Abstract
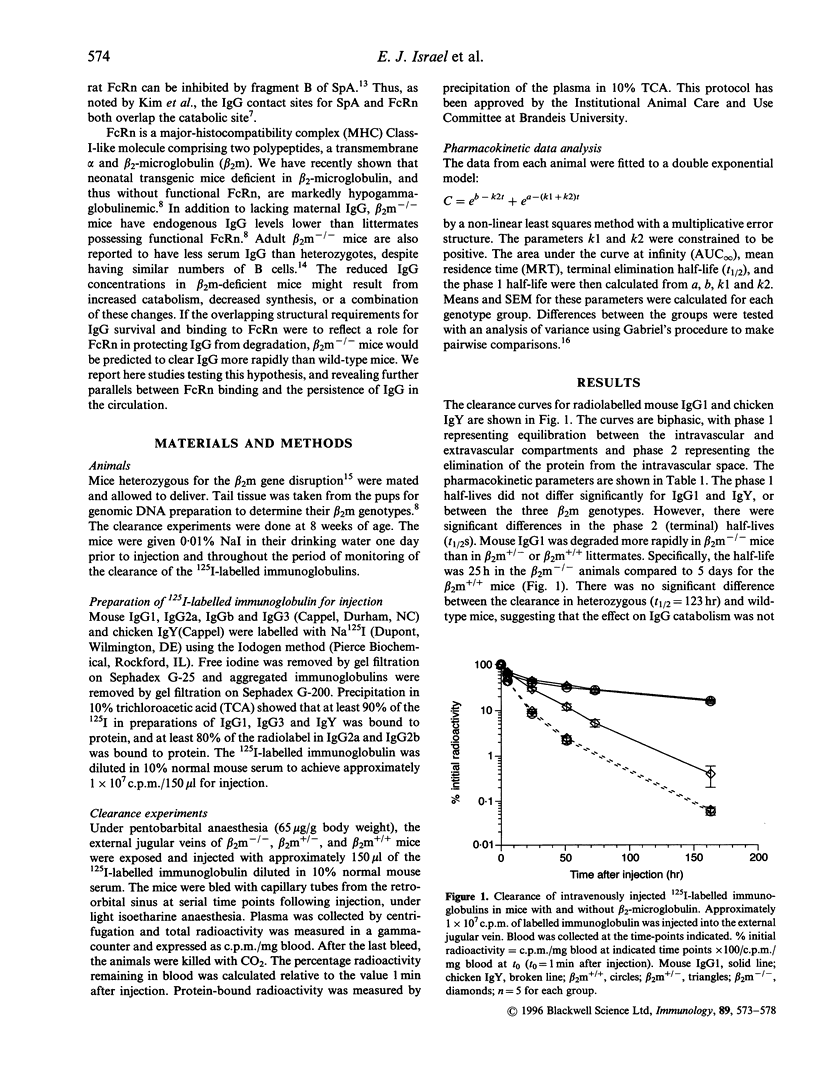
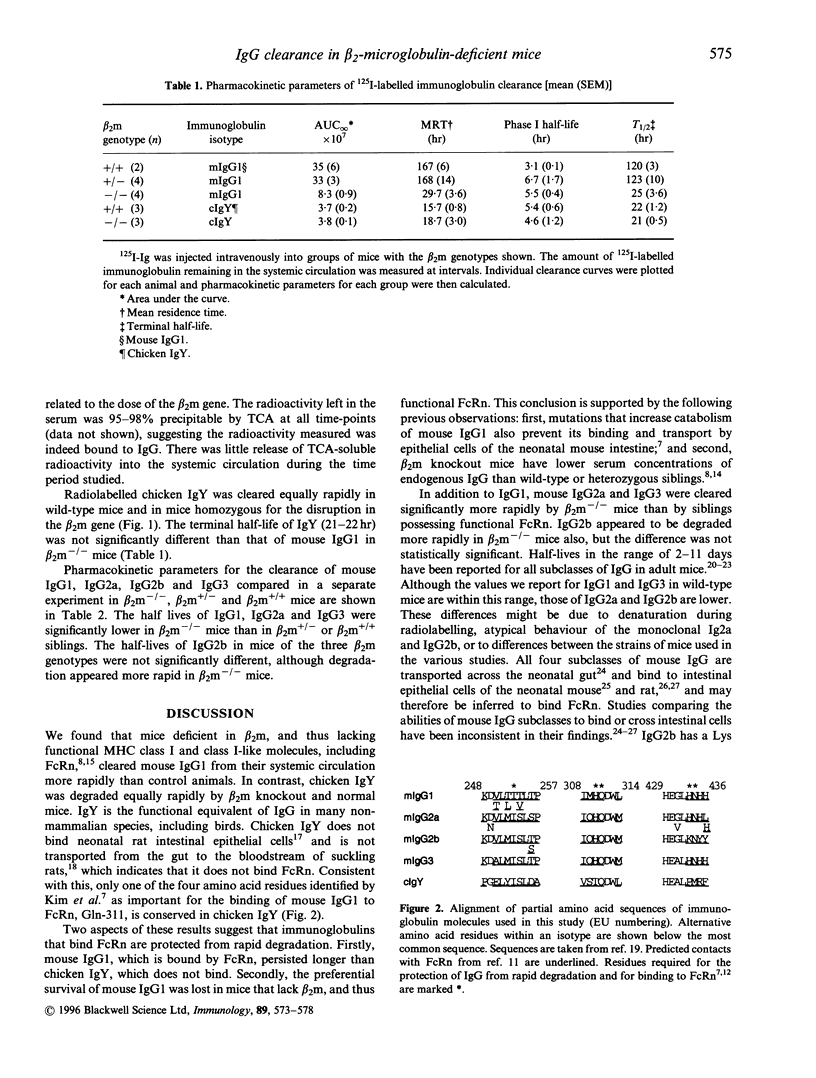
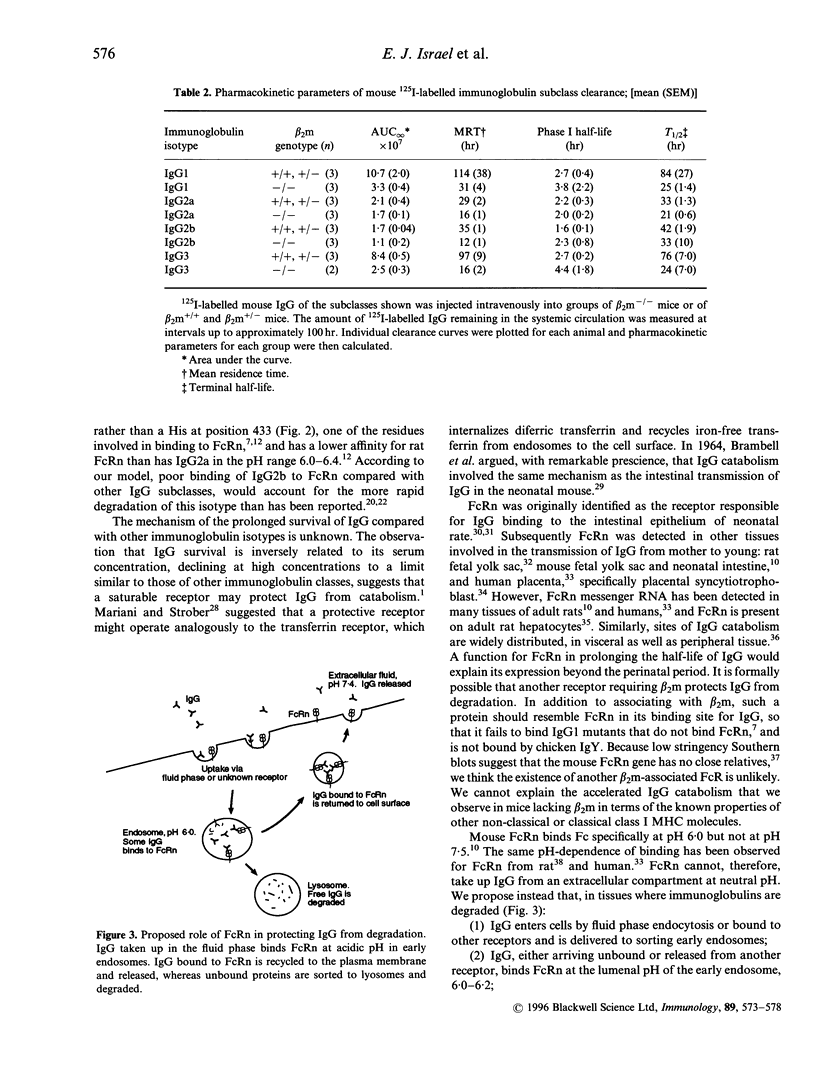
The mechanisms that regulate immunoglobulin G (IgG) catabolism are little understood. We have previously found unusually low IgG concentrations in sera of mice homozygous for a targeted disruption of the beta 2-microglobulin gene. We therefore investigated whether this might result, at least in part, from increased clearance of IgG from the systemic circulation in mice lacking beta 2-microglobulin. We compared the half-lives of radiolabelled mouse IgG1 injected intravenously into beta 2-microglobulin-/- mice and wild-type or heterozygous siblings. The clearance of 125I-labelled IgG1 was strikingly more rapid in the mice lacking beta 2-microglobulin. beta 2-microglobulin-/- mice lack functional molecules of the MHC class I-related Fc receptor, FcRn. Some mutations in mouse IgG1 that increase its clearance have recently been shown to prevent binding to FcRn in the gut. To determine whether the slower degradation of immunoglobulin in mice with beta 2-microglobulin correlated with the ability of the antibody to bind FcRn, we measured the clearance of chicken IgY, which does not bind this receptor. The 125I-labelled IgY was catabolized equally rapidly in beta 2-microglobulin-deficient and wild-type mice. We compared the half-lives of the four subclasses of mouse IgG in beta 2-microglobulin-/-, +/-, and +/+ mice to determine whether the difference we had noted for IgG1 was peculiar to this subclass. The 125I-labelled IgG of all subclasses, with the possible exception of IgG2b. was degraded more rapidly in the beta 2-microglobulin-deficient mice than in heterozygous or wild-type siblings. These data suggest that FcRn can protect IgG from degradation, and is therefore important in maintaining IgG levels in the circulation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahouse J. J., Hagerman C. L., Mittal P., Gilbert D. J., Copeland N. G., Jenkins N. A., Simister N. E. Mouse MHC class I-like Fc receptor encoded outside the MHC. J Immunol. 1993 Dec 1;151(11):6076–6088. [PubMed] [Google Scholar]
- BRAMBELL F. W., HEMMINGS W. A., MORRIS I. G. A THEORETICAL MODEL OF GAMMA-GLOBULIN CATABOLISM. Nature. 1964 Sep 26;203:1352–1354. doi: 10.1038/2031352a0. [DOI] [PubMed] [Google Scholar]
- Benlounes N., Chedid R., Thuillier F., Desjeux J. F., Rousselet F., Heyman M. Intestinal transport and processing of immunoglobulin G in the neonatal and adult rat. Biol Neonate. 1995;67(4):254–263. doi: 10.1159/000244173. [DOI] [PubMed] [Google Scholar]
- Borthistle B. K., Kubo R. T., Brown W. R., Grey H. M. Studies on receptors for IgG on epithelial cells of the rat intestine. J Immunol. 1977 Aug;119(2):471–476. [PubMed] [Google Scholar]
- Broen J. J., Cafruny W. A. Immunoglobulin transfer from immune-reconstituted SCID mice to nursing neonates: blood distribution of antibody and association with perinatal virus protection. Reg Immunol. 1993 Jan-Feb;5(1):44–52. [PubMed] [Google Scholar]
- Burmeister W. P., Huber A. H., Bjorkman P. J. Crystal structure of the complex of rat neonatal Fc receptor with Fc. Nature. 1994 Nov 24;372(6504):379–383. doi: 10.1038/372379a0. [DOI] [PubMed] [Google Scholar]
- Deisenhofer J. Crystallographic refinement and atomic models of a human Fc fragment and its complex with fragment B of protein A from Staphylococcus aureus at 2.9- and 2.8-A resolution. Biochemistry. 1981 Apr 28;20(9):2361–2370. [PubMed] [Google Scholar]
- Dima S., Medeşan C., Moţa G., Moraru I., Sjöquist J., Gheţie V. Effect of protein A and its fragment B on the catabolic and Fc receptor sites of IgG. Eur J Immunol. 1983 Aug;13(8):605–614. doi: 10.1002/eji.1830130802. [DOI] [PubMed] [Google Scholar]
- Ellerson J. R., Yasmeen D., Painter R. H., Dorrington K. J. Structure and function of immunoglobulin domains. III. Isolation and characterization of a fragment corresponding to the Cgamma2 homology region of human immunoglobin G1. J Immunol. 1976 Feb;116(2):510–517. [PubMed] [Google Scholar]
- FAHEY J. L., SELL S. THE IMMUNOGLOBULINS OF MICE. V. THE METABOLIC (CATABOLIC) PROPERTIES OF FIVE IMMUNOGLOBULIN CLASSES. J Exp Med. 1965 Jul 1;122:41–58. doi: 10.1084/jem.122.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghetie V., Hubbard J. G., Kim J. K., Tsen M. F., Lee Y., Ward E. S. Abnormally short serum half-lives of IgG in beta 2-microglobulin-deficient mice. Eur J Immunol. 1996 Mar;26(3):690–696. doi: 10.1002/eji.1830260327. [DOI] [PubMed] [Google Scholar]
- Guyer R. L., Koshland M. E., Knopf P. M. Immunoglobulin binding by mouse intestinal epithelial cell receptors. J Immunol. 1976 Aug;117(2):587–593. [PubMed] [Google Scholar]
- HALLIDAY R. The absorption of antibodies from immune sera by the gut of the young rat. Proc R Soc Lond B Biol Sci. 1955 Mar 15;143(912):408–413. doi: 10.1098/rspb.1955.0020. [DOI] [PubMed] [Google Scholar]
- Haba S., Ovary Z., Nisonoff A. Clearance of IgE from serum of normal and hybridoma-bearing mice. J Immunol. 1985 May;134(5):3291–3297. [PubMed] [Google Scholar]
- Henderson L. A., Baynes J. W., Thorpe S. R. Identification of the sites of IgG catabolism in the rat. Arch Biochem Biophys. 1982 Apr 15;215(1):1–11. doi: 10.1016/0003-9861(82)90272-7. [DOI] [PubMed] [Google Scholar]
- Israel E. J., Patel V. K., Taylor S. F., Marshak-Rothstein A., Simister N. E. Requirement for a beta 2-microglobulin-associated Fc receptor for acquisition of maternal IgG by fetal and neonatal mice. J Immunol. 1995 Jun 15;154(12):6246–6251. [PubMed] [Google Scholar]
- Jakoi E. R., Cambier J., Saslow S. Transepithelial transport of maternal antibody: purification of IgG receptor from newborn rat intestine. J Immunol. 1985 Nov;135(5):3360–3364. [PubMed] [Google Scholar]
- Junghans R. P., Anderson C. L. The protection receptor for IgG catabolism is the beta2-microglobulin-containing neonatal intestinal transport receptor. Proc Natl Acad Sci U S A. 1996 May 28;93(11):5512–5516. doi: 10.1073/pnas.93.11.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandil E., Noguchi M., Ishibashi T., Kasahara M. Structural and phylogenetic analysis of the MHC class I-like Fc receptor gene. J Immunol. 1995 Jun 1;154(11):5907–5918. [PubMed] [Google Scholar]
- Kim J. K., Tsen M. F., Ghetie V., Ward E. S. Identifying amino acid residues that influence plasma clearance of murine IgG1 fragments by site-directed mutagenesis. Eur J Immunol. 1994 Mar;24(3):542–548. doi: 10.1002/eji.1830240308. [DOI] [PubMed] [Google Scholar]
- Kim J. K., Tsen M. F., Ghetie V., Ward E. S. Localization of the site of the murine IgG1 molecule that is involved in binding to the murine intestinal Fc receptor. Eur J Immunol. 1994 Oct;24(10):2429–2434. doi: 10.1002/eji.1830241025. [DOI] [PubMed] [Google Scholar]
- Mackenzie N. M., Keeler K. D. Isotope specificity in the binding of mouse immunoglobulins to brush borders isolated from the neonatal mouse small intestine. Biochem Soc Trans. 1984 Oct;12(5):747–747. doi: 10.1042/bst0120747. [DOI] [PubMed] [Google Scholar]
- Press O. W., Eary J. F., Appelbaum F. R., Martin P. J., Nelp W. B., Glenn S., Fisher D. R., Porter B., Matthews D. C., Gooley T. Phase II trial of 131I-B1 (anti-CD20) antibody therapy with autologous stem cell transplantation for relapsed B cell lymphomas. Lancet. 1995 Aug 5;346(8971):336–340. doi: 10.1016/s0140-6736(95)92225-3. [DOI] [PubMed] [Google Scholar]
- Racadot E., Mousson C., Tanter Y., Wijdenes J., Rifle G., Herve P. Outcome of lymphocyte subsets and cytokines during combined CD4/anti-IL-2R monoclonal antibody therapy in kidney transplantation. Transplant Proc. 1995 Apr;27(2):1672–1673. [PubMed] [Google Scholar]
- Raghavan M., Chen M. Y., Gastinel L. N., Bjorkman P. J. Investigation of the interaction between the class I MHC-related Fc receptor and its immunoglobulin G ligand. Immunity. 1994 Jul;1(4):303–315. doi: 10.1016/1074-7613(94)90082-5. [DOI] [PubMed] [Google Scholar]
- Ravetch J. V., Kinet J. P. Fc receptors. Annu Rev Immunol. 1991;9:457–492. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- Roberts D. M., Guenthert M., Rodewald R. Isolation and characterization of the Fc receptor from the fetal yolk sac of the rat. J Cell Biol. 1990 Nov;111(5 Pt 1):1867–1876. doi: 10.1083/jcb.111.5.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodewald R., Kraehenbuhl J. P. Receptor-mediated transport of IgG. J Cell Biol. 1984 Jul;99(1 Pt 2):159s–164s. doi: 10.1083/jcb.99.1.159s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodewald R. pH-dependent binding of immunoglobulins to intestinal cells of the neonatal rat. J Cell Biol. 1976 Nov;71(2):666–669. doi: 10.1083/jcb.71.2.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPIEGELBERG H. L., WEIGLE W. O. THE CATABOLISM OF HOMOLOGOUS AND HETEROLOGOUS 7S GAMMA GLOBULIN FRAGMENTS. J Exp Med. 1965 Mar 1;121:323–338. doi: 10.1084/jem.121.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simister N. E., Mostov K. E. An Fc receptor structurally related to MHC class I antigens. Nature. 1989 Jan 12;337(6203):184–187. doi: 10.1038/337184a0. [DOI] [PubMed] [Google Scholar]
- Simister N. E., Mostov K. E. Cloning and expression of the neonatal rat intestinal Fc receptor, a major histocompatibility complex class I antigen homolog. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):571–580. doi: 10.1101/sqb.1989.054.01.068. [DOI] [PubMed] [Google Scholar]
- Simister N. E., Rees A. R. Isolation and characterization of an Fc receptor from neonatal rat small intestine. Eur J Immunol. 1985 Jul;15(7):733–738. doi: 10.1002/eji.1830150718. [DOI] [PubMed] [Google Scholar]
- Simister N. E., Story C. M., Chen H. L., Hunt J. S. An IgG-transporting Fc receptor expressed in the syncytiotrophoblast of human placenta. Eur J Immunol. 1996 Jul;26(7):1527–1531. doi: 10.1002/eji.1830260718. [DOI] [PubMed] [Google Scholar]
- Sipe D. M., Murphy R. F. High-resolution kinetics of transferrin acidification in BALB/c 3T3 cells: exposure to pH 6 followed by temperature-sensitive alkalinization during recycling. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7119–7123. doi: 10.1073/pnas.84.20.7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spriggs M. K., Koller B. H., Sato T., Morrissey P. J., Fanslow W. C., Smithies O., Voice R. F., Widmer M. B., Maliszewski C. R. Beta 2-microglobulin-, CD8+ T-cell-deficient mice survive inoculation with high doses of vaccinia virus and exhibit altered IgG responses. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6070–6074. doi: 10.1073/pnas.89.13.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Story C. M., Mikulska J. E., Simister N. E. A major histocompatibility complex class I-like Fc receptor cloned from human placenta: possible role in transfer of immunoglobulin G from mother to fetus. J Exp Med. 1994 Dec 1;180(6):2377–2381. doi: 10.1084/jem.180.6.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira P., Rajewsky K. The half-lives of serum immunoglobulins in adult mice. Eur J Immunol. 1988 Feb;18(2):313–316. doi: 10.1002/eji.1830180221. [DOI] [PubMed] [Google Scholar]
- Waldmann T. A., Strober W. Metabolism of immunoglobulins. Prog Allergy. 1969;13:1–110. doi: 10.1159/000385919. [DOI] [PubMed] [Google Scholar]
- Wawrzynczak E. J., Denham S., Parnell G. D., Cumber A. J., Jones P. T., Winter G. Recombinant mouse monoclonal antibodies with single amino acid substitutions affecting Clq and high affinity Fc receptor binding have identical serum half-lives in the BALB/c mouse. Mol Immunol. 1992 Feb;29(2):221–227. doi: 10.1016/0161-5890(92)90103-5. [DOI] [PubMed] [Google Scholar]
- Zijlstra M., Bix M., Simister N. E., Loring J. M., Raulet D. H., Jaenisch R. Beta 2-microglobulin deficient mice lack CD4-8+ cytolytic T cells. Nature. 1990 Apr 19;344(6268):742–746. doi: 10.1038/344742a0. [DOI] [PubMed] [Google Scholar]
- van der Lubbe P. A., Dijkmans B. A., Markusse H. M., Nässander U., Breedveld F. C. A randomized, double-blind, placebo-controlled study of CD4 monoclonal antibody therapy in early rheumatoid arthritis. Arthritis Rheum. 1995 Aug;38(8):1097–1106. doi: 10.1002/art.1780380812. [DOI] [PubMed] [Google Scholar]


