Abstract
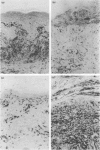
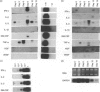
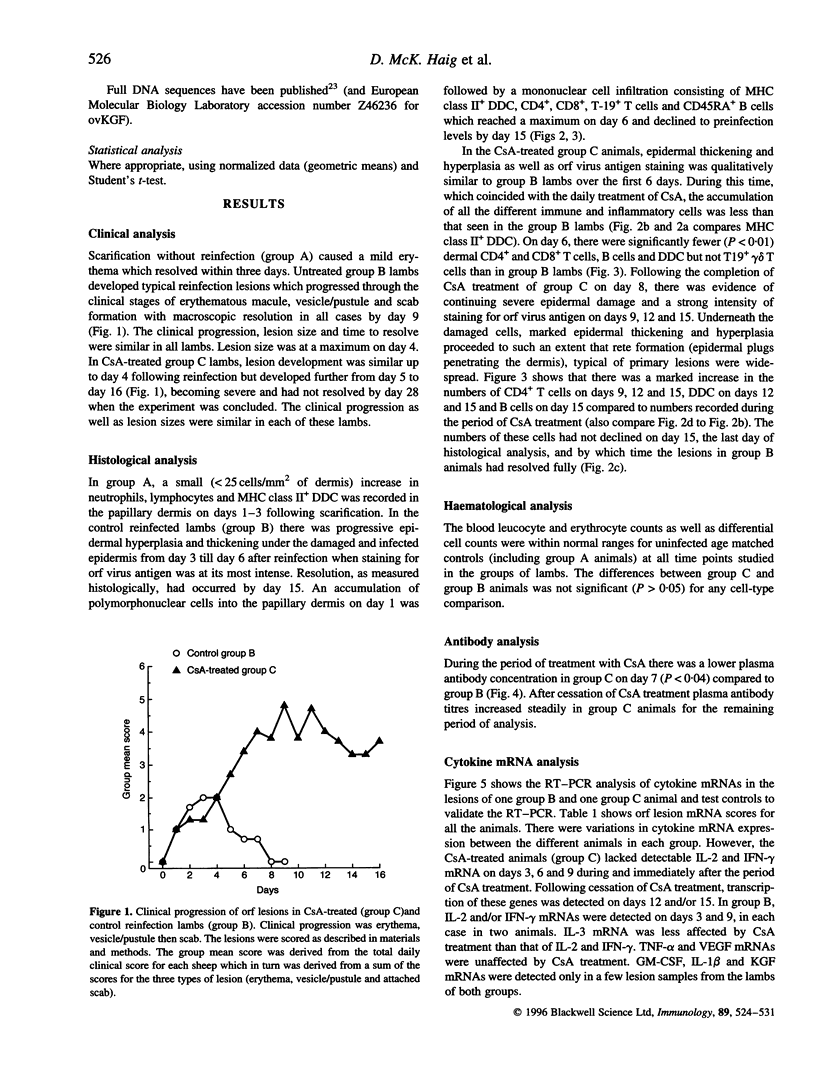
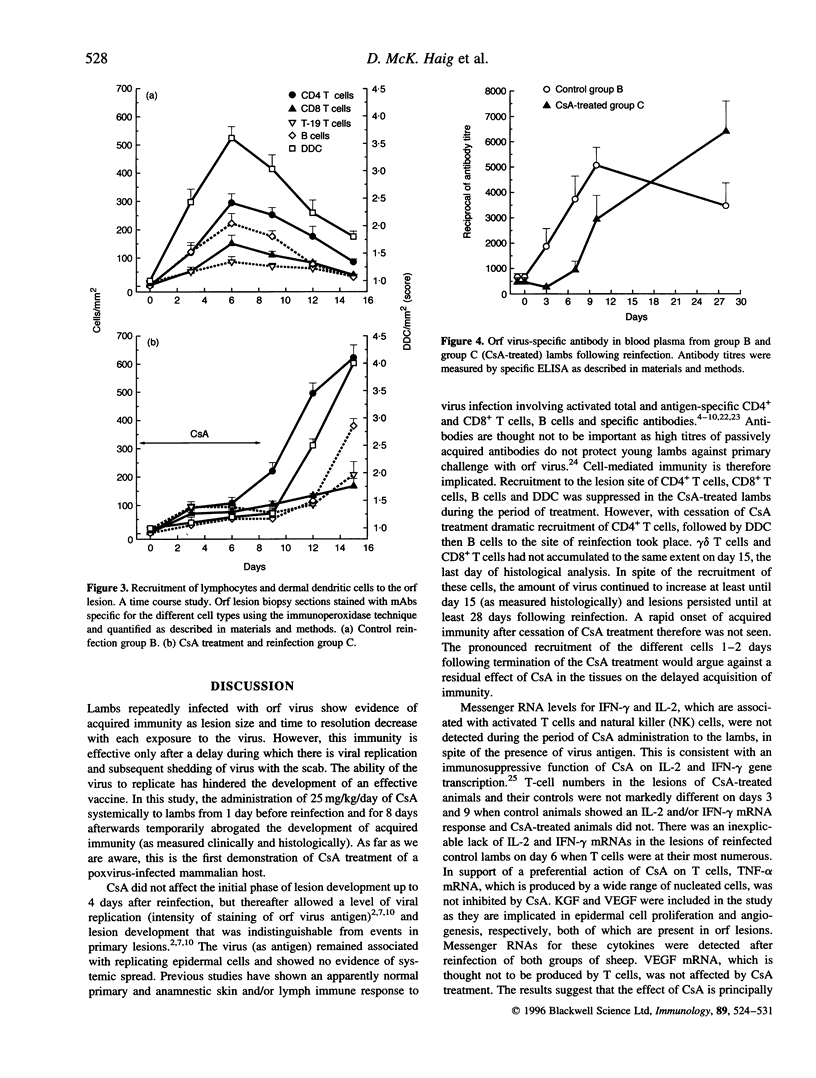
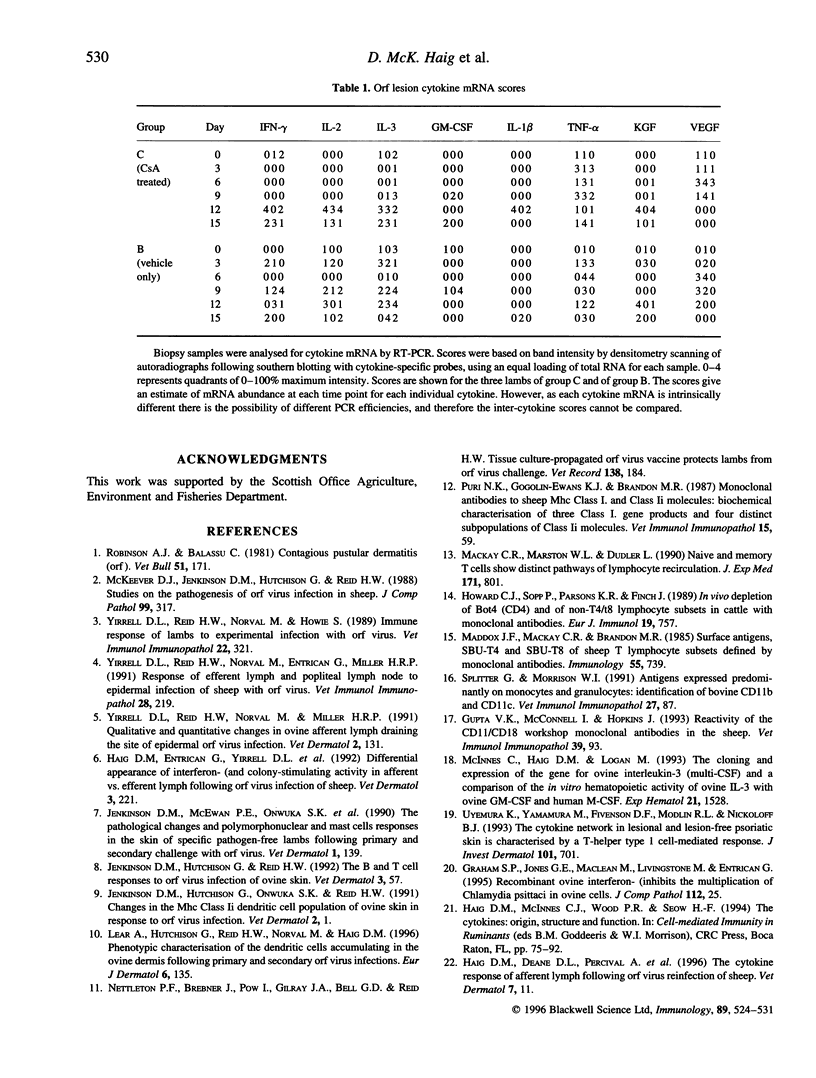
The effect of cyclosporin A (CsA) on host immunity to cutaneous reinfection with the parapoxvirus orf virus was studied in 6-month-old lambs. In control reinfected animals, clinical lesions and viral replication (measured by the presence of vesicular/pustular lesions and viral antigen) in regenerating epidermal cells were at a maximum on day 4 with resolution by day 9. Lesion histology revealed recruitment of T cells, B cells and dermal dendritic cells (DDC) which increased and decreased in parallel with the clinical course of the reinfection. In animals treated with CsA (25 mg/kg/day) 1 day before and for 8 days after reinfection, more severe clinical lesions and viral replication typical of primary infections were recorded and had not resolved by 28 days following reinfection. During CsA treatment, the recruitment of T cells, B cells and DDC was inhibited. With cessation of CsA treatment there was dramatic recruitment of CD4+ T cells followed by DDC then B cells to the lesion site but rapid onset of acquired immunity was not recorded. Reverse transcription-polymerase chain reaction (RT-PCR) analysis of cytokine mRNAs from lesion biopsies showed individual sheep variations. However, interleukin-2 (IL-2) and interferon-gamma (IFN-gamma) mRNAs were detected in the control reinfected animals on days 3 and/or 9 after reinfection but not on these days in animals undergoing treatment with CsA. In the untreated lambs there was an inexplicable lack of IL-2 and IFN-gamma mRNAs on day 6 after reinfection. Tumour necrosis factor-alpha (TNF-alpha) and vascular endothelial growth factor (VEGF) mRNAs were unaffected by CsA treatment. The data suggest that CsA abrogates acquired immunity to orf virus reinfection by targetting T-cell lymphokine production.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcamí A., Smith G. L. Cytokine receptors encoded by poxviruses: a lesson in cytokine biology. Immunol Today. 1995 Oct;16(10):474–478. doi: 10.1016/0167-5699(95)80030-1. [DOI] [PubMed] [Google Scholar]
- Haig D. M., Hutchinson G., Thomson J., Yirrell D., Reid H. W. Cytolytic activity and associated serine protease expression by skin and afferent lymph CD8+ T cells during orf virus reinfection. J Gen Virol. 1996 May;77(Pt 5):953–961. doi: 10.1099/0022-1317-77-5-953. [DOI] [PubMed] [Google Scholar]
- Howard C. J., Sopp P., Parsons K. R., Finch J. In vivo depletion of BoT4 (CD4) and of non-T4/T8 lymphocyte subsets in cattle with monoclonal antibodies. Eur J Immunol. 1989 Apr;19(4):757–764. doi: 10.1002/eji.1830190428. [DOI] [PubMed] [Google Scholar]
- Mackay C. R., Marston W. L., Dudler L. Naive and memory T cells show distinct pathways of lymphocyte recirculation. J Exp Med. 1990 Mar 1;171(3):801–817. doi: 10.1084/jem.171.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox J. F., Mackay C. R., Brandon M. R. Surface antigens, SBU-T4 and SBU-T8, of sheep T lymphocyte subsets defined by monoclonal antibodies. Immunology. 1985 Aug;55(4):739–748. [PMC free article] [PubMed] [Google Scholar]
- McInnes C., Haig D., Logan M. The cloning and expression of the gene for ovine interleukin-3 (multi-CSF) and a comparison of the in vitro hematopoietic activity of ovine IL-3 with ovine GM-CSF and human M-CSF. Exp Hematol. 1993 Nov;21(12):1528–1534. [PubMed] [Google Scholar]
- McKeever D. J., Jenkinson D. M., Hutchison G., Reid H. W. Studies of the pathogenesis of orf virus infection in sheep. J Comp Pathol. 1988 Oct;99(3):317–328. doi: 10.1016/0021-9975(88)90052-7. [DOI] [PubMed] [Google Scholar]
- Nettleton P. F., Brebner J., Pow I., Gilray J. A., Bell G. D., Reid H. W. Tissue culture-propagated orf virus vaccine protects lambs from orf virus challenge. Vet Rec. 1996 Feb 24;138(8):184–186. doi: 10.1136/vr.138.8.184. [DOI] [PubMed] [Google Scholar]
- Uyemura K., Yamamura M., Fivenson D. F., Modlin R. L., Nickoloff B. J. The cytokine network in lesional and lesion-free psoriatic skin is characterized by a T-helper type 1 cell-mediated response. J Invest Dermatol. 1993 Nov;101(5):701–705. doi: 10.1111/1523-1747.ep12371679. [DOI] [PubMed] [Google Scholar]
- Yirrell D. L., Reid H. W., Norval M., Entrican G., Miller H. R. Response of efferent lymph and popliteal lymph node to epidermal infection of sheep with orf virus. Vet Immunol Immunopathol. 1991 Jul;28(3-4):219–235. doi: 10.1016/0165-2427(91)90116-t. [DOI] [PubMed] [Google Scholar]
- Yirrell D. L., Reid H. W., Norval M., Howie S. E. Immune response of lambs to experimental infection with Orf virus. Vet Immunol Immunopathol. 1989 Nov 15;22(4):321–332. doi: 10.1016/0165-2427(89)90168-2. [DOI] [PubMed] [Google Scholar]