Abstract
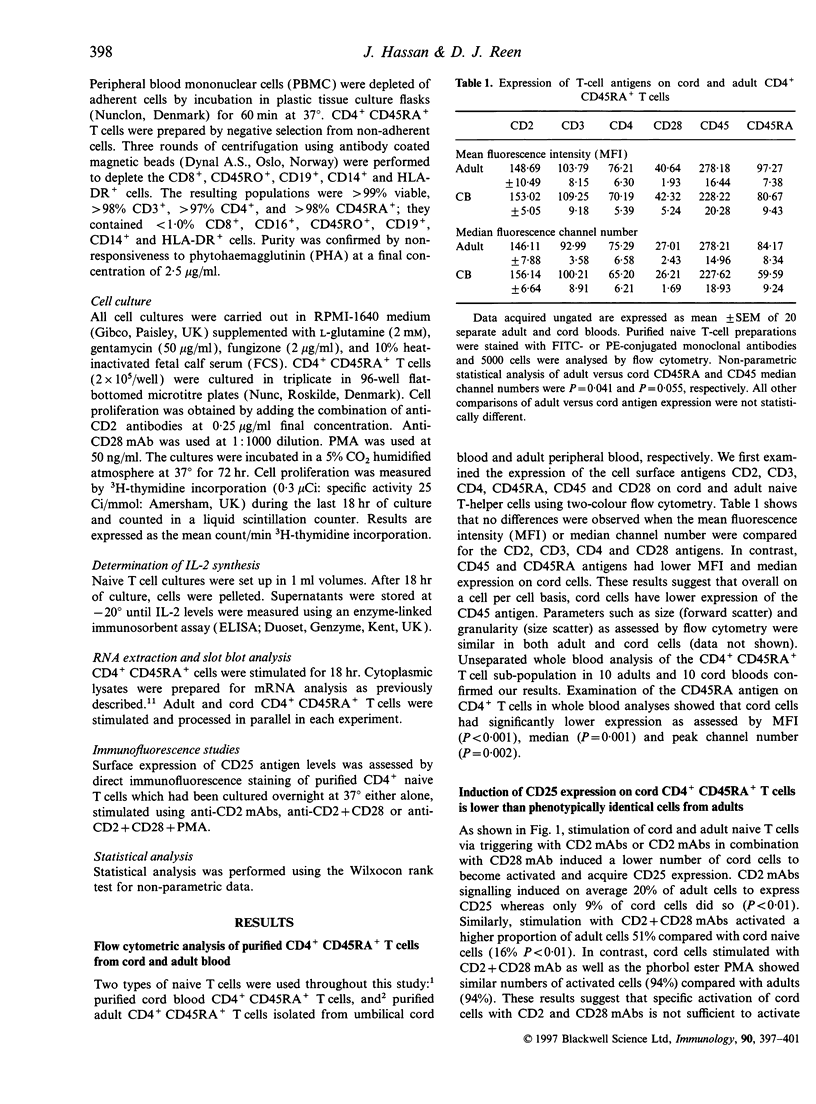
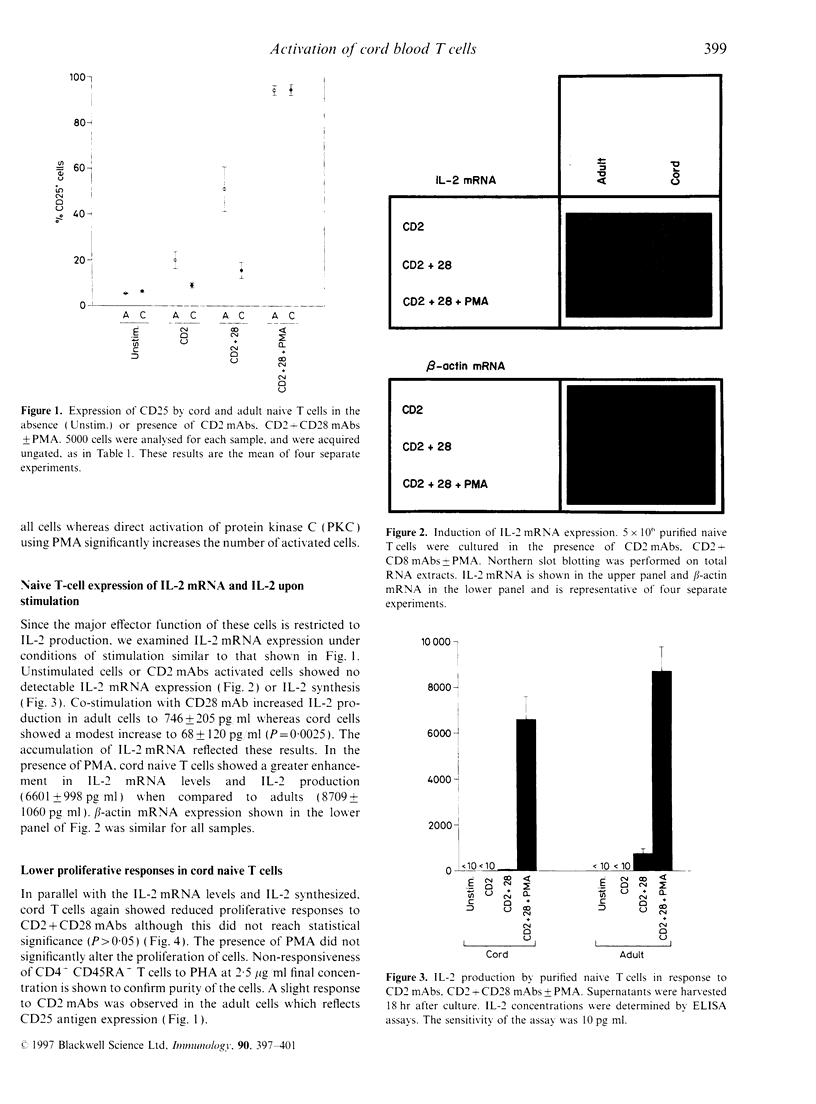
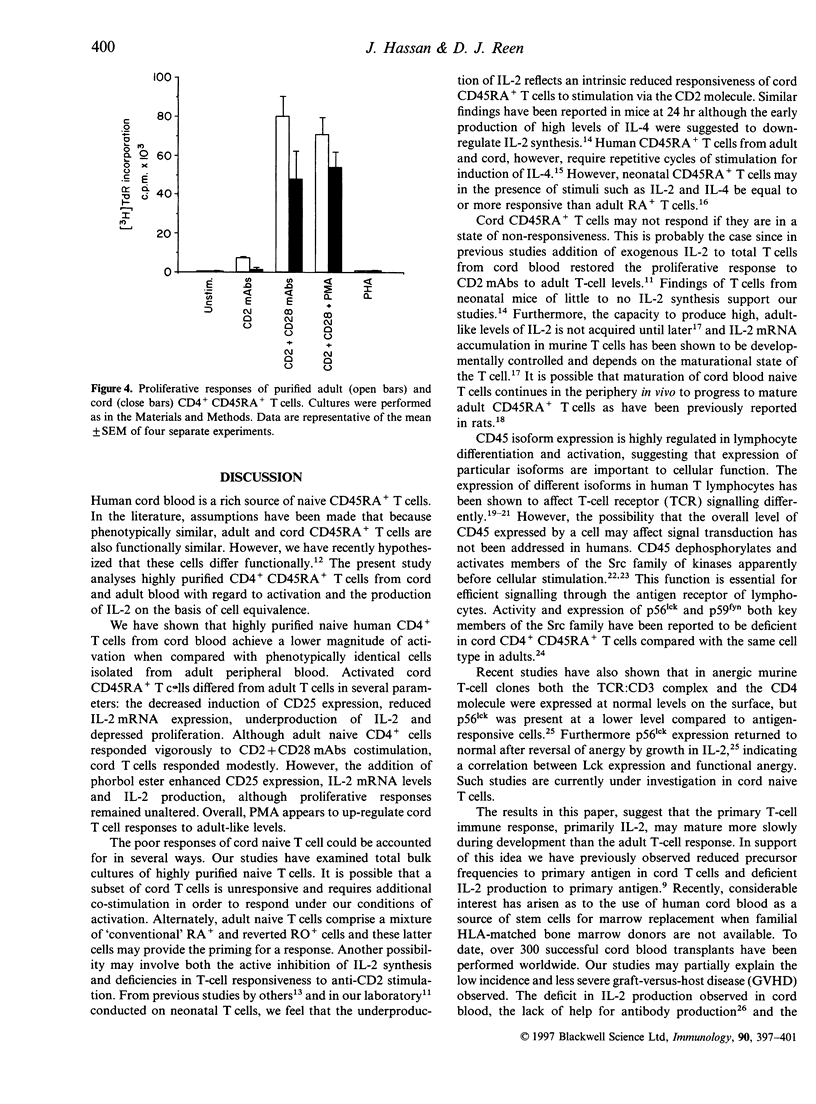
Highly purified CD4+ CD45RA+ cells from cord blood and peripheral blood from healthy adults were studied. The levels of expression of the CD2, CD3, CD4 and CD28 antigens were similar; however, CD45 and CD45RA antigen expression were slightly lower in cord cells. The reduced expression of the CD45RA antigen on cord CD4+ T cells was confirmed in whole blood. Functional assessment revealed deficiencies in cord CD4+ CD45RA+ T cells. Interleukin-2 (IL-2) production in response to specific triggering via CD2 monoclonal antibody (mAb) alone, or CD2 mAb in combination with CD28 mAb showed marked underproduction (about 10% of adult production). When CD25 expression was examined, it was observed that the proportion of activated CD4+ CD45RA+ T cells in cord blood was lower than in adult (about 20% of adult expression). Proliferation to CD2 mAbs or CD2 + 28 mAbs of cord blood native cells was similarly depressed. Investigation of IL-2 mRNA expression under these stimulatory conditions paralleled the results observed for CD25 expression, IL-2 production and proliferation. When phorbol 12-myristate 13-acetate (PMA) was added to the cells triggered with CD2 + 28mAbs, the responses examined were enhanced in both cord and adult blood with no significant differences between the groups. These findings suggest that under identical conditions of stimulation, purified cord blood CD4+ CD45RA+ T cells do not acquire similar activation status as their adult counterparts. These findings may help in understanding the reduced graft-versus-host disease (GVHD) observed in cord blood stem cell transplantation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adkins B., Ghanei A., Hamilton K. Developmental regulation of IL-4, IL-2, and IFN-gamma production by murine peripheral T lymphocytes. J Immunol. 1993 Dec 15;151(12):6617–6626. [PubMed] [Google Scholar]
- Akbar A. N., Salmon M., Ivory K., Taki S., Pilling D., Janossy G. Human CD4+CD45R0+ and CD4+CD45RA+ T cells synergize in response to alloantigens. Eur J Immunol. 1991 Oct;21(10):2517–2522. doi: 10.1002/eji.1830211031. [DOI] [PubMed] [Google Scholar]
- Cho E. A., Riley M. P., Sillman A. L., Quill H. Altered protein tyrosine phosphorylation in anergic Th1 cells. J Immunol. 1993 Jul 1;151(1):20–28. [PubMed] [Google Scholar]
- Chui D., Ong C. J., Johnson P., Teh H. S., Marth J. D. Specific CD45 isoforms differentially regulate T cell receptor signaling. EMBO J. 1994 Feb 15;13(4):798–807. doi: 10.1002/j.1460-2075.1994.tb06322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeure C. E., Yang L. P., Byun D. G., Ishihara H., Vezzio N., Delespesse G. Human naive CD4 T cells produce interleukin-4 at priming and acquire a Th2 phenotype upon repetitive stimulations in neutral conditions. Eur J Immunol. 1995 Sep;25(9):2722–2725. doi: 10.1002/eji.1830250950. [DOI] [PubMed] [Google Scholar]
- Early E. M., Reen D. J. Antigen-independent responsiveness to interleukin-4 demonstrates differential regulation of newborn human T cells. Eur J Immunol. 1996 Dec;26(12):2885–2889. doi: 10.1002/eji.1830261212. [DOI] [PubMed] [Google Scholar]
- Gerli R., Bertotto A., Crupi S., Arcangeli C., Marinelli I., Spinozzi F., Cernetti C., Angelella P., Rambotti P. Activation of cord T lymphocytes. I. Evidence for a defective T cell mitogenesis induced through the CD2 molecule. J Immunol. 1989 Apr 15;142(8):2583–2589. [PubMed] [Google Scholar]
- Gerli R., Rambotti P., Cernetti C., Velardi A., Spinozzi F. Evidence for phenotypic t precursor cells in human cord blood. Br J Haematol. 1983 Apr;53(4):685–686. doi: 10.1111/j.1365-2141.1983.tb07321.x. [DOI] [PubMed] [Google Scholar]
- Griffiths-Chu S., Patterson J. A., Berger C. L., Edelson R. L., Chu A. C. Characterization of immature T cell subpopulations in neonatal blood. Blood. 1984 Jul;64(1):296–300. [PubMed] [Google Scholar]
- Hannet I., Erkeller-Yuksel F., Lydyard P., Deneys V., DeBruyère M. Developmental and maturational changes in human blood lymphocyte subpopulations. Immunol Today. 1992 Jun;13(6):215–218. doi: 10.1016/0167-5699(92)90157-3. [DOI] [PubMed] [Google Scholar]
- Hassan J., O'Neill S., O'Neill L. A., Pattison U., Reen D. J. Signalling via CD28 of human naive neonatal T lymphocytes. Clin Exp Immunol. 1995 Oct;102(1):192–198. doi: 10.1111/j.1365-2249.1995.tb06655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan J., Reen D. J. Neonatal CD4+ CD45RA+ T cells: precursors of adult CD4+ CD45RA+ T cells? Res Immunol. 1993 Feb;144(2):87–92. doi: 10.1016/0923-2494(93)80064-6. [DOI] [PubMed] [Google Scholar]
- Hassan J., Reen D. J. Reduced primary antigen-specific T-cell precursor frequencies in neonates is associated with deficient interleukin-2 production. Immunology. 1996 Apr;87(4):604–608. doi: 10.1046/j.1365-2567.1996.476587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseinzadeh H., Goldschneider I. Recent thymic emigrants in the rat express a unique antigenic phenotype and undergo post-thymic maturation in peripheral lymphoid tissues. J Immunol. 1993 Mar 1;150(5):1670–1679. [PubMed] [Google Scholar]
- Michie C. A., McLean A., Alcock C., Beverley P. C. Lifespan of human lymphocyte subsets defined by CD45 isoforms. Nature. 1992 Nov 19;360(6401):264–265. doi: 10.1038/360264a0. [DOI] [PubMed] [Google Scholar]
- Mustelin T., Coggeshall K. M., Altman A. Rapid activation of the T-cell tyrosine protein kinase pp56lck by the CD45 phosphotyrosine phosphatase. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6302–6306. doi: 10.1073/pnas.86.16.6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustelin T., Pessa-Morikawa T., Autero M., Gassmann M., Andersson L. C., Gahmberg C. G., Burn P. Regulation of the p59fyn protein tyrosine kinase by the CD45 phosphotyrosine phosphatase. Eur J Immunol. 1992 May;22(5):1173–1178. doi: 10.1002/eji.1830220510. [DOI] [PubMed] [Google Scholar]
- Novak T. J., Farber D., Leitenberg D., Hong S. C., Johnson P., Bottomly K. Isoforms of the transmembrane tyrosine phosphatase CD45 differentially affect T cell recognition. Immunity. 1994 May;1(2):109–119. doi: 10.1016/1074-7613(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Ong C. J., Chui D., Teh H. S., Marth J. D. Thymic CD45 tyrosine phosphatase regulates apoptosis and MHC-restricted negative selection. J Immunol. 1994 Apr 15;152(8):3793–3805. [PubMed] [Google Scholar]
- Plebanski M., Saunders M., Burtles S. S., Crowe S., Hooper D. C. Primary and secondary human in vitro T-cell responses to soluble antigens are mediated by subsets bearing different CD45 isoforms. Immunology. 1992 Jan;75(1):86–91. [PMC free article] [PubMed] [Google Scholar]
- Sanders M. E., Makgoba M. W., Shaw S. Human naive and memory T cells: reinterpretation of helper-inducer and suppressor-inducer subsets. Immunol Today. 1988 Jul-Aug;9(7-8):195–199. doi: 10.1016/0167-5699(88)91212-1. [DOI] [PubMed] [Google Scholar]
- Sparshott S. M., Bell E. B. Membrane CD45R isoform exchange on CD4 T cells is rapid, frequent and dynamic in vivo. Eur J Immunol. 1994 Nov;24(11):2573–2578. doi: 10.1002/eji.1830241102. [DOI] [PubMed] [Google Scholar]
- Wesselborg S., Prüfer U., Wild M., Schraven B., Meuer S. C., Kabelitz D. Triggering via the alternative CD2 pathway induces apoptosis in activated human T lymphocytes. Eur J Immunol. 1993 Oct;23(10):2707–2710. doi: 10.1002/eji.1830231050. [DOI] [PubMed] [Google Scholar]



