Abstract
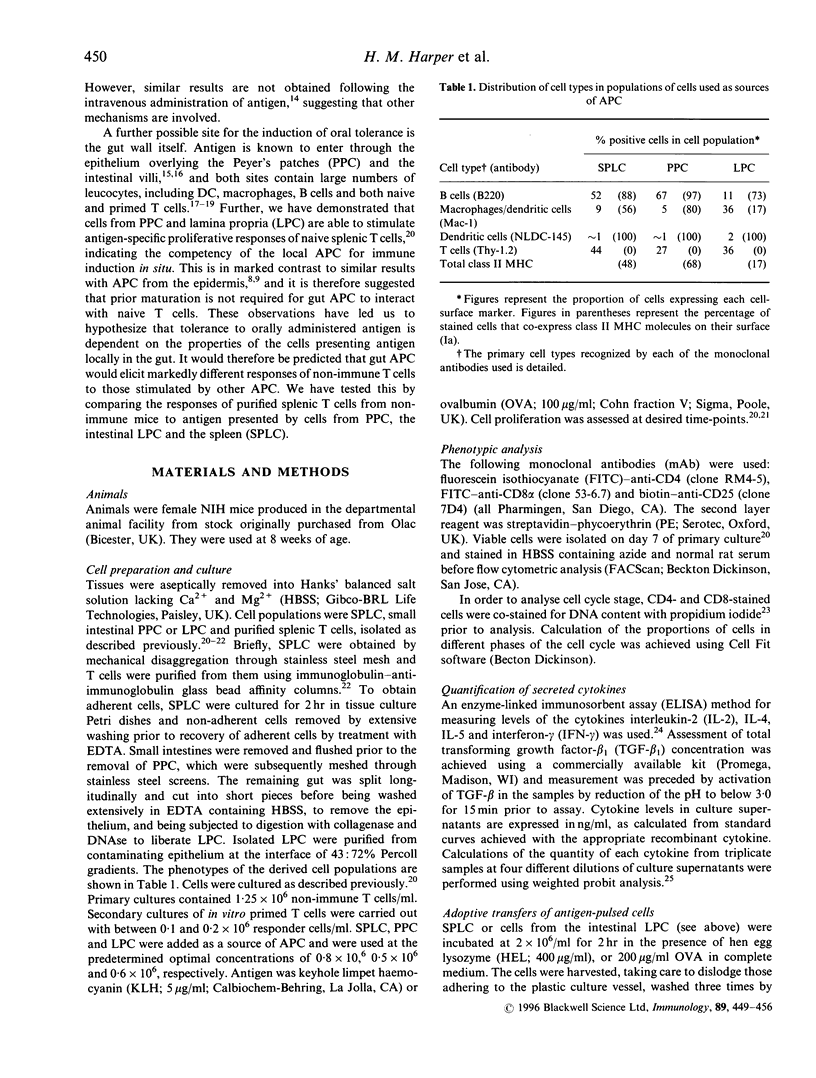
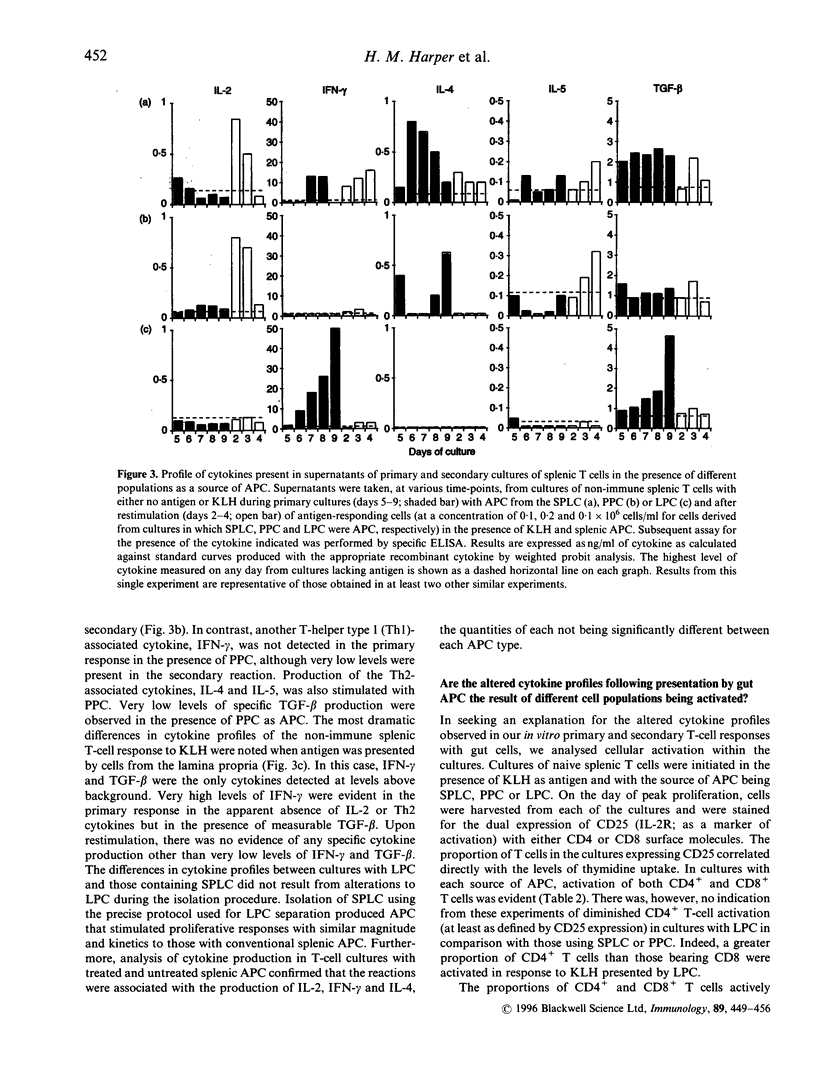
The oral administration of soluble protein antigen results in profound immunological tolerance. However, the tissue location and function of antigen-presenting cells (APC) that stimulate this response remain unclear. We have hypothesized that the properties of cells presenting antigen to naive T cells within the gut are involved, and therefore gut APC should stimulate T-cell responses with different characteristics to those induced by other APC. To test this, we studied in vitro primary T-cell responses following presentation of soluble protein antigen by cells from the Peyer's patches (PPC) and lamina propria (LPC) of the murine small intestine and the spleen (SPLC). Each APC population stimulated antigen-specific proliferative responses with similar anamnestic characteristics; however, analysis of the cytokines produced revealed marked differences. Whereas SPLC stimulated the balanced production of T-helper type 1 (Th1) and Th2 cytokines, PPC induced a profile consistent with the provision of T-cell help for IgA production. Interestingly, presentation of antigen by LPC stimulated high levels of interferon-gamma (IFN-gamma) and transforming growth factor-beta (TGF-beta) in the absence of other cytokines [interleukin-2 (IL-2), IL-4, IL-5]. Evidence from analysis of cell activation and division within the cultures suggested that this profile may result from the preferential activation of CD8+ T cells by LPC; however, the lack of conventional CD4+ T-cell cytokines indicated a defect in the normal function of these cells. Adoptive transfer of antigen-pulsed LPC to syngeneic animals abrogated the induction of delayed-type hypersensitivity (DTH) responsiveness, which followed a subsequent conventional antigen challenge further suggesting a role for lamina propria APC in tolerance induction.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey M., Williams N. A., Wilson A. D., Stokes C. R. PROBIT: weighted probit regression analysis for estimation of biological activity. J Immunol Methods. 1992 Aug 30;153(1-2):261–262. doi: 10.1016/0022-1759(92)90329-r. [DOI] [PubMed] [Google Scholar]
- Bland P. W., Whiting C. V. Differential control of major histocompatibility complex class II I-Ek alpha protein expression in the epithelium and in subsets of lamina propria antigen-presenting cells of the gut. Immunology. 1993 May;79(1):107–111. [PMC free article] [PubMed] [Google Scholar]
- Bruce M. G., Ferguson A. Oral tolerance to ovalbumin in mice: studies of chemically modified and 'biologically filtered' antigen. Immunology. 1986 Apr;57(4):627–630. [PMC free article] [PubMed] [Google Scholar]
- Bucy R. P., Panoskaltsis-Mortari A., Huang G. Q., Li J., Karr L., Ross M., Russell J. H., Murphy K. M., Weaver C. T. Heterogeneity of single cell cytokine gene expression in clonal T cell populations. J Exp Med. 1994 Oct 1;180(4):1251–1262. doi: 10.1084/jem.180.4.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Inobe J., Marks R., Gonnella P., Kuchroo V. K., Weiner H. L. Peripheral deletion of antigen-reactive T cells in oral tolerance. Nature. 1995 Jul 13;376(6536):177–180. doi: 10.1038/376177a0. [DOI] [PubMed] [Google Scholar]
- Firestein G. S., Roeder W. D., Laxer J. A., Townsend K. S., Weaver C. T., Hom J. T., Linton J., Torbett B. E., Glasebrook A. L. A new murine CD4+ T cell subset with an unrestricted cytokine profile. J Immunol. 1989 Jul 15;143(2):518–525. [PubMed] [Google Scholar]
- Friedman A., Weiner H. L. Induction of anergy or active suppression following oral tolerance is determined by antigen dosage. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6688–6692. doi: 10.1073/pnas.91.14.6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant E. P., Rock K. L. MHC class I-restricted presentation of exogenous antigen by thymic antigen-presenting cells in vitro and in vivo. J Immunol. 1992 Jan 1;148(1):13–18. [PubMed] [Google Scholar]
- Halstensen T. S., Farstad I. N., Scott H., Fausa O., Brandtzaeg P. Intraepithelial TcR alpha/beta+ lymphocytes express CD45RO more often than the TcR gamma/delta+ counterparts in coeliac disease. Immunology. 1990 Dec;71(4):460–466. [PMC free article] [PubMed] [Google Scholar]
- Halstensen T. S., Scott H., Brandtzaeg P. Human CD8+ intraepithelial T lymphocytes are mainly CD45RA-RB+ and show increased co-expression of CD45R0 in celiac disease. Eur J Immunol. 1990 Aug;20(8):1825–1830. doi: 10.1002/eji.1830200829. [DOI] [PubMed] [Google Scholar]
- Inaba K., Schuler G., Witmer M. D., Valinksy J., Atassi B., Steinman R. M. Immunologic properties of purified epidermal Langerhans cells. Distinct requirements for stimulation of unprimed and sensitized T lymphocytes. J Exp Med. 1986 Aug 1;164(2):605–613. doi: 10.1084/jem.164.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins M. K. The role of cell division in the induction of clonal anergy. Immunol Today. 1992 Feb;13(2):69–73. doi: 10.1016/0167-5699(92)90137-V. [DOI] [PubMed] [Google Scholar]
- Liu L. M., MacPherson G. G. Antigen acquisition by dendritic cells: intestinal dendritic cells acquire antigen administered orally and can prime naive T cells in vivo. J Exp Med. 1993 May 1;177(5):1299–1307. doi: 10.1084/jem.177.5.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L. M., MacPherson G. G. Lymph-borne (veiled) dendritic cells can acquire and present intestinally administered antigens. Immunology. 1991 Jul;73(3):281–286. [PMC free article] [PubMed] [Google Scholar]
- Mackay C. R., Marston W. L., Dudler L. Naive and memory T cells show distinct pathways of lymphocyte recirculation. J Exp Med. 1990 Mar 1;171(3):801–817. doi: 10.1084/jem.171.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melamed D., Friedman A. Direct evidence for anergy in T lymphocytes tolerized by oral administration of ovalbumin. Eur J Immunol. 1993 Apr;23(4):935–942. doi: 10.1002/eji.1830230426. [DOI] [PubMed] [Google Scholar]
- Melamed D., Friedman A. In vivo tolerization of Th1 lymphocytes following a single feeding with ovalbumin: anergy in the absence of suppression. Eur J Immunol. 1994 Sep;24(9):1974–1981. doi: 10.1002/eji.1830240906. [DOI] [PubMed] [Google Scholar]
- Miller A., Lider O., Roberts A. B., Sporn M. B., Weiner H. L. Suppressor T cells generated by oral tolerization to myelin basic protein suppress both in vitro and in vivo immune responses by the release of transforming growth factor beta after antigen-specific triggering. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):421–425. doi: 10.1073/pnas.89.1.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A., Lider O., Weiner H. L. Antigen-driven bystander suppression after oral administration of antigens. J Exp Med. 1991 Oct 1;174(4):791–798. doi: 10.1084/jem.174.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Schumacher J. H., Street N. F., Budd R., O'Garra A., Fong T. A., Bond M. W., Moore K. W., Sher A., Fiorentino D. F. Diversity of cytokine synthesis and function of mouse CD4+ T cells. Immunol Rev. 1991 Oct;123:209–229. doi: 10.1111/j.1600-065x.1991.tb00612.x. [DOI] [PubMed] [Google Scholar]
- Murray P. D., Swain S. L., Kagnoff M. P. Regulation of the IgM and IgA anti-dextran B1355S response: synergy between IFN-gamma, BCGF II, and IL 2. J Immunol. 1985 Dec;135(6):4015–4020. [PubMed] [Google Scholar]
- Norbury C. C., Hewlett L. J., Prescott A. R., Shastri N., Watts C. Class I MHC presentation of exogenous soluble antigen via macropinocytosis in bone marrow macrophages. Immunity. 1995 Dec;3(6):783–791. doi: 10.1016/1074-7613(95)90067-5. [DOI] [PubMed] [Google Scholar]
- O'Connor P. M., Jackman J., Jondle D., Bhatia K., Magrath I., Kohn K. W. Role of the p53 tumor suppressor gene in cell cycle arrest and radiosensitivity of Burkitt's lymphoma cell lines. Cancer Res. 1993 Oct 15;53(20):4776–4780. [PubMed] [Google Scholar]
- Ramshaw I., Ruby J., Ramsay A., Ada G., Karupiah G. Expression of cytokines by recombinant vaccinia viruses: a model for studying cytokines in virus infections in vivo. Immunol Rev. 1992 Jun;127:157–182. doi: 10.1111/j.1600-065x.1992.tb01413.x. [DOI] [PubMed] [Google Scholar]
- Strobel S., Mowat A. M., Drummond H. E., Pickering M. G., Ferguson A. Immunological responses to fed protein antigens in mice. II. Oral tolerance for CMI is due to activation of cyclophosphamide-sensitive cells by gut-processed antigen. Immunology. 1983 Jul;49(3):451–456. [PMC free article] [PubMed] [Google Scholar]
- Warshaw A. L., Walker W. A., Cornell R., Isselbacher K. J. Small intestinal permeability to macromolecules. Transmission of horseradish peroxidase into mesenteric lymph and portal blood. Lab Invest. 1971 Dec;25(6):675–684. [PubMed] [Google Scholar]
- Whitacre C. C., Gienapp I. E., Orosz C. G., Bitar D. M. Oral tolerance in experimental autoimmune encephalomyelitis. III. Evidence for clonal anergy. J Immunol. 1991 Oct 1;147(7):2155–2163. [PubMed] [Google Scholar]
- Williams N. A., Hill T. J., Hooper D. C. Murine epidermal antigen-presenting cells in primary and secondary T-cell proliferative responses to a soluble protein antigen in vitro. Immunology. 1990 Nov;71(3):411–416. [PMC free article] [PubMed] [Google Scholar]
- Williams N. A., Hill T. J., Hooper D. C. Murine epidermal antigen-presenting cells in primary and secondary T-cell proliferative responses to herpes simplex virus in vitro. Immunology. 1991 Jan;72(1):34–39. [PMC free article] [PubMed] [Google Scholar]
- Williams N. A., Wilson A. D., Bailey M., Bland P. W., Stokes C. R. Primary antigen-specific T-cell proliferative responses following presentation of soluble protein antigen by cells from the murine small intestine. Immunology. 1992 Apr;75(4):608–613. [PMC free article] [PubMed] [Google Scholar]
- Wilson A. D., Clarke C. J., Stokes C. R. Whole cholera toxin and B subunit act synergistically as an adjuvant for the mucosal immune response of mice to keyhole limpet haemocyanin. Scand J Immunol. 1990 Apr;31(4):443–451. doi: 10.1111/j.1365-3083.1990.tb02791.x. [DOI] [PubMed] [Google Scholar]