Abstract
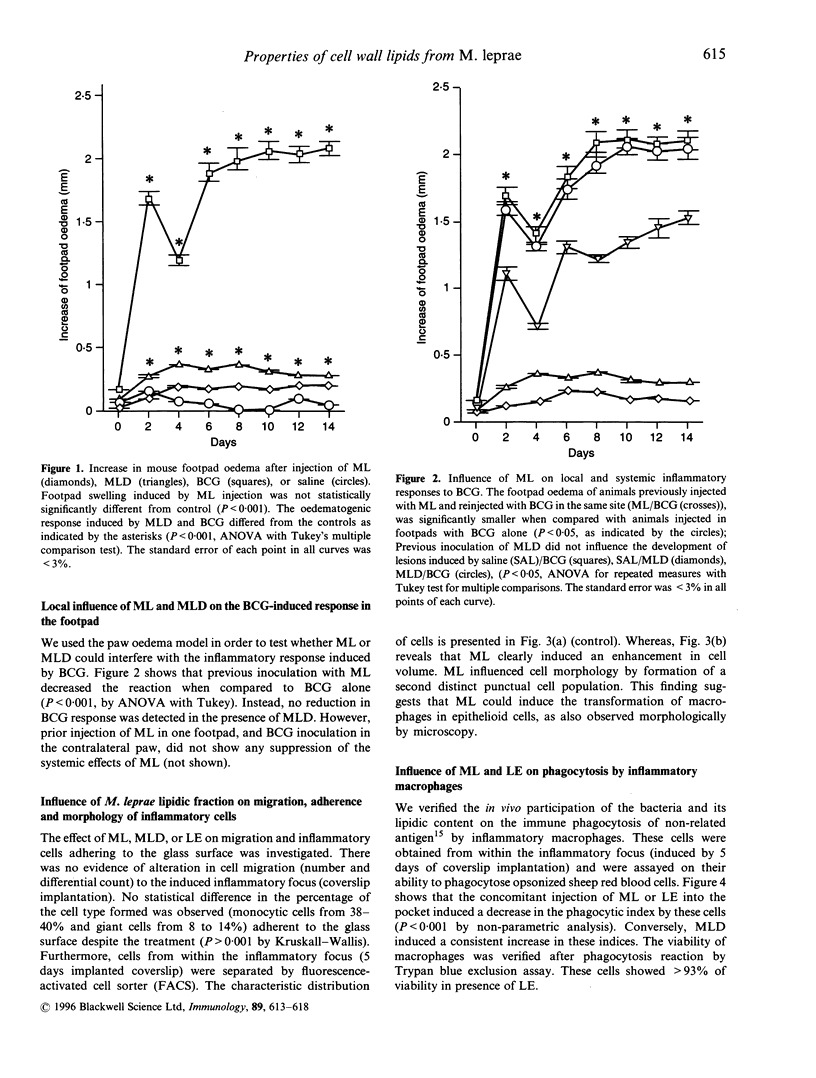
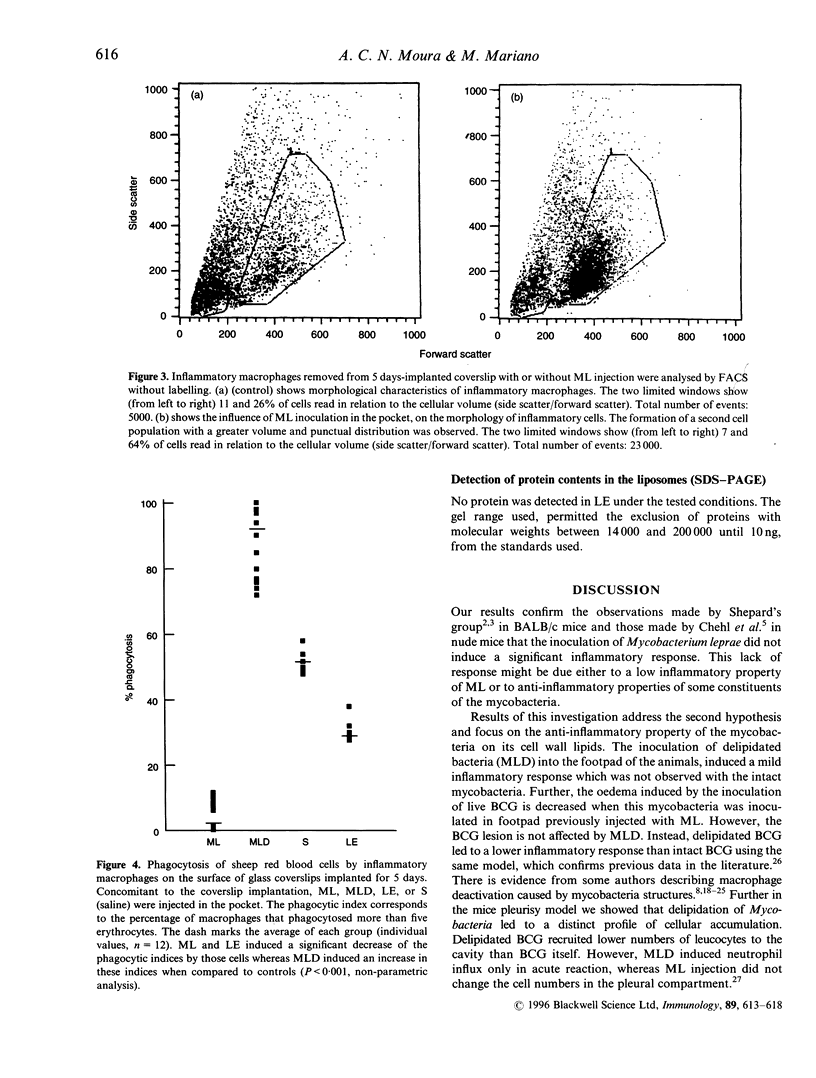
In general, the majority of bacteria are pre-inflammatory when injected in experimental animals. However, Mycobacterium leprae has no inflammatory effect when injected into mouse footpad, but using the delipidated mycobacteria we observed a mild significant increase in footpad oedema. Other mycobacteria, Mycobacterium bovis-BCG or M. tuberculosis induce a strong paw oedema. Furthermore, M. leprae reduced locally the BCG-induced inflammatory reaction in mouse footpad, whereas delipidated M. leprae did not influence this reaction. Both M. leprae and M. leprae cell wall lipids blocked immune phagocytosis in vivo by inflammatory macrophages (from an induced focus). In contrast delipidated M. leprae stimulated the phagocytosis reaction. Neither intact M. leprae. delipidated M. leprae, nor its lipids had any toxic effect on macrophages or on cell migration. Although M. leprae did not interfere on cell influx and cell type in an induced-inflammatory site, this mycobacterium led to the appearance of a distinct cell population in vivo. The hypothesis is that M. leprae would transform macrophages in epithelioid cells, suggested by morphology analysis of cells by fluorescence-activated cell sorter and observed under optic microscopy.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelberg R., Soares R., Ferreira P., Silva M. T. Induction of non-specific immunosuppression in mice by mycobacterial infections and its relationship to macrophage activation. Scand J Immunol. 1989 Aug;30(2):165–174. doi: 10.1111/j.1365-3083.1989.tb01198.x. [DOI] [PubMed] [Google Scholar]
- BLOCH H. Studies on the virulence of tubercle bacilli; isolation and biological properties of a constituent of virulent organisms. J Exp Med. 1950 Feb;91(2):197-218, pl. doi: 10.1084/jem.91.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beiguelman B. Some remarks on the genetics of leprosy resistance. Acta Genet Med Gemellol (Roma) 1968 Oct;17(4):584–594. doi: 10.1017/s1120962300012452. [DOI] [PubMed] [Google Scholar]
- Chehl S., Job C. K., Hastings R. C. Transmission of leprosy in nude mice. Am J Trop Med Hyg. 1985 Nov;34(6):1161–1166. doi: 10.4269/ajtmh.1985.34.1161. [DOI] [PubMed] [Google Scholar]
- Colston M. J., Hilson G. R. Growth of Mycobacterium leprae and M. marinum in congenitally athymic (nude) mice. Nature. 1976 Jul 29;262(5567):399–401. doi: 10.1038/262399a0. [DOI] [PubMed] [Google Scholar]
- Desai S. D., Birdi T. J., Antia N. H. Correlation between macrophage activation and bactericidal function and Mycobacterium leprae antigen presentation in macrophages of leprosy patients and normal individuals. Infect Immun. 1989 Apr;57(4):1311–1317. doi: 10.1128/iai.57.4.1311-1317.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer T. J., Kizlaitis L., Vachula M., Weaver C. W., Andersen B. R. Human phagocytic cell responses to Mycobacterium leprae and Mycobacterium bovis Bacillus Calmette-Guérin. An in vitro comparison of leprosy vaccine components. J Immunol. 1988 Sep 1;141(5):1701–1708. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Launois P., Niang M., Dieye A., Sarthou J. L. TNF-alpha failed to reverse the M. leprae-induced defective chemiluminescence response of human mononuclear cells. FEMS Microbiol Immunol. 1992 Jan;4(2):91–96. doi: 10.1111/j.1574-6968.1992.tb04974.x. [DOI] [PubMed] [Google Scholar]
- Mariano M. Does macrophage deactivating factor play a role in the maintenance and fate of infectious granulomata? Mem Inst Oswaldo Cruz. 1991 Oct-Dec;86(4):485–487. doi: 10.1590/s0074-02761991000400024. [DOI] [PubMed] [Google Scholar]
- Mariano M., Nikitin T., Malucelli B. E. Immunological and non-immunological phagocytosis by inflammatory macrophages, epithelioid cells and macrophage polykaryons from foreign body granulomata. J Pathol. 1976 Nov;120(3):151–159. doi: 10.1002/path.1711200304. [DOI] [PubMed] [Google Scholar]
- Mariano M., Spector W. G. The formation and properties of macrophage polykaryons (inflammatory giant cells). J Pathol. 1974 May;113(1):1–19. doi: 10.1002/path.1711130102. [DOI] [PubMed] [Google Scholar]
- Moura A. C., Mariano M. Dead Mycobacterium leprae inhibits phagocytosis by inflammatory macrophages in vivo. Participation of the bacteria cell lipids in the phenomenon. Mem Inst Oswaldo Cruz. 1990 Jul-Sep;85(3):381–382. doi: 10.1590/s0074-02761990000300020. [DOI] [PubMed] [Google Scholar]
- Narayanan R. B. Immunopathology of leprosy granulomas--current status: a review. Lepr Rev. 1988 Mar;59(1):75–82. [PubMed] [Google Scholar]
- Porichha D., Bhatia V. N. Epithelioid and polygonal cells in histoid leprosy. Indian J Lepr. 1987 Apr-Jun;59(2):191–193. [PubMed] [Google Scholar]
- Rabinovitch M. The dissociation of the attachment and ingestion phases of phagocytosis by macrophages. Exp Cell Res. 1967 Apr;46(1):19–28. doi: 10.1016/0014-4827(67)90405-3. [DOI] [PubMed] [Google Scholar]
- Rastogi N., Cadou S., Hellio R. Differential handling of bacterial antigens in macrophages infected with Mycobacterium leprae as studied by immunogold labeling of ultrathin sections. Int J Lepr Other Mycobact Dis. 1991 Jun;59(2):278–291. [PubMed] [Google Scholar]
- Rook G. A. The immunogenicity of killed mycobacteria. Lepr Rev. 1980 Dec;51(4):295–301. doi: 10.5935/0305-7518.19800030. [DOI] [PubMed] [Google Scholar]
- Salgame P. R., Mahadevan P. R., Antia N. H. Mechanism of immunosuppression in leprosy: presence of suppressor factor(s) from macrophages of lepromatous patients. Infect Immun. 1983 Jun;40(3):1119–1126. doi: 10.1128/iai.40.3.1119-1126.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley L. D., Krahenbuhl J. L. Induction of unresponsiveness to gamma interferon in macrophages infected with Mycobacterium leprae. Infect Immun. 1988 Aug;56(8):1912–1919. doi: 10.1128/iai.56.8.1912-1919.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva C. L., Ekizlerian S. M., Fazioli R. A. Role of cord factor in the modulation of infection caused by mycobacteria. Am J Pathol. 1985 Feb;118(2):238–247. [PMC free article] [PubMed] [Google Scholar]
- Spector W. G. The granulomatous inflammatory exudate. Int Rev Exp Pathol. 1969;8:1–55. [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Verghese S., Curtis J., Turk J. L. Epithelioid cell granuloma induction in the guinea pig by haptenated Mycobacterium leprae. Cell Immunol. 1987 Jul;107(2):307–316. doi: 10.1016/0008-8749(87)90239-5. [DOI] [PubMed] [Google Scholar]



