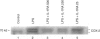Abstract
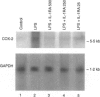
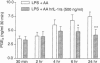
Cyclooxygenase (COX) is the key rate-limiting enzyme in the synthesis of prostanoids from arachidonic acid. Two isoforms of COX have been described in mammalian cells, referred to as cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2). COX-1 is a constitutively expressed enzyme; COX-2 is an inducible enzyme that appears to be expressed in inflamed tissue and following exposure to growth factors or cytokines, such as interleukin-1 (IL-1). The aim of the present study was to test if the antagonism on the binding of IL-1 to its cell-surface receptor by human recombinant IL-1 receptor antagonist (hrIL-1ra) may control the COX mRNA expression and prostaglandin E2 (PGE2) production by human monocyte cultures. Northern blot studies showed that hrIL-ra (500 ng/ml) had a strong inhibitory effect on inducible COX activity. The effect was evident after 6 hr incubation (2.7-fold decrease of mRNA COX-2 transcripts); and about a threefold decrease at 24hr incubation. A non-significant effect was observed with COX-1 transcripts. Induced PGE2 production by monocyte cultures treated with lipopolysaccharide (LPS) or interleukin-1 beta (IL-1 beta) was strongly inhibited in the presence of hrIL-1ra (500 ng/ml). In addition, a significant inhibition of COX-2 protein expression, as evaluated by Western blotting, was also observed. These data suggest that hrIL-1ra may be the key mediator in the down-regulation of the COX-2 inducible pathway.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arend W. P. Interleukin 1 receptor antagonist. A new member of the interleukin 1 family. J Clin Invest. 1991 Nov;88(5):1445–1451. doi: 10.1172/JCI115453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arend W. P., Joslin F. G., Massoni R. J. Effects of immune complexes on production by human monocytes of interleukin 1 or an interleukin 1 inhibitor. J Immunol. 1985 Jun;134(6):3868–3875. [PubMed] [Google Scholar]
- Arend W. P., Smith M. F., Jr, Janson R. W., Joslin F. G. IL-1 receptor antagonist and IL-1 beta production in human monocytes are regulated differently. J Immunol. 1991 Sep 1;147(5):1530–1536. [PubMed] [Google Scholar]
- Arend W. P., Welgus H. G., Thompson R. C., Eisenberg S. P. Biological properties of recombinant human monocyte-derived interleukin 1 receptor antagonist. J Clin Invest. 1990 May;85(5):1694–1697. doi: 10.1172/JCI114622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balavoine J. F., de Rochemonteix B., Williamson K., Seckinger P., Cruchaud A., Dayer J. M. Prostaglandin E2 and collagenase production by fibroblasts and synovial cells is regulated by urine-derived human interleukin 1 and inhibitor(s). J Clin Invest. 1986 Oct;78(4):1120–1124. doi: 10.1172/JCI112669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breviario F., Proserpio P., Bertocchi F., Lampugnani M. G., Mantovani A., Dejana E. Interleukin-1 stimulates prostacyclin production by cultured human endothelial cells by increasing arachidonic acid mobilization and conversion. Arteriosclerosis. 1990 Jan-Feb;10(1):129–134. doi: 10.1161/01.atv.10.1.129. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Chang J., Gilman S. C., Lewis A. J. Interleukin 1 activates phospholipase A2 in rabbit chondrocytes: a possible signal for IL 1 action. J Immunol. 1986 Feb 15;136(4):1283–1287. [PubMed] [Google Scholar]
- Conti P., Panara M. R., Barbacane R. C., Placido F. C., Bongrazio M., Reale M., Dempsey R. A., Fiore S. Blocking the interleukin-1 receptor inhibits leukotriene B4 and prostaglandin E2 generation in human monocyte cultures. Cell Immunol. 1992 Nov;145(1):199–209. doi: 10.1016/0008-8749(92)90323-h. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and interleukin-1 antagonism. Blood. 1991 Apr 15;77(8):1627–1652. [PubMed] [Google Scholar]
- Dinarello C. A. Modalities for reducing interleukin 1 activity in disease. Trends Pharmacol Sci. 1993 May;14(5):155–159. doi: 10.1016/0165-6147(93)90200-4. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Thompson R. C. Blocking IL-1: interleukin 1 receptor antagonist in vivo and in vitro. Immunol Today. 1991 Nov;12(11):404–410. doi: 10.1016/0167-5699(91)90142-G. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Wolff S. M. The role of interleukin-1 in disease. N Engl J Med. 1993 Jan 14;328(2):106–113. doi: 10.1056/NEJM199301143280207. [DOI] [PubMed] [Google Scholar]
- Fu J. Y., Masferrer J. L., Seibert K., Raz A., Needleman P. The induction and suppression of prostaglandin H2 synthase (cyclooxygenase) in human monocytes. J Biol Chem. 1990 Oct 5;265(28):16737–16740. [PubMed] [Google Scholar]
- Godfrey R. W., Johnson W. J., Hoffstein S. T. Recombinant tumor necrosis factor and interleukin-1 both stimulate human synovial cell arachidonic acid release and phospholipid metabolism. Biochem Biophys Res Commun. 1987 Jan 15;142(1):235–241. doi: 10.1016/0006-291x(87)90476-1. [DOI] [PubMed] [Google Scholar]
- Habenicht A. J., Goerig M., Grulich J., Rothe D., Gronwald R., Loth U., Schettler G., Kommerell B., Ross R. Human platelet-derived growth factor stimulates prostaglandin synthesis by activation and by rapid de novo synthesis of cyclooxygenase. J Clin Invest. 1985 Apr;75(4):1381–1387. doi: 10.1172/JCI111839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempel S. L., Monick M. M., Hunninghake G. W. Lipopolysaccharide induces prostaglandin H synthase-2 protein and mRNA in human alveolar macrophages and blood monocytes. J Clin Invest. 1994 Jan;93(1):391–396. doi: 10.1172/JCI116971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hla T., Neilson K. Human cyclooxygenase-2 cDNA. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7384–7388. doi: 10.1073/pnas.89.16.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujubu D. A., Fletcher B. S., Varnum B. C., Lim R. W., Herschman H. R. TIS10, a phorbol ester tumor promoter-inducible mRNA from Swiss 3T3 cells, encodes a novel prostaglandin synthase/cyclooxygenase homologue. J Biol Chem. 1991 Jul 15;266(20):12866–12872. [PubMed] [Google Scholar]
- Libby P., Warner S. J., Friedman G. B. Interleukin 1: a mitogen for human vascular smooth muscle cells that induces the release of growth-inhibitory prostanoids. J Clin Invest. 1988 Feb;81(2):487–498. doi: 10.1172/JCI113346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier J. A., Hla T., Maciag T. Cyclooxygenase is an immediate-early gene induced by interleukin-1 in human endothelial cells. J Biol Chem. 1990 Jul 5;265(19):10805–10808. [PubMed] [Google Scholar]
- Masferrer J. L., Seibert K., Zweifel B., Needleman P. Endogenous glucocorticoids regulate an inducible cyclooxygenase enzyme. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3917–3921. doi: 10.1073/pnas.89.9.3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masferrer J. L., Zweifel B. S., Seibert K., Needleman P. Selective regulation of cellular cyclooxygenase by dexamethasone and endotoxin in mice. J Clin Invest. 1990 Oct;86(4):1375–1379. doi: 10.1172/JCI114850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade E. A., Smith W. L., DeWitt D. L. Differential inhibition of prostaglandin endoperoxide synthase (cyclooxygenase) isozymes by aspirin and other non-steroidal anti-inflammatory drugs. J Biol Chem. 1993 Mar 25;268(9):6610–6614. [PubMed] [Google Scholar]
- Mincione G., Cirafici A. M., Lazzareschi D., Pepe S., Ciardiello F., Colletta G. Loss of thyrotropin regulation and transforming growth factor beta-induced growth arrest in erbB-2 overexpressing rat thyroid cells. Cancer Res. 1993 Nov 15;53(22):5548–5553. [PubMed] [Google Scholar]
- Mitchell J. A., Akarasereenont P., Thiemermann C., Flower R. J., Vane J. R. Selectivity of nonsteroidal antiinflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11693–11697. doi: 10.1073/pnas.90.24.11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Banion M. K., Winn V. D., Young D. A. cDNA cloning and functional activity of a glucocorticoid-regulated inflammatory cyclooxygenase. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4888–4892. doi: 10.1073/pnas.89.11.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan M. G., Chilton F. H., Huggins E. M., Jr, McCall C. E. Lipopolysaccharide priming of alveolar macrophages for enhanced synthesis of prostanoids involves induction of a novel prostaglandin H synthase. J Biol Chem. 1992 Jul 25;267(21):14547–14550. [PubMed] [Google Scholar]
- O'Sullivan M. G., Huggins E. M., Jr, Meade E. A., DeWitt D. L., McCall C. E. Lipopolysaccharide induces prostaglandin H synthase-2 in alveolar macrophages. Biochem Biophys Res Commun. 1992 Sep 16;187(2):1123–1127. doi: 10.1016/0006-291x(92)91313-f. [DOI] [PubMed] [Google Scholar]
- Raz A., Wyche A., Siegel N., Needleman P. Regulation of fibroblast cyclooxygenase synthesis by interleukin-1. J Biol Chem. 1988 Feb 25;263(6):3022–3028. [PubMed] [Google Scholar]
- Ristimäki A., Garfinkel S., Wessendorf J., Maciag T., Hla T. Induction of cyclooxygenase-2 by interleukin-1 alpha. Evidence for post-transcriptional regulation. J Biol Chem. 1994 Apr 22;269(16):11769–11775. [PubMed] [Google Scholar]
- Rossi V., Breviario F., Ghezzi P., Dejana E., Mantovani A. Prostacyclin synthesis induced in vascular cells by interleukin-1. Science. 1985 Jul 12;229(4709):174–176. doi: 10.1126/science.2409598. [DOI] [PubMed] [Google Scholar]
- Sano H., Hla T., Maier J. A., Crofford L. J., Case J. P., Maciag T., Wilder R. L. In vivo cyclooxygenase expression in synovial tissues of patients with rheumatoid arthritis and osteoarthritis and rats with adjuvant and streptococcal cell wall arthritis. J Clin Invest. 1992 Jan;89(1):97–108. doi: 10.1172/JCI115591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalkwijk C. G., de Vet E., Pfeilschifter J., van den Bosch H. Interleukin-1 beta and transforming growth factor-beta 2 enhance cytosolic high-molecular-mass phospholipase A2 activity and induce prostaglandin E2 formation in rat mesangial cells. Eur J Biochem. 1992 Nov 15;210(1):169–176. doi: 10.1111/j.1432-1033.1992.tb17405.x. [DOI] [PubMed] [Google Scholar]
- Smith W. L. The eicosanoids and their biochemical mechanisms of action. Biochem J. 1989 Apr 15;259(2):315–324. doi: 10.1042/bj2590315. [DOI] [PMC free article] [PubMed] [Google Scholar]