterization of a gene encoding a G-protein alpha subunit from Schizosaccharomyces pombe: involvement in mating and sporulation pathways. Proc. Natl. Acad. Sci. USA 88: 5877–5881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha, C. R. C., K. Schröppel, D. Harcus, A. Marcil, D. Dignard et al., 2001. Signaling through adenylyl cyclase is essential for hyphal growth and virulence in the pathogenic fungus Candida albicans. Mol. Biol. Cell 12: 3631–3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen, S., J. H. Yu and T. H. Adams, 1999. The Aspergillus nidulans sfaD gene encodes a G protein beta subunit that is required for normal growth and repression of sporulation. EMBO J. 18: 5592–5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarese, J. J., 1974. Germination studies on pure yeast ascospores. Can. J. Microbiol. 20: 1517–1522. [DOI] [PubMed] [Google Scholar]
- Seo, J.-A., K.-H. Han and J.-H. Yu, 2005. Multiple roles of a heterotrimeric G-protein γ-subunit in governing growth and development of Aspergillus nidulans. Genetics 171: 81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu, K., and N. P. Keller, 2001. Genetic involvement of a cAMP-dependent protein kinase in a G-protein signaling pathway regulating morphological and chemical transitions in Aspergillus nidulans. Genetics 157: 591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Som, T., and V. S. Kolaparthi, 1994. Developmental decisions in Aspergillus nidulans are modulated by Ras activity. Mol. Cell. Biol. 14: 5333–5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevelein, J. M., 1984. Regulation of trehalose mobilization in fungi. Microbiol. Rev. 48: 42–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevelein, J. M., M. Beullens, F. Honshoven, G. Hoebeeck, K. Detremerie et al., 1987. Regulation of the cAMP level in the yeast Saccharomyces cerevisiae: the glucose-induced cAMP signal is not mediated by a transient drop in the intracellular pH. J. Gen. Microbiol. 133 (8): 2197–2205. [DOI] [PubMed] [Google Scholar]
- Tingle, M. A., M. T. Kuenzi and H. O. Halvorson, 1974. Germination of yeast spores lacking mitochondrial deoxyribonucleic acid. J. Bacteriol. 117: 89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versele, M., J. H. de Winde and J. M. Thevelein, 1999. A novel regulator of G protein signalling in yeast, Rgs2, downregulates glucose-activation of the cAMP pathway through direct inhibition of Gpa2. EMBO J. 18: 5577–5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, P., J. R. Perfect and J. Heitman, 2000. The G-protein beta subunit GPB1 is required for mating and haploid fruiting in Cryptococcus neoformans. Mol. Cell. Biol. 20: 352–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welton, R. M., and C. S. Hoffman, 2000. Glucose monitoring in fission yeast via the gpa2 Gα, the git5 Gβ and the git3 putative glucose receptor. Genetics 156: 513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland, J., 2001. Comparison of morphogenetic networks of filamentous fungi and yeast. Fungal Genet. Biol. 34: 63–82. [DOI] [PubMed] [Google Scholar]
- Whiteway, M., L. Hougan, D. Dignard, D. Y. Thomas, L. Bell et al., 1989. The STE4 and STE18 genes of yeast encode potential beta and gamma subunits of the mating factor receptor-coupled G protein. Cell 56: 467–477. [DOI] [PubMed] [Google Scholar]
- Xu, H. P., M. White, S. Marcus and M. Wigler, 1994. Concerted action of RAS and G proteins in the sexual response pathways of Schizosaccharomyces pombe. Mol. Cell. Biol. 14: 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, J. R., and J. E. Hamer, 1996. MAP kinase and cAMP signaling regulate infection structure formation and pathogenic growth in the rice blast fungus Magnaporthe grisea. Genes Dev. 10: 2696–2706. [DOI] [PubMed] [Google Scholar]
- Xue, Y., M. Batlle and J. P. Hirsch, 1998. GPR1 encodes a putative G protein-coupled receptor that associates with the Gpa2p Galpha subunit and functions in a Ras-independent pathway. EMBO J. 17: 1996–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, J. H., J. Wieser and T. H. Adams, 1996. The Aspergillus FlbA RGS domain protein antagonizes G protein signaling to block proliferation and allow development. EMBO J. 15: 5184–5190. [PMC free article] [PubMed] [Google Scholar]
- Zuber, S., M. J. Hynes and A. Andrianopoulos, 2002. G-protein signaling mediates asexual development at 25° C but has no effect on yeast-like growth at 37° C in the dimorphic fungus Penicillium mameffei. Eukaryot. Cell 1: 440–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber, S., M. J. Hynes and A. Andrianopoulos, 2003. The G-protein α-subunit GasC plays a major role in germination in the dimorphic fungus Penicillium marneffei. Genetics 164: 487–499. [DOI] [PMC free article] [PubMed] [Google Scholar]