Abstract
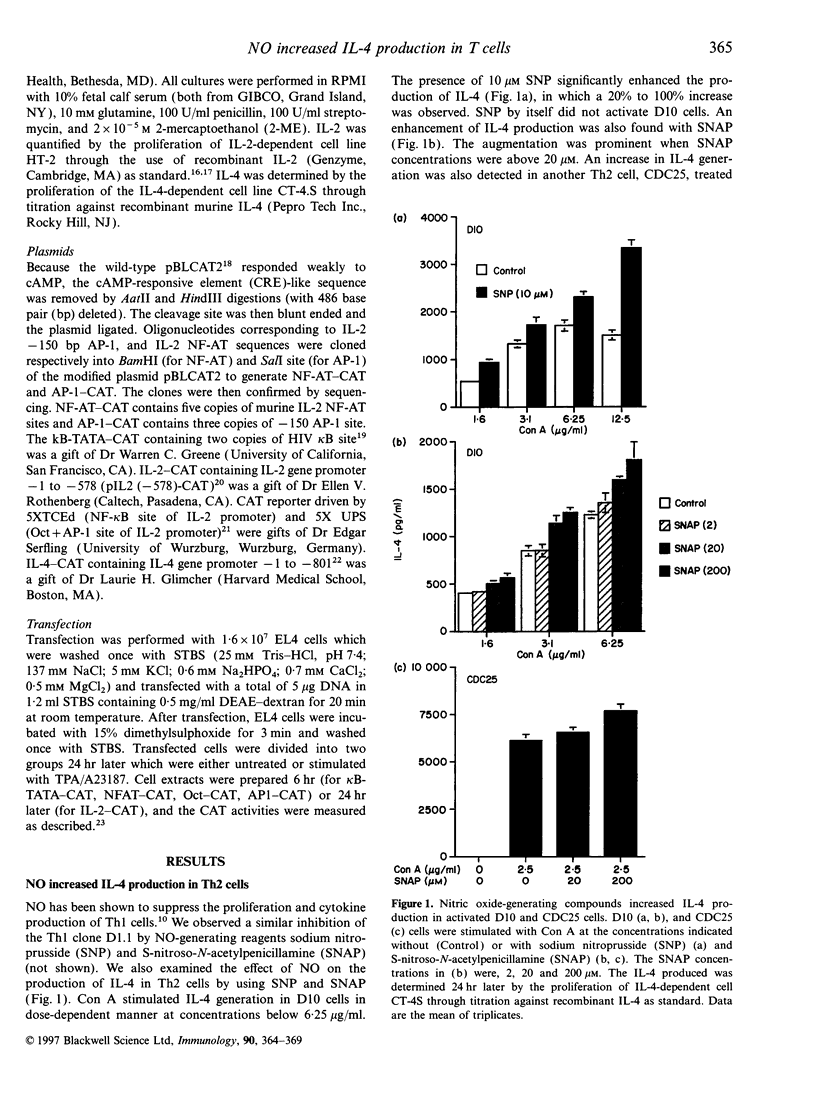
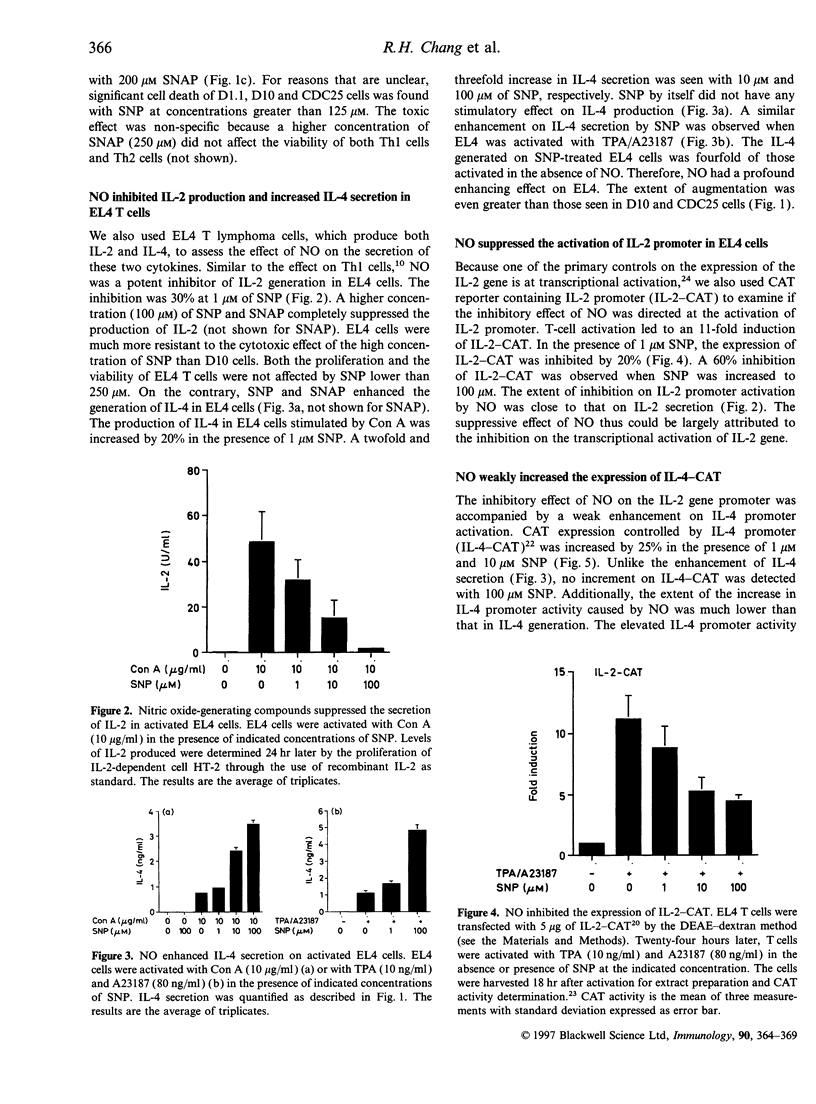
Nitric oxide (NO) is a regulator of many biological functions including T helper 1 (Th1)/T helper 2 cells balance. It has been demonstrated that NO inhibits the secretion of interleukin-2 (IL-2) and interferon-gamma on Th1 cells. Here we showed that, in addition to the suppression of IL-2 production, NO-generating agents sodium nitroprusside (SNP) and S-nitroso-N-acetylpenicillamine (SNAP) increased the secretion of IL-4 both in Th2 clones and EL4 T cells. The additive effect was dependent on the dose of SNP and SNAP. Augmentation of IL-4 production was detected with 1 microM SNP, and up to threefold increase in IL-4 secretion could be observed with higher concentrations of SNP/SNAP. NO also weakly increased the activation of IL-4 promoter. In contrast, NO markedly inhibited the induction of IL-2 promoter, which could account for most of the reduction in IL-2 production. Analysis of the transcriptional elements on IL-2 and IL-4 promoters revealed a selective inactivation of NF-kappa B and NF-AT. It is suggested that despite the complex feedback network regulating NO production, the enhanced IL-4 expression would lead to the expansion of Th2 cells once NO is generated.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballard D. W., Dixon E. P., Peffer N. J., Bogerd H., Doerre S., Stein B., Greene W. C. The 65-kDa subunit of human NF-kappa B functions as a potent transcriptional activator and a target for v-Rel-mediated repression. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1875–1879. doi: 10.1073/pnas.89.5.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes P. J., Liew F. Y. Nitric oxide and asthmatic inflammation. Immunol Today. 1995 Mar;16(3):128–130. doi: 10.1016/0167-5699(95)80128-6. [DOI] [PubMed] [Google Scholar]
- Chen D., Rothenberg E. V. Interleukin 2 transcription factors as molecular targets of cAMP inhibition: delayed inhibition kinetics and combinatorial transcription roles. J Exp Med. 1994 Mar 1;179(3):931–942. doi: 10.1084/jem.179.3.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrity P. A., Chen D., Rothenberg E. V., Wold B. J. Interleukin-2 transcription is regulated in vivo at the level of coordinated binding of both constitutive and regulated factors. Mol Cell Biol. 1994 Mar;14(3):2159–2169. doi: 10.1128/mcb.14.3.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollob K. J., Coffman R. L. A minority subpopulation of CD4+ T cells directs the development of naive CD4+ T cells into IL-4-secreting cells. J Immunol. 1994 Jun 1;152(11):5180–5188. [PubMed] [Google Scholar]
- Hodge M. R., Ranger A. M., Charles de la Brousse F., Hoey T., Grusby M. J., Glimcher L. H. Hyperproliferation and dysregulation of IL-4 expression in NF-ATp-deficient mice. Immunity. 1996 Apr;4(4):397–405. doi: 10.1016/s1074-7613(00)80253-8. [DOI] [PubMed] [Google Scholar]
- Jain J., Loh C., Rao A. Transcriptional regulation of the IL-2 gene. Curr Opin Immunol. 1995 Jun;7(3):333–342. doi: 10.1016/0952-7915(95)80107-3. [DOI] [PubMed] [Google Scholar]
- Kaye J., Gillis S., Mizel S. B., Shevach E. M., Malek T. R., Dinarello C. A., Lachman L. B., Janeway C. A., Jr Growth of a cloned helper T cell line induced by a monoclonal antibody specific for the antigen receptor: interleukin 1 is required for the expression of receptors for interleukin 2. J Immunol. 1984 Sep;133(3):1339–1345. [PubMed] [Google Scholar]
- Kurt-Jones E. A., Hamberg S., Ohara J., Paul W. E., Abbas A. K. Heterogeneity of helper/inducer T lymphocytes. I. Lymphokine production and lymphokine responsiveness. J Exp Med. 1987 Dec 1;166(6):1774–1787. doi: 10.1084/jem.166.6.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M. Z., Huang S. Y., Briner T. J., Guillet J. G., Smith J. A., Gefter M. L. T cell receptor gene usage in the response to lambda repressor cI protein. An apparent bias in the usage of a V alpha gene element. J Exp Med. 1988 Sep 1;168(3):1081–1097. doi: 10.1084/jem.168.3.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander H. M., Sehajpal P., Levine D. M., Novogrodsky A. Activation of human peripheral blood mononuclear cells by nitric oxide-generating compounds. J Immunol. 1993 Feb 15;150(4):1509–1516. [PubMed] [Google Scholar]
- Lee M. R., Chung C. S., Liou M. L., Wu M., Li W. F., Hsueh Y. P., Lai M. Z. Isolation and characterization of nuclear proteins that bind to T cell receptor V beta decamer motif. J Immunol. 1992 Mar 15;148(6):1906–1912. [PubMed] [Google Scholar]
- Liang H. E., Chen C. C., Chou D. L., Lai M. Z. Flexibility of the T cell receptor repertoire. Eur J Immunol. 1994 Jul;24(7):1604–1611. doi: 10.1002/eji.1830240723. [DOI] [PubMed] [Google Scholar]
- Liew F. Y. Regulation of lymphocyte functions by nitric oxide. Curr Opin Immunol. 1995 Jun;7(3):396–399. doi: 10.1016/0952-7915(95)80116-2. [DOI] [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMicking J. D., Nathan C., Hom G., Chartrain N., Fletcher D. S., Trumbauer M., Stevens K., Xie Q. W., Sokol K., Hutchinson N. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995 May 19;81(4):641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- Marletta M. A. Nitric oxide synthase: aspects concerning structure and catalysis. Cell. 1994 Sep 23;78(6):927–930. doi: 10.1016/0092-8674(94)90268-2. [DOI] [PubMed] [Google Scholar]
- Modolell M., Corraliza I. M., Link F., Soler G., Eichmann K. Reciprocal regulation of the nitric oxide synthase/arginase balance in mouse bone marrow-derived macrophages by TH1 and TH2 cytokines. Eur J Immunol. 1995 Apr;25(4):1101–1104. doi: 10.1002/eji.1830250436. [DOI] [PubMed] [Google Scholar]
- Neumann M., Grieshammer T., Chuvpilo S., Kneitz B., Lohoff M., Schimpl A., Franza B. R., Jr, Serfling E. RelA/p65 is a molecular target for the immunosuppressive action of protein kinase A. EMBO J. 1995 May 1;14(9):1991–2004. doi: 10.1002/j.1460-2075.1995.tb07191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak T. J., White P. M., Rothenberg E. V. Regulatory anatomy of the murine interleukin-2 gene. Nucleic Acids Res. 1990 Aug 11;18(15):4523–4533. doi: 10.1093/nar/18.15.4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nukaya I., Takagi K., Kawabe T., Suketa Y. Suppression of cytokine production in T helper type 2 cells by nitric oxide in comparison with T helper type 1 cells. Microbiol Immunol. 1995;39(9):709–714. doi: 10.1111/j.1348-0421.1995.tb03246.x. [DOI] [PubMed] [Google Scholar]
- O'Garra A., Murphy K. Role of cytokines in determining T-lymphocyte function. Curr Opin Immunol. 1994 Jun;6(3):458–466. doi: 10.1016/0952-7915(94)90128-7. [DOI] [PubMed] [Google Scholar]
- Peng H. B., Libby P., Liao J. K. Induction and stabilization of I kappa B alpha by nitric oxide mediates inhibition of NF-kappa B. J Biol Chem. 1995 Jun 9;270(23):14214–14219. doi: 10.1074/jbc.270.23.14214. [DOI] [PubMed] [Google Scholar]
- Peunova N., Enikolopov G. Amplification of calcium-induced gene transcription by nitric oxide in neuronal cells. Nature. 1993 Jul 29;364(6436):450–453. doi: 10.1038/364450a0. [DOI] [PubMed] [Google Scholar]
- Reiner S. L., Zheng S., Wang Z. E., Stowring L., Locksley R. M. Leishmania promastigotes evade interleukin 12 (IL-12) induction by macrophages and stimulate a broad range of cytokines from CD4+ T cells during initiation of infection. J Exp Med. 1994 Feb 1;179(2):447–456. doi: 10.1084/jem.179.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney J. W., Hoey T., Glimcher L. H. Coordinate and cooperative roles for NF-AT and AP-1 in the regulation of the murine IL-4 gene. Immunity. 1995 May;2(5):473–483. doi: 10.1016/1074-7613(95)90028-4. [DOI] [PubMed] [Google Scholar]
- Rooney J. W., Sun Y. L., Glimcher L. H., Hoey T. Novel NFAT sites that mediate activation of the interleukin-2 promoter in response to T-cell receptor stimulation. Mol Cell Biol. 1995 Nov;15(11):6299–6310. doi: 10.1128/mcb.15.11.6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H. H., Walter U. NO at work. Cell. 1994 Sep 23;78(6):919–925. doi: 10.1016/0092-8674(94)90267-4. [DOI] [PubMed] [Google Scholar]
- Schmitz J., Thiel A., Kühn R., Rajewsky K., Müller W., Assenmacher M., Radbruch A. Induction of interleukin 4 (IL-4) expression in T helper (Th) cells is not dependent on IL-4 from non-Th cells. J Exp Med. 1994 Apr 1;179(4):1349–1353. doi: 10.1084/jem.179.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seder R. A., Paul W. E. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu Rev Immunol. 1994;12:635–673. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- Stamler J. S. Redox signaling: nitrosylation and related target interactions of nitric oxide. Cell. 1994 Sep 23;78(6):931–936. doi: 10.1016/0092-8674(94)90269-0. [DOI] [PubMed] [Google Scholar]
- Taylor-Robinson A. W., Liew F. Y., Severn A., Xu D., McSorley S. J., Garside P., Padron J., Phillips R. S. Regulation of the immune response by nitric oxide differentially produced by T helper type 1 and T helper type 2 cells. Eur J Immunol. 1994 Apr;24(4):980–984. doi: 10.1002/eji.1830240430. [DOI] [PubMed] [Google Scholar]
- Todd M. D., Grusby M. J., Lederer J. A., Lacy E., Lichtman A. H., Glimcher L. H. Transcription of the interleukin 4 gene is regulated by multiple promoter elements. J Exp Med. 1993 Jun 1;177(6):1663–1674. doi: 10.1084/jem.177.6.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tony H. P., Parker D. C. Major histocompatibility complex-restricted, polyclonal B cell responses resulting from helper T cell recognition of antiimmunoglobulin presented by small B lymphocytes. J Exp Med. 1985 Jan 1;161(1):223–241. doi: 10.1084/jem.161.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X. Q., Charles I. G., Smith A., Ure J., Feng G. J., Huang F. P., Xu D., Muller W., Moncada S., Liew F. Y. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995 Jun 1;375(6530):408–411. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- Weinberg J. B., Granger D. L., Pisetsky D. S., Seldin M. F., Misukonis M. A., Mason S. N., Pippen A. M., Ruiz P., Wood E. R., Gilkeson G. S. The role of nitric oxide in the pathogenesis of spontaneous murine autoimmune disease: increased nitric oxide production and nitric oxide synthase expression in MRL-lpr/lpr mice, and reduction of spontaneous glomerulonephritis and arthritis by orally administered NG-monomethyl-L-arginine. J Exp Med. 1994 Feb 1;179(2):651–660. doi: 10.1084/jem.179.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worrall N. K., Lazenby W. D., Misko T. P., Lin T. S., Rodi C. P., Manning P. T., Tilton R. G., Williamson J. R., Ferguson T. B., Jr Modulation of in vivo alloreactivity by inhibition of inducible nitric oxide synthase. J Exp Med. 1995 Jan 1;181(1):63–70. doi: 10.1084/jem.181.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q. W., Cho H. J., Calaycay J., Mumford R. A., Swiderek K. M., Lee T. D., Ding A., Troso T., Nathan C. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992 Apr 10;256(5054):225–228. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T., Paul W. E. CD4pos, NK1.1pos T cells promptly produce interleukin 4 in response to in vivo challenge with anti-CD3. J Exp Med. 1994 Apr 1;179(4):1285–1295. doi: 10.1084/jem.179.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]


