Abstract
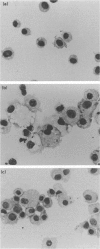
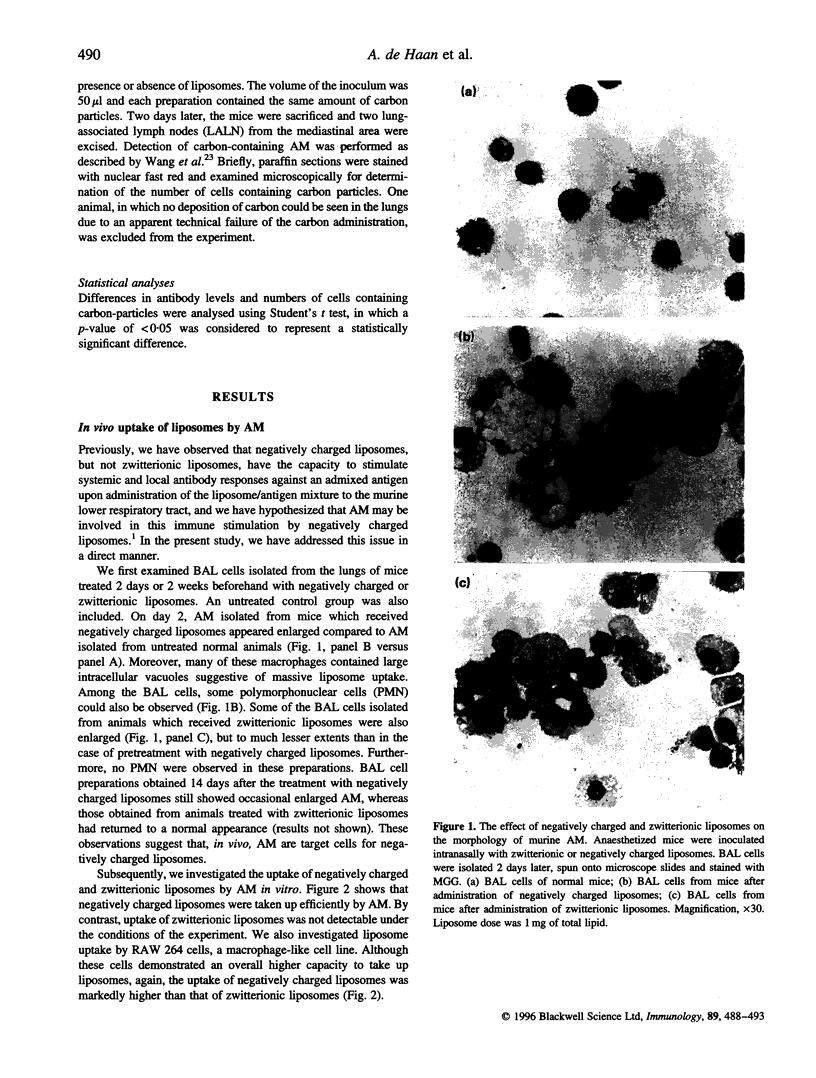
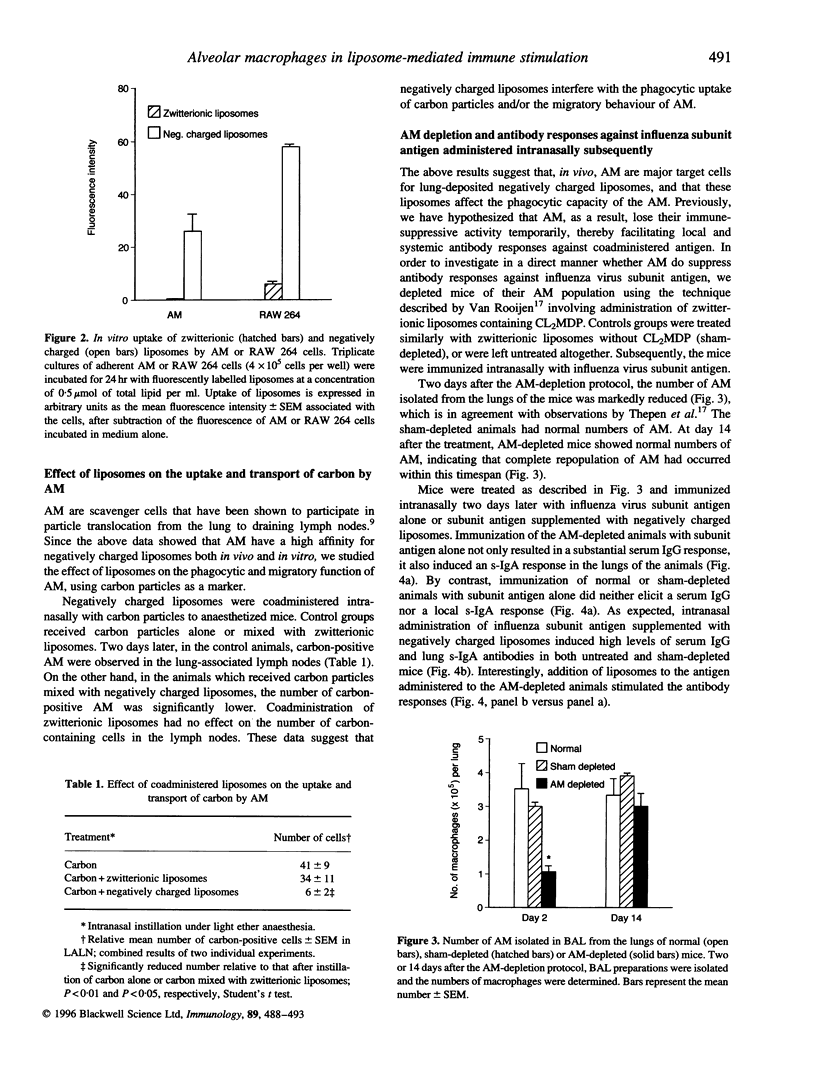
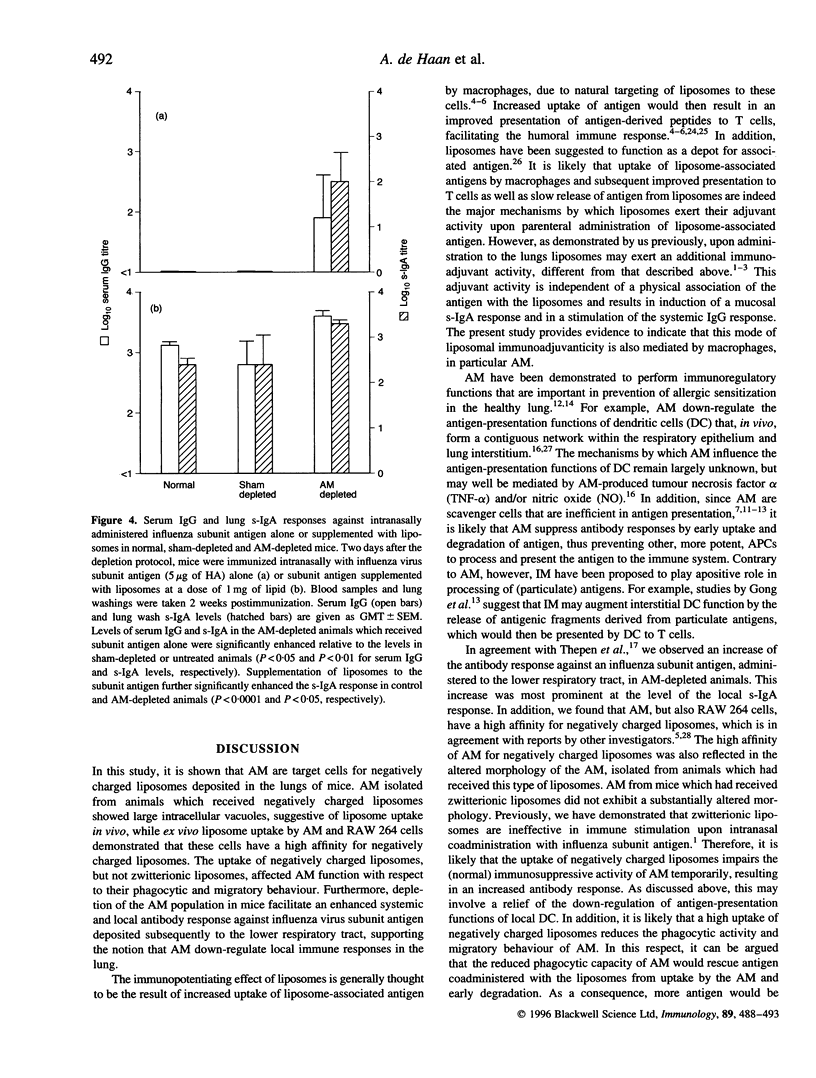
Previously, we have reported on a liposomal adjuvant system for stimulation of both systemic IgG and mucosal s-IgA responses against viral antigens (influenza virus subunit antigen or whole inactivated measles virus) administered intranasally to mice. Immune stimulation is observed with negatively charged, but not with zwitterionic, liposomes and is independent of a physical association of the antigen with the liposomes. Furthermore, liposome-mediated immune stimulation requires deposition of the liposomes and the antigen in the lower respiratory tract. In the present study, it is shown that alveolar macrophages (AM) are the main target cells for negatively charged liposomes administered to the lungs of mice. AM isolated from animals, to which negatively charged liposomes were administered beforehand, showed large intracellular vacuoles, suggestive of massive liposome uptake. Under ex vivo conditions, both AM and RAW 264 cells exhibited a high capacity to take up negatively charged liposomes. The deposition of negatively charged liposomes, but not zwitterionic, liposomes in the lung reduced the phagocytic and migratory behaviour of AM, as assessed on the basis of transport of carbon particles to the draining lymph nodes of the lungs. Depletion of AM in vivo with dichloromethylene diphosphonate, facilitated an enhanced systemic and local antibody response against influenza subunit antigen deposited subsequently to the lower respiratory tract. In conclusion, these data provide support for the hypothesis that uptake of negatively charged liposomes blocks the immunosuppressive activity of AM, thereby facilitating local and systemic antibody responses.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alving C. R. Immunologic aspects of liposomes: presentation and processing of liposomal protein and phospholipid antigens. Biochim Biophys Acta. 1992 Dec 11;1113(3-4):307–322. doi: 10.1016/0304-4157(92)90004-t. [DOI] [PubMed] [Google Scholar]
- Brain J. D. Lung macrophages: how many kinds are there? What do they do? Am Rev Respir Dis. 1988 Mar;137(3):507–509. doi: 10.1164/ajrccm/137.3.507. [DOI] [PubMed] [Google Scholar]
- Dal Monte P. R., Szoka F. C., Jr Antigen presentation by B cells and macrophages of cytochrome c and its antigenic fragment when conjugated to the surface of liposomes. Vaccine. 1989 Oct;7(5):401–408. doi: 10.1016/0264-410x(89)90153-9. [DOI] [PubMed] [Google Scholar]
- Dal Monte P., Szoka F. C., Jr Effect of liposome encapsulation on antigen presentation in vitro. Comparison of presentation by peritoneal macrophages and B cell tumors. J Immunol. 1989 Mar 1;142(5):1437–1443. [PubMed] [Google Scholar]
- Ferro T. J., Kern J. A., Elias J. A., Kamoun M., Daniele R. P., Rossman M. D. Alveolar macrophages, blood monocytes, and density-fractionated alveolar macrophages differ in their ability to promote lymphocyte proliferation to mitogen and antigen. Am Rev Respir Dis. 1987 Mar;135(3):682–687. doi: 10.1164/arrd.1987.135.3.682. [DOI] [PubMed] [Google Scholar]
- Fortin A., Thérien H. M. Mechanism of liposome adjuvanticity: an in vivo approach. Immunobiology. 1993 Jul;188(3):316–322. doi: 10.1016/S0171-2985(11)80239-1. [DOI] [PubMed] [Google Scholar]
- Gong J. L., McCarthy K. M., Rogers R. A., Schneeberger E. E. Interstitial lung macrophages interact with dendritic cells to present antigenic peptides derived from particulate antigens to T cells. Immunology. 1994 Mar;81(3):343–351. [PMC free article] [PubMed] [Google Scholar]
- Harmsen A. G., Muggenburg B. A., Snipes M. B., Bice D. E. The role of macrophages in particle translocation from lungs to lymph nodes. Science. 1985 Dec 13;230(4731):1277–1280. doi: 10.1126/science.4071052. [DOI] [PubMed] [Google Scholar]
- Holt P. G., Degebrodt A., O'Leary C., Krska K., Plozza T. T cell activation by antigen-presenting cells from lung tissue digests: suppression by endogenous macrophages. Clin Exp Immunol. 1985 Dec;62(3):586–593. [PMC free article] [PubMed] [Google Scholar]
- Holt P. G. Down-regulation of immune responses in the lower respiratory tract: the role of alveolar macrophages. Clin Exp Immunol. 1986 Feb;63(2):261–270. [PMC free article] [PubMed] [Google Scholar]
- Holt P. G., McMenamin C. Defence against allergic sensitization in the healthy lung: the role of inhalation tolerance. Clin Exp Allergy. 1989 May;19(3):255–262. doi: 10.1111/j.1365-2222.1989.tb02380.x. [DOI] [PubMed] [Google Scholar]
- Holt P. G., Oliver J., Bilyk N., McMenamin C., McMenamin P. G., Kraal G., Thepen T. Downregulation of the antigen presenting cell function(s) of pulmonary dendritic cells in vivo by resident alveolar macrophages. J Exp Med. 1993 Feb 1;177(2):397–407. doi: 10.1084/jem.177.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G. W. Immunoregulatory functions of human alveolar macrophages. Am Rev Respir Dis. 1987 Aug;136(2):253–254. doi: 10.1164/ajrccm/136.2.253. [DOI] [PubMed] [Google Scholar]
- Strickland D. H., Thepen T., Kees U. R., Kraal G., Holt P. G. Regulation of T-cell function in lung tissue by pulmonary alveolar macrophages. Immunology. 1993 Oct;80(2):266–272. [PMC free article] [PubMed] [Google Scholar]
- Thepen T., Van Rooijen N., Kraal G. Alveolar macrophage elimination in vivo is associated with an increase in pulmonary immune response in mice. J Exp Med. 1989 Aug 1;170(2):499–509. doi: 10.1084/jem.170.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Winter J. B., Dam M., Wildevuur C. R., Prop J. Influence of interrupted pulmonary lymph drainage on antibody responses in hilar-stripped lungs. J Heart Lung Transplant. 1992 Jul-Aug;11(4 Pt 2):S215–S220. [PubMed] [Google Scholar]
- Wood J. M., Schild G. C., Newman R. W., Seagroatt V. An improved single-radial-immunodiffusion technique for the assay of influenza haemagglutinin antigen: application for potency determinations of inactivated whole virus and subunit vaccines. J Biol Stand. 1977;5(3):237–247. doi: 10.1016/s0092-1157(77)80008-5. [DOI] [PubMed] [Google Scholar]
- Yetter R. A., Lehrer S., Ramphal R., Small P. A., Jr Outcome of influenza infection: effect of site of initial infection and heterotypic immunity. Infect Immun. 1980 Aug;29(2):654–662. doi: 10.1128/iai.29.2.654-662.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haan A., Geerligs H. J., Huchshorn J. P., van Scharrenburg G. J., Palache A. M., Wilschut J. Mucosal immunoadjuvant activity of liposomes: induction of systemic IgG and secretory IgA responses in mice by intranasal immunization with an influenza subunit vaccine and coadministered liposomes. Vaccine. 1995 Feb;13(2):155–162. doi: 10.1016/0264-410x(95)93129-w. [DOI] [PubMed] [Google Scholar]
- de Haan A., Renegar K. B., Small P. A., Jr, Wilschut J. Induction of a secretory IgA response in the murine female urogenital tract by immunization of the lungs with liposome-supplemented viral subunit antigen. Vaccine. 1995 May;13(7):613–616. doi: 10.1016/0264-410x(94)00062-r. [DOI] [PubMed] [Google Scholar]
- de Haan A., Tomee J. F., Huchshorn J. P., Wilschut J. Liposomes as an immunoadjuvant system for stimulation of mucosal and systemic antibody responses against inactivated measles virus administered intranasally to mice. Vaccine. 1995 Oct;13(14):1320–1324. doi: 10.1016/0264-410x(95)00037-2. [DOI] [PubMed] [Google Scholar]