Abstract
Peptides binding to a murine, human immunodeficiency virus type 1 (HIV-1) neutralizing monoclonal antibody (F58/H3) were isolated from two random peptide libraries expressed on the surface of phage. The antibody was originally elicited by immunization with HIV-1 envelope protein gp120LAI, and has previously been shown to interact with the -I-GPGRA- motif of the V3 loop. The peptide libraries consisted of nine or 15 random amino acid residues flanked by two cysteines, and fused to the amino terminal end of the cpIII protein on the filamentous phage. Selection of specific peptides was carried out in three rounds, with decreasing antibody concentration. An expected peptide motif -GPGRA-, a similar segment, -GPAR-, and two unrelated motifs -FRLLG- and -WRM/ALG- were selected. Binding of antibody was tested both to synthetic peptides in solution, and the corresponding peptide on phage. The GPXR motifs bound in both formats, while the FRLLG bound antibody only when present on the phage The reactivity of peptides on phage was highly dependent on an intact disulphide bond between the cysteines flanking the peptide. The molecular mimicry of the found motifs was tested by immunizing mice and rabbits with conjugated synthetic peptides or peptide on phage. In mice, peptide-specific antisera were raised, but no reactivity to the whole protein (gp120) was detected. In rabbits, however, this was accomplished with the -GPGRA- containing peptide when present on phage. In addition, this antisera precipitated virus particles, and neutralized HIV-1SF2 virus in vitro.
Full text
PDF



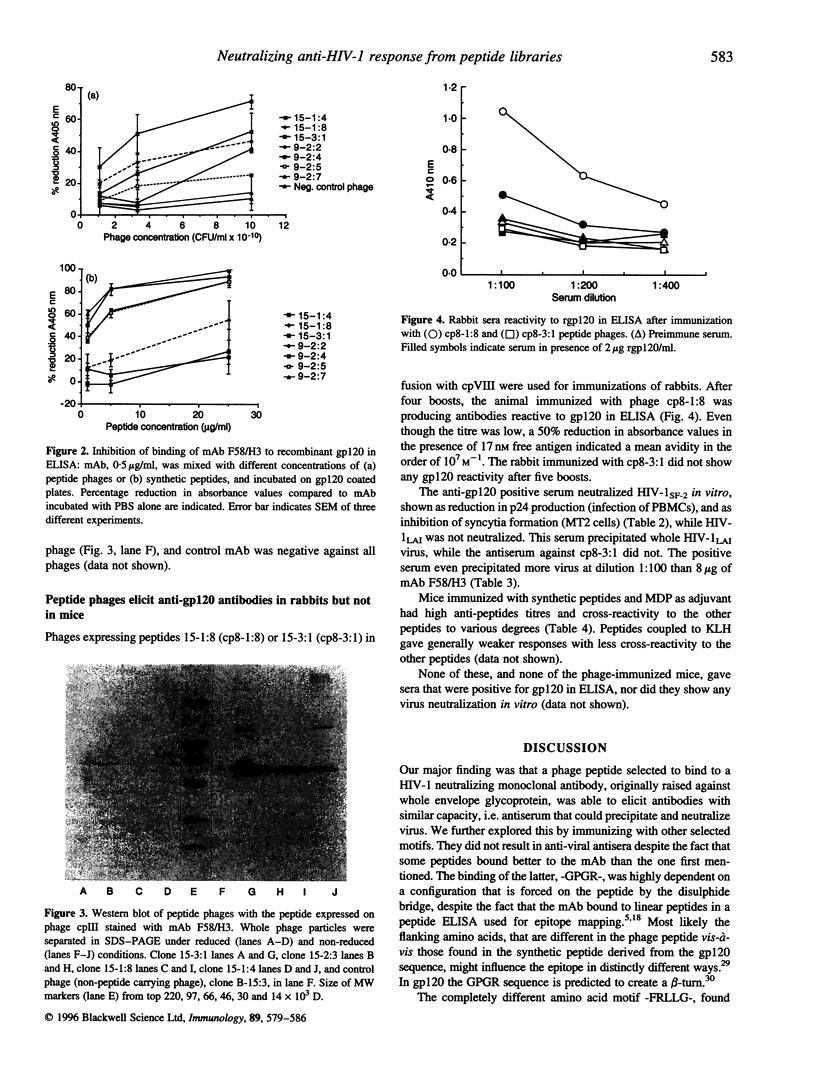


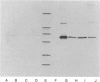
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerblom L., Hinkula J., Broliden P. A., Mäkitalo B., Fridberger T., Rosen J., Villacres-Eriksson M., Morein B., Wahren B. Neutralizing cross-reactive and non-neutralizing monoclonal antibodies to HIV-1 gp120. AIDS. 1990 Oct;4(10):953–960. doi: 10.1097/00002030-199010000-00002. [DOI] [PubMed] [Google Scholar]
- Arnon R. Synthetic peptides as the basis for vaccine design. Mol Immunol. 1991 Mar;28(3):209–215. doi: 10.1016/0161-5890(91)90063-p. [DOI] [PubMed] [Google Scholar]
- Barbas C. F., 3rd, Kang A. S., Lerner R. A., Benkovic S. J. Assembly of combinatorial antibody libraries on phage surfaces: the gene III site. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):7978–7982. doi: 10.1073/pnas.88.18.7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blond-Elguindi S., Cwirla S. E., Dower W. J., Lipshutz R. J., Sprang S. R., Sambrook J. F., Gething M. J. Affinity panning of a library of peptides displayed on bacteriophages reveals the binding specificity of BiP. Cell. 1993 Nov 19;75(4):717–728. doi: 10.1016/0092-8674(93)90492-9. [DOI] [PubMed] [Google Scholar]
- Broliden P. A., Mäkitalo B., Akerblom L., Rosen J., Broliden K., Utter G., Jondal M., Norrby E., Wahren B. Identification of amino acids in the V3 region of gp120 critical for virus neutralization by human HIV-1-specific antibodies. Immunology. 1991 Aug;73(4):371–376. [PMC free article] [PubMed] [Google Scholar]
- Broliden P. A., von Gegerfelt A., Clapham P., Rosen J., Fenyö E. M., Wahren B., Broliden K. Identification of human neutralization-inducing regions of the human immunodeficiency virus type 1 envelope glycoproteins. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):461–465. doi: 10.1073/pnas.89.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cwirla S. E., Peters E. A., Barrett R. W., Dower W. J. Peptides on phage: a vast library of peptides for identifying ligands. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6378–6382. doi: 10.1073/pnas.87.16.6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkula J., Rosen J., Sundqvist V. A., Stigbrand T., Wahren B. Epitope mapping of the HIV-1 gag region with monoclonal antibodies. Mol Immunol. 1990 May;27(5):395–403. doi: 10.1016/0161-5890(90)90163-t. [DOI] [PubMed] [Google Scholar]
- Ho D. D., McKeating J. A., Li X. L., Moudgil T., Daar E. S., Sun N. C., Robinson J. E. Conformational epitope on gp120 important in CD4 binding and human immunodeficiency virus type 1 neutralization identified by a human monoclonal antibody. J Virol. 1991 Jan;65(1):489–493. doi: 10.1128/jvi.65.1.489-493.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang A. S., Barbas C. F., Janda K. D., Benkovic S. J., Lerner R. A. Linkage of recognition and replication functions by assembling combinatorial antibody Fab libraries along phage surfaces. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4363–4366. doi: 10.1073/pnas.88.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller P. M., Arnold B. A., Shaw A. R., Tolman R. L., Van Middlesworth F., Bondy S., Rusiecki V. K., Koenig S., Zolla-Pazner S., Conard P. Identification of HIV vaccine candidate peptides by screening random phage epitope libraries. Virology. 1993 Apr;193(2):709–716. doi: 10.1006/viro.1993.1179. [DOI] [PubMed] [Google Scholar]
- Mathiesen T., Broliden P. A., Rosen J., Wahren B. Mapping of IgG subclass and T-cell epitopes on HIV proteins by synthetic peptides. Immunology. 1989 Aug;67(4):453–459. [PMC free article] [PubMed] [Google Scholar]
- Mathiesen T., Sönnerborg A., Wahren B. Detection of antibodies against myelin basic protein and increased levels of HIV-IgG antibodies and HIV antigen after solubilization of immune complexes in sera and CSF of HIV infected patients. Viral Immunol. 1989;2(1):1–9. doi: 10.1089/vim.1989.2.1. [DOI] [PubMed] [Google Scholar]
- Moore J. P., Cao Y., Conley A. J., Wyatt R., Robinson J., Gorny M. K., Zolla-Pazner S., Ho D. D., Koup R. A. Studies with monoclonal antibodies to the V3 region of HIV-1 gp120 reveal limitations to the utility of solid-phase peptide binding assays. J Acquir Immune Defic Syndr. 1994 Apr;7(4):332–339. [PubMed] [Google Scholar]
- Nara P. L., Hatch W. C., Dunlop N. M., Robey W. G., Arthur L. O., Gonda M. A., Fischinger P. J. Simple, rapid, quantitative, syncytium-forming microassay for the detection of human immunodeficiency virus neutralizing antibody. AIDS Res Hum Retroviruses. 1987 Fall;3(3):283–302. doi: 10.1089/aid.1987.3.283. [DOI] [PubMed] [Google Scholar]
- Palker T. J., Clark M. E., Langlois A. J., Matthews T. J., Weinhold K. J., Randall R. R., Bolognesi D. P., Haynes B. F. Type-specific neutralization of the human immunodeficiency virus with antibodies to env-encoded synthetic peptides. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1932–1936. doi: 10.1073/pnas.85.6.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmley S. F., Smith G. P. Antibody-selectable filamentous fd phage vectors: affinity purification of target genes. Gene. 1988 Dec 20;73(2):305–318. doi: 10.1016/0378-1119(88)90495-7. [DOI] [PubMed] [Google Scholar]
- Persson M. A., Caothien R. H., Burton D. R. Generation of diverse high-affinity human monoclonal antibodies by repertoire cloning. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2432–2436. doi: 10.1073/pnas.88.6.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Profy A. T., Salinas P. A., Eckler L. I., Dunlop N. M., Nara P. L., Putney S. D. Epitopes recognized by the neutralizing antibodies of an HIV-1-infected individual. J Immunol. 1990 Jun 15;144(12):4641–4647. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steimer K. S., Scandella C. J., Skiles P. V., Haigwood N. L. Neutralization of divergent HIV-1 isolates by conformation-dependent human antibodies to Gp120. Science. 1991 Oct 4;254(5028):105–108. doi: 10.1126/science.1718036. [DOI] [PubMed] [Google Scholar]
- Steward M. W., Stanley C. M., Obeid O. E. A mimotope from a solid-phase peptide library induces a measles virus-neutralizing and protective antibody response. J Virol. 1995 Dec;69(12):7668–7673. doi: 10.1128/jvi.69.12.7668-7673.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ståhl S., Hultman T., Olsson A., Moks T., Uhlén M. Solid phase DNA sequencing using the biotin-avidin system. Nucleic Acids Res. 1988 Apr 11;16(7):3025–3038. doi: 10.1093/nar/16.7.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundqvist V. A., Albert J., Ohlsson E., Hinkula J., Fenyö E. M., Wahren B. Human immunodeficiency virus type 1 p24 production and antigenic variation in tissue culture of isolates with various growth characteristics. J Med Virol. 1989 Nov;29(3):170–175. doi: 10.1002/jmv.1890290305. [DOI] [PubMed] [Google Scholar]
- VanCott T. C., Bethke F. R., Polonis V. R., Gorny M. K., Zolla-Pazner S., Redfield R. R., Birx D. L. Dissociation rate of antibody-gp120 binding interactions is predictive of V3-mediated neutralization of HIV-1. J Immunol. 1994 Jul 1;153(1):449–459. [PubMed] [Google Scholar]
- Zacher A. N., 3rd, Stock C. A., Golden J. W., 2nd, Smith G. P. A new filamentous phage cloning vector: fd-tet. Gene. 1980 Apr;9(1-2):127–140. doi: 10.1016/0378-1119(80)90171-7. [DOI] [PubMed] [Google Scholar]
- Zhong G., Smith G. P., Berry J., Brunham R. C. Conformational mimicry of a chlamydial neutralization epitope on filamentous phage. J Biol Chem. 1994 Sep 30;269(39):24183–24188. [PubMed] [Google Scholar]
- de la Cruz V. F., Lal A. A., McCutchan T. F. Immunogenicity and epitope mapping of foreign sequences via genetically engineered filamentous phage. J Biol Chem. 1988 Mar 25;263(9):4318–4322. [PubMed] [Google Scholar]