Abstract
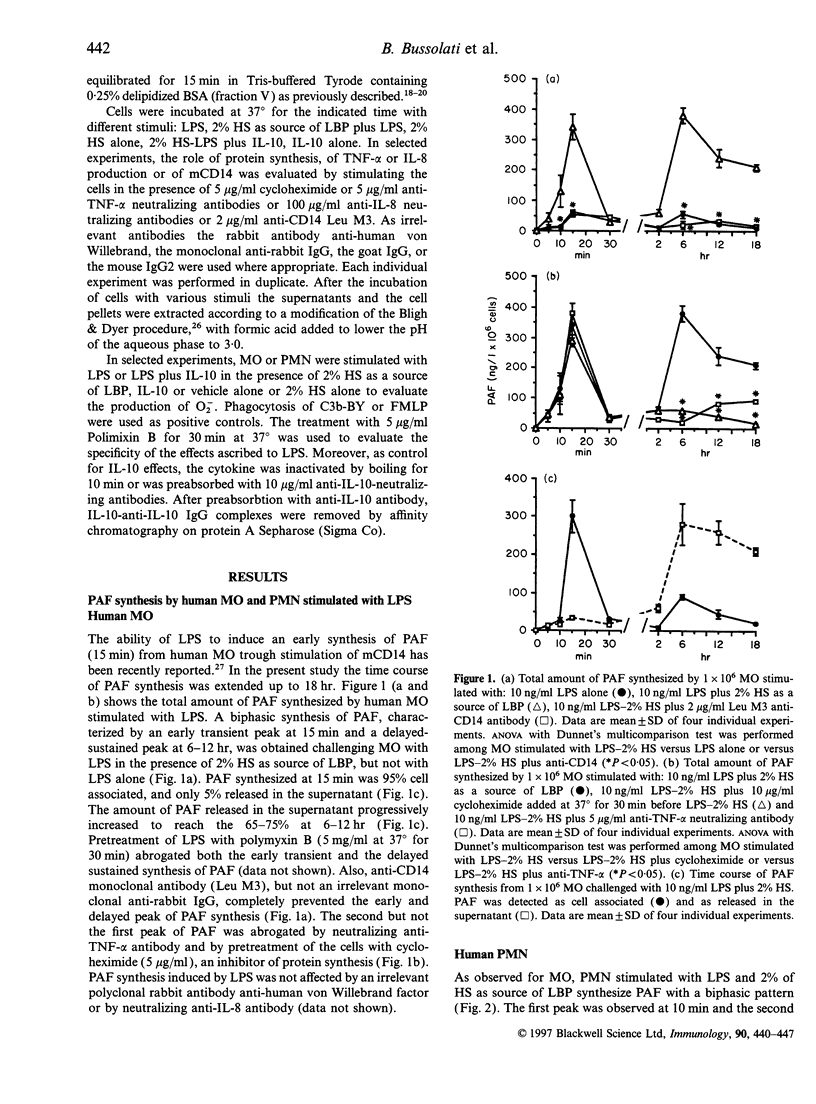
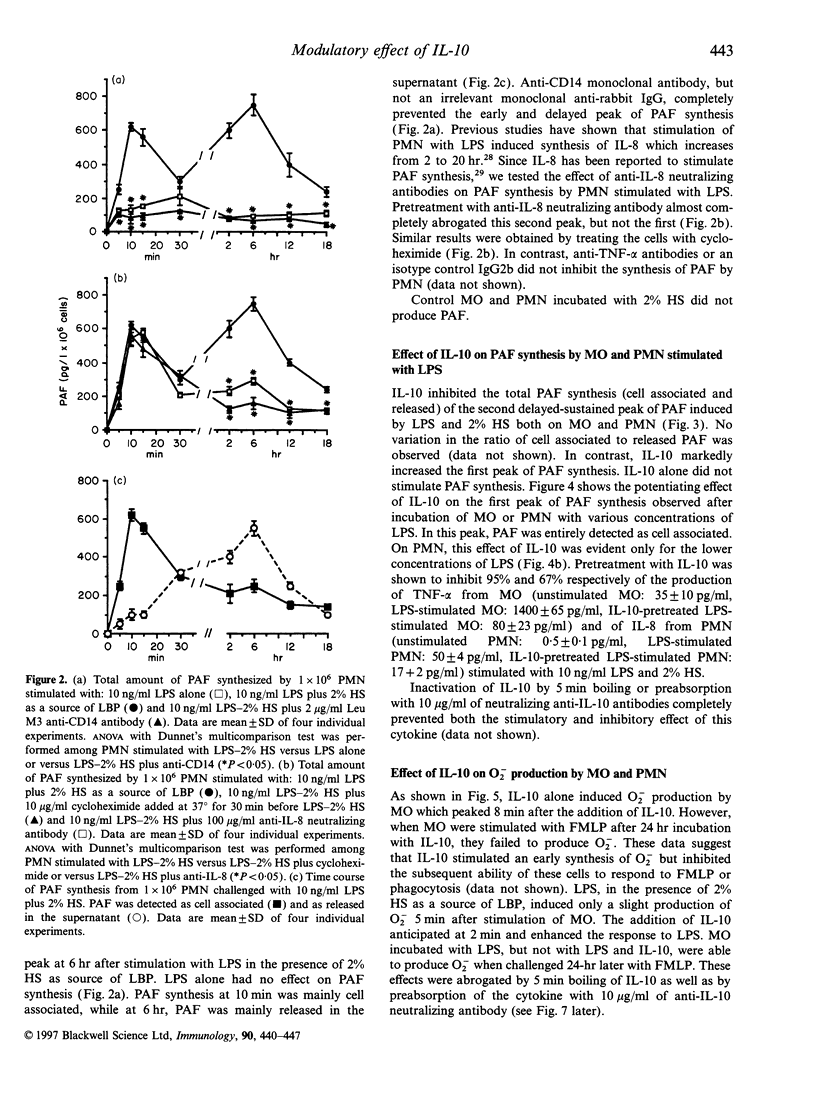
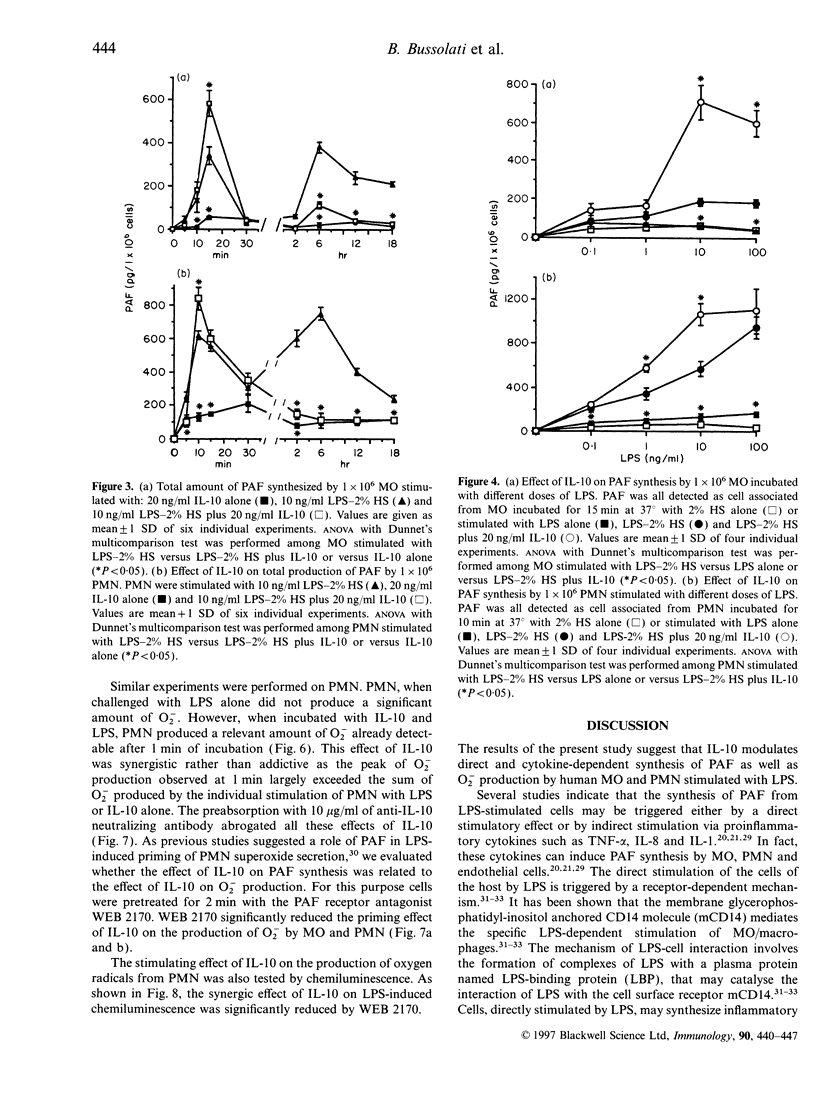
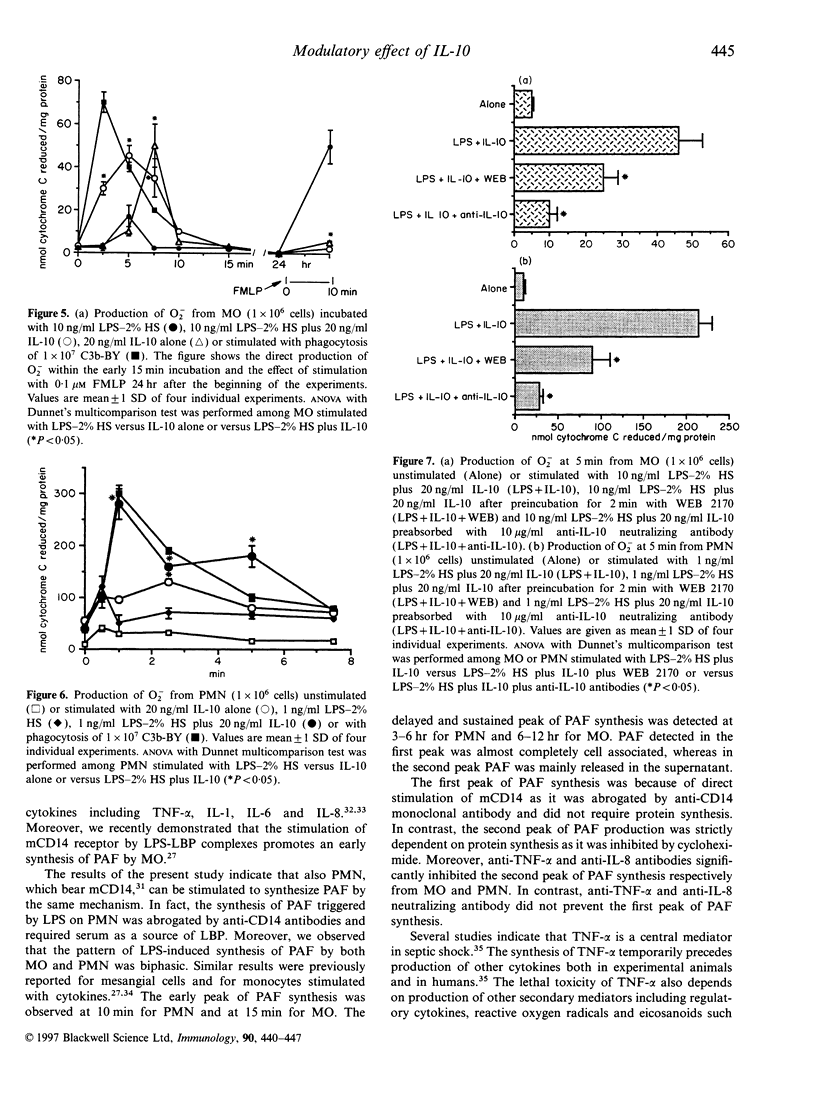
We observed that human monocytes (MO) and polymorphonuclear neutrophils (PMN) stimulated by lipopolysaccharide (LPS) produce platelet-activating factor (PAF) in a pattern characterized by an early and a delayed peak of synthesis. The early peak of PAF synthesis was due to a direct stimulation of these cells through mCD14 receptor as it was inhibited by anti-CD14 monoclonal antibody. The delayed and sustained peak of PAF synthesis was dependent on protein synthesis and cytokine production as shown by the inhibitory effect of cycloheximide on both MO and PMN, and of anti-tumour necrosis factor-alpha (anti-TNF-alpha) and of anti-interleukin-8 (anti-IL-8) neutralizing antibodies on MO and PMN respectively. IL-10 completely prevented this second, cytokine-dependent peak of PAF synthesis. In contrast, IL-10 markedly enhanced the first peak of PAF synthesis both in MO and PMN. Moreover, IL-10 was shown to modulate the production of superoxide anions (O2-) on both MO and PMN. As suggested by previous studies, IL-10 inhibited the delayed production of O2-. In the present study, we observed that IL-10 directly stimulated an early production of O2-. In addition, IL-10 enhanced the synthesis of O2- by MO and PMN challenged with LPS. The IL-10-induced O2- production was dependent, at least in part, from its effect on PAF synthesis, as it was inhibited by the PAF receptor antagonist WEB 2170. These results suggest that IL-10 may upregulate the early synthesis of PAF and O2- triggered by direct LPS stimulation, whereas it may downregulate the delayed production of these mediators.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson B. O., Bensard D. D., Harken A. H. The role of platelet activating factor and its antagonists in shock, sepsis and multiple organ failure. Surg Gynecol Obstet. 1991 May;172(5):415–424. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Baggiolini M., Walz A., Kunkel S. L. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989 Oct;84(4):1045–1049. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste J., Le Couedic J. P., Polonsky J., Tence M. Structural analysis of purified platelet-activating factor by lipases. Nature. 1977 Sep 8;269(5624):170–171. doi: 10.1038/269170a0. [DOI] [PubMed] [Google Scholar]
- Bogdan C., Paik J., Vodovotz Y., Nathan C. Contrasting mechanisms for suppression of macrophage cytokine release by transforming growth factor-beta and interleukin-10. J Biol Chem. 1992 Nov 15;267(32):23301–23308. [PubMed] [Google Scholar]
- Bogdan C., Vodovotz Y., Nathan C. Macrophage deactivation by interleukin 10. J Exp Med. 1991 Dec 1;174(6):1549–1555. doi: 10.1084/jem.174.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camussi G., Bussolino F., Salvidio G., Baglioni C. Tumor necrosis factor/cachectin stimulates peritoneal macrophages, polymorphonuclear neutrophils, and vascular endothelial cells to synthesize and release platelet-activating factor. J Exp Med. 1987 Nov 1;166(5):1390–1404. doi: 10.1084/jem.166.5.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camussi G. Interactive effects of tumor necrosis factor and platelet-activating factor in the pathogenesis of glomerular injury. Lab Invest. 1994 Apr;70(4):435–436. [PubMed] [Google Scholar]
- Camussi G., Mariano F., Biancone L., De Martino A., Bussolati B., Montrucchio G., Tobias P. S. Lipopolysaccharide binding protein and CD14 modulate the synthesis of platelet-activating factor by human monocytes and mesangial and endothelial cells stimulated with lipopolysaccharide. J Immunol. 1995 Jul 1;155(1):316–324. [PubMed] [Google Scholar]
- Camussi G., Montrucchio G., Dominioni L., Dionigi R. Septic shock--the unravelling of molecular mechanisms. Nephrol Dial Transplant. 1995 Oct;10(10):1808–1813. [PubMed] [Google Scholar]
- Camussi G., Tetta C., Bussolino F., Baglioni C. Synthesis and release of platelet-activating factor is inhibited by plasma alpha 1-proteinase inhibitor or alpha 1-antichymotrypsin and is stimulated by proteinases. J Exp Med. 1988 Oct 1;168(4):1293–1306. doi: 10.1084/jem.168.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassatella M. A., Meda L., Bonora S., Ceska M., Constantin G. Interleukin 10 (IL-10) inhibits the release of proinflammatory cytokines from human polymorphonuclear leukocytes. Evidence for an autocrine role of tumor necrosis factor and IL-1 beta in mediating the production of IL-8 triggered by lipopolysaccharide. J Exp Med. 1993 Dec 1;178(6):2207–2211. doi: 10.1084/jem.178.6.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassatella M. A., Meda L., Gasperini S., Calzetti F., Bonora S. Interleukin 10 (IL-10) upregulates IL-1 receptor antagonist production from lipopolysaccharide-stimulated human polymorphonuclear leukocytes by delaying mRNA degradation. J Exp Med. 1994 May 1;179(5):1695–1699. doi: 10.1084/jem.179.5.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao W., Olson M. S. Platelet-activating factor: receptors and signal transduction. Biochem J. 1993 Jun 15;292(Pt 3):617–629. doi: 10.1042/bj2920617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doebber T. W., Wu M. S., Robbins J. C., Choy B. M., Chang M. N., Shen T. Y. Platelet activating factor (PAF) involvement in endotoxin-induced hypotension in rats. Studies with PAF-receptor antagonist kadsurenone. Biochem Biophys Res Commun. 1985 Mar 29;127(3):799–808. doi: 10.1016/s0006-291x(85)80014-0. [DOI] [PubMed] [Google Scholar]
- Douglas J. G. Angiotensin receptor subtypes of the kidney cortex. Am J Physiol. 1987 Jul;253(1 Pt 2):F1–F7. doi: 10.1152/ajprenal.1987.253.1.F1. [DOI] [PubMed] [Google Scholar]
- Gazzinelli R. T., Oswald I. P., James S. L., Sher A. IL-10 inhibits parasite killing and nitrogen oxide production by IFN-gamma-activated macrophages. J Immunol. 1992 Mar 15;148(6):1792–1796. [PubMed] [Google Scholar]
- Gegner J. A., Ulevitch R. J., Tobias P. S. Lipopolysaccharide (LPS) signal transduction and clearance. Dual roles for LPS binding protein and membrane CD14. J Biol Chem. 1995 Mar 10;270(10):5320–5325. doi: 10.1074/jbc.270.10.5320. [DOI] [PubMed] [Google Scholar]
- Gérard C., Bruyns C., Marchant A., Abramowicz D., Vandenabeele P., Delvaux A., Fiers W., Goldman M., Velu T. Interleukin 10 reduces the release of tumor necrosis factor and prevents lethality in experimental endotoxemia. J Exp Med. 1993 Feb 1;177(2):547–550. doi: 10.1084/jem.177.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M., Muchamuel T., Andrade S., Menon S. Interleukin 10 protects mice from lethal endotoxemia. J Exp Med. 1993 Apr 1;177(4):1205–1208. doi: 10.1084/jem.177.4.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñarrea P., Gomez-Cambronero J., Pascual J., Ponte M. C., Hernando L., Sánchez-Crespo M. Synthesis of PAF-acether and blood volume changes in gram-negative sepsis. Immunopharmacology. 1985 Feb;9(1):45–52. doi: 10.1016/0162-3109(85)90046-3. [DOI] [PubMed] [Google Scholar]
- Lopez Diez F., Nieto M. L., Fernandez-Gallardo S., Gijon M. A., Sanchez Crespo M. Occupancy of platelet receptors for platelet-activating factor in patients with septicemia. J Clin Invest. 1989 May;83(5):1733–1740. doi: 10.1172/JCI114074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. S., Leal-Berumen I., Nielsen L., Glibetic M., Jordana M. Interleukin (IL)-10 inhibits long-term IL-6 production but not preformed mediator release from rat peritoneal mast cells. J Clin Invest. 1996 Feb 15;97(4):1122–1128. doi: 10.1172/JCI118506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus L. M., Woodard D. S., Deavers S. I., Pinckard R. N. PAF molecular heterogeneity: pathobiological implications. Lab Invest. 1993 Dec;69(6):639–650. [PubMed] [Google Scholar]
- Moore K. W., O'Garra A., de Waal Malefyt R., Vieira P., Mosmann T. R. Interleukin-10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- Pugin J., Ulevitch R. J., Tobias P. S. A critical role for monocytes and CD14 in endotoxin-induced endothelial cell activation. J Exp Med. 1993 Dec 1;178(6):2193–2200. doi: 10.1084/jem.178.6.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovin B. H., Wurst E., Kohan D. E. Production of reactive oxygen species by tubular epithelial cells in culture. Kidney Int. 1990 Jun;37(6):1509–1514. doi: 10.1038/ki.1990.142. [DOI] [PubMed] [Google Scholar]
- Silvestro L., Da Col R., Scappaticci E., Libertucci D., Biancone L., Camussi G. Development of a high-performance liquid chromatographic-mass spectrometric technique, with an ionspray interface, for the determination of platelet-activating factor (PAF) and lyso-PAF in biological samples. J Chromatogr. 1993 Sep 24;647(2):261–269. doi: 10.1016/0021-9673(93)83406-i. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Vlassara H., Cerami A. Cachectin/tumour necrosis factor. Lancet. 1989 May 20;1(8647):1122–1126. doi: 10.1016/s0140-6736(89)92394-5. [DOI] [PubMed] [Google Scholar]
- Valone F. H., Epstein L. B. Biphasic platelet-activating factor synthesis by human monocytes stimulated with IL-1-beta, tumor necrosis factor, or IFN-gamma. J Immunol. 1988 Dec 1;141(11):3945–3950. [PubMed] [Google Scholar]
- Valone F. H., Epstein L. B. Biphasic platelet-activating factor synthesis by human monocytes stimulated with IL-1-beta, tumor necrosis factor, or IFN-gamma. J Immunol. 1988 Dec 1;141(11):3945–3950. [PubMed] [Google Scholar]
- Worthen G. S., Seccombe J. F., Clay K. L., Guthrie L. A., Johnston R. B., Jr The priming of neutrophils by lipopolysaccharide for production of intracellular platelet-activating factor. Potential role in mediation of enhanced superoxide secretion. J Immunol. 1988 May 15;140(10):3553–3559. [PubMed] [Google Scholar]
- Wright S. D., Ramos R. A., Tobias P. S., Ulevitch R. J., Mathison J. C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990 Sep 21;249(4975):1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- Ziegler-Heitbrock H. W., Ulevitch R. J. CD14: cell surface receptor and differentiation marker. Immunol Today. 1993 Mar;14(3):121–125. doi: 10.1016/0167-5699(93)90212-4. [DOI] [PubMed] [Google Scholar]


