Abstract
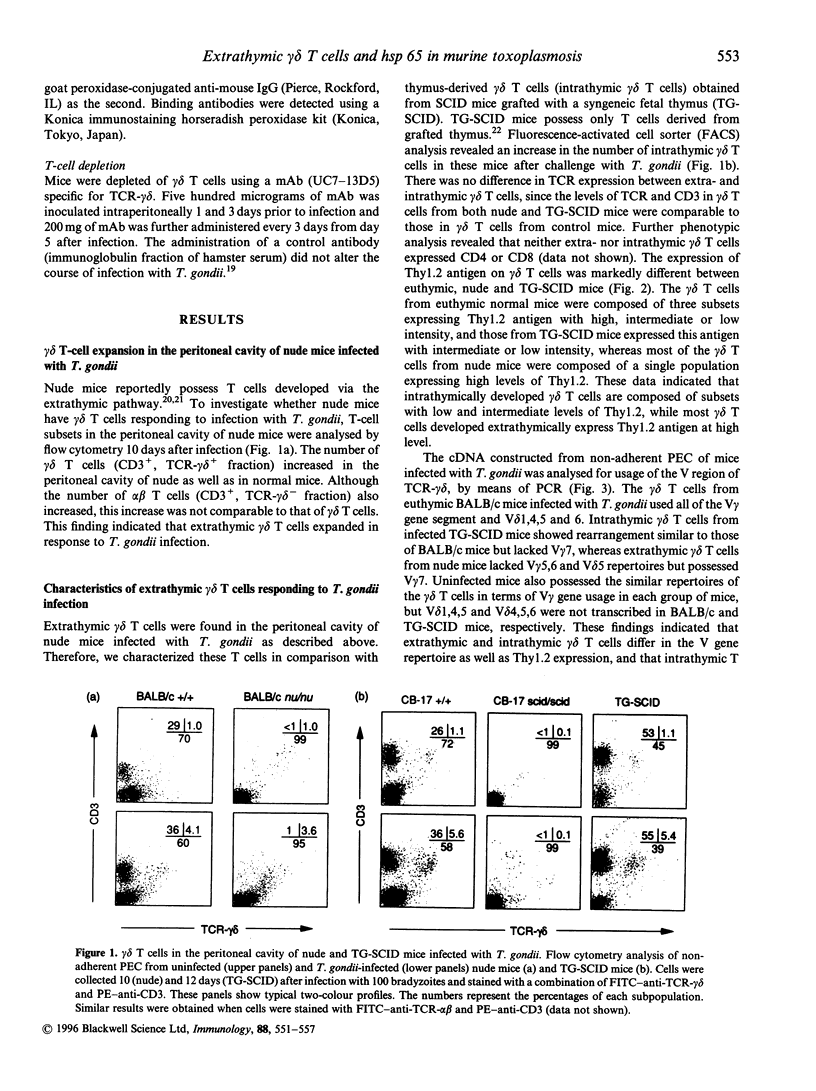
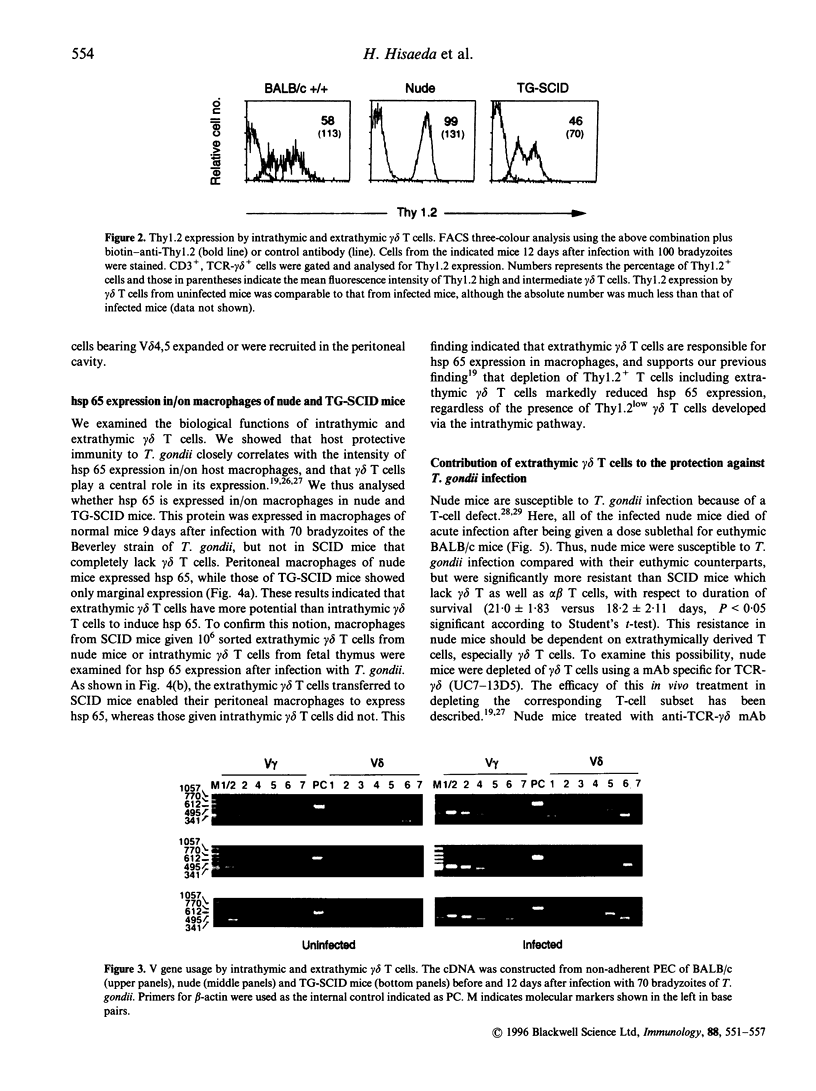
We demonstrated that gamma delta T cells contribute to protective immunity against Toxoplasma gondii by inducing the expression of a 65,000 MW heat-shock protein (hsp 65) in host macrophages. Here we examined the role of extrathymic and intrathymic gamma delta T cells in protective immunity and hsp 65 expression in mice infected with T. gondii. Intrathymic gamma delta T cells were obtained from severe combined immunodeficiency (SCID) mice grafted with syngeneic fetal thymus (TG-SCID), in which only T cells derived from the donor thymus developed, whereas extrathymic gamma delta T cells were obtained from nude mice that lack thymus. Extrathymic gamma delta T cells from T. gondii-infected nude mice differed from intrathymic gamma delta T cells of infected TG-SCID mice, in terms of Thy1.2 expression and V-region gene usage of T-cell receptor (TCR) gamma delta. Extrathymic gamma delta T cells expressed extremely high levels of Thy1.2, and had V gamma 7 repertoire but lacked V gamma 5,6 and V delta 1,5. On the other hand, intrathymic gamma delta T cells express intermediate and low levels of Thy1,2. These cells possessed V gamma 5,6 and V delta 1,5 but failed to rearrange the V gamma 7 gene. Peritoneal macrophages from infected nude mice contained hsp 65, whereas this protein was scarcely expressed in those of infected TG-SCID mice. Transfer of extrathymic, but not of intrathymic gamma delta T cells to SCID mice enabled their macrophages to express hsp 65. Athymic nude mice were significantly resistant to the infection compared with SCID mice which lack gamma delta T as well as alpha beta T cells. The resistance was dependent upon extrathymic gamma delta T cells, since nude mice depleted of gamma delta T cells using a corresponding monoclonal antibody became extremely susceptible. These results indicated that extrathymic rather than intrathymic gamma delta T cells play some crucial roles in protection against T. gondii and in hsp 65 expression.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderton S. M., van der Zee R., Goodacre J. A. Inflammation activates self hsp60-specific T cells. Eur J Immunol. 1993 Jan;23(1):33–38. doi: 10.1002/eji.1830230107. [DOI] [PubMed] [Google Scholar]
- Anderton S. M., van der Zee R., Prakken B., Noordzij A., van Eden W. Activation of T cells recognizing self 60-kD heat shock protein can protect against experimental arthritis. J Exp Med. 1995 Mar 1;181(3):943–952. doi: 10.1084/jem.181.3.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beagley K. W., Fujihashi K., Black C. A., Lagoo A. S., Yamamoto M., McGhee J. R., Kiyono H. The Mycobacterium tuberculosis 71-kDa heat-shock protein induces proliferation and cytokine secretion by murine gut intraepithelial lymphocytes. Eur J Immunol. 1993 Aug;23(8):2049–2052. doi: 10.1002/eji.1830230852. [DOI] [PubMed] [Google Scholar]
- Bonneville M., Itohara S., Krecko E. G., Mombaerts P., Ishida I., Katsuki M., Berns A., Farr A. G., Janeway C. A., Jr, Tonegawa S. Transgenic mice demonstrate that epithelial homing of gamma/delta T cells is determined by cell lineages independent of T cell receptor specificity. J Exp Med. 1990 Apr 1;171(4):1015–1026. doi: 10.1084/jem.171.4.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Paoli P., Basaglia G., Gennari D., Crovatto M., Modolo M. L., Santini G. Phenotypic profile and functional characteristics of human gamma and delta T cells during acute toxoplasmosis. J Clin Microbiol. 1992 Mar;30(3):729–731. doi: 10.1128/jcm.30.3.729-731.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denkers E. Y., Gazzinelli R. T., Martin D., Sher A. Emergence of NK1.1+ cells as effectors of IFN-gamma dependent immunity to Toxoplasma gondii in MHC class I-deficient mice. J Exp Med. 1993 Nov 1;178(5):1465–1472. doi: 10.1084/jem.178.5.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emoto M., Danbara H., Yoshikai Y. Induction of gamma/delta T cells in murine salmonellosis by an avirulent but not by a virulent strain of Salmonella choleraesuis. J Exp Med. 1992 Aug 1;176(2):363–372. doi: 10.1084/jem.176.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzinelli R., Xu Y., Hieny S., Cheever A., Sher A. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J Immunol. 1992 Jul 1;149(1):175–180. [PubMed] [Google Scholar]
- Goodman T., Lefrancois L. Intraepithelial lymphocytes. Anatomical site, not T cell receptor form, dictates phenotype and function. J Exp Med. 1989 Nov 1;170(5):1569–1581. doi: 10.1084/jem.170.5.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas W., Pereira P., Tonegawa S. Gamma/delta cells. Annu Rev Immunol. 1993;11:637–685. doi: 10.1146/annurev.iy.11.040193.003225. [DOI] [PubMed] [Google Scholar]
- Hiromatsu K., Yoshikai Y., Matsuzaki G., Ohga S., Muramori K., Matsumoto K., Bluestone J. A., Nomoto K. A protective role of gamma/delta T cells in primary infection with Listeria monocytogenes in mice. J Exp Med. 1992 Jan 1;175(1):49–56. doi: 10.1084/jem.175.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisaeda H., Nagasawa H., Maeda K., Maekawa Y., Ishikawa H., Ito Y., Good R. A., Himeno K. Gamma delta T cells play an important role in hsp65 expression and in acquiring protective immune responses against infection with Toxoplasma gondii. J Immunol. 1995 Jul 1;155(1):244–251. [PubMed] [Google Scholar]
- Kasper L. H., Khan I. A., Ely K. H., Buelow R., Boothroyd J. C. Antigen-specific (p30) mouse CD8+ T cells are cytotoxic against Toxoplasma gondii-infected peritoneal macrophages. J Immunol. 1992 Mar 1;148(5):1493–1498. [PubMed] [Google Scholar]
- Kobayashi N., Matsuzaki G., Yoshikai Y., Seki R., Ivanyi J., Nomoto K. V delta 5+ T cells of BALB/c mice recognize the murine heat shock protein 60 target cell specificity. Immunology. 1994 Feb;81(2):240–246. [PMC free article] [PubMed] [Google Scholar]
- Koga T., Wand-Württenberger A., DeBruyn J., Munk M. E., Schoel B., Kaufmann S. H. T cells against a bacterial heat shock protein recognize stressed macrophages. Science. 1989 Sep 8;245(4922):1112–1115. doi: 10.1126/science.2788923. [DOI] [PubMed] [Google Scholar]
- Lefrancois L., Goodman T. In vivo modulation of cytolytic activity and Thy-1 expression in TCR-gamma delta+ intraepithelial lymphocytes. Science. 1989 Mar 31;243(4899):1716–1718. doi: 10.1126/science.2564701. [DOI] [PubMed] [Google Scholar]
- Lindberg R. E., Frenkel J. K. Toxoplasmosis in nude mice. J Parasitol. 1977 Apr;63(2):219–221. [PubMed] [Google Scholar]
- Maeda K., Nagasawa H., Furukawa A., Hisaeda H., Maekawa Y., Manabe T., Kudo E., Good R. A., Himeno K. Development of T cells in SCID mice grafted with fetal thymus from AKR mice or F344 rats. Eur J Immunol. 1993 Dec;23(12):3151–3157. doi: 10.1002/eji.1830231217. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Teitelbaum R., Hariprashad J. Response to treatment for an intracellular infection in a T cell-deficient host: toxoplasmosis in nude mice. J Infect Dis. 1993 May;167(5):1173–1177. doi: 10.1093/infdis/167.5.1173. [DOI] [PubMed] [Google Scholar]
- Nagasawa H., Hisaeda H., Maekawa Y., Fujioka H., Ito Y., Aikawa M., Himeno K. gamma delta T cells play a crucial role in the expression of 65,000 MW heat-shock protein in mice immunized with Toxoplasma antigen. Immunology. 1994 Nov;83(3):347–352. [PMC free article] [PubMed] [Google Scholar]
- Nagasawa H., Manabe T., Maekawa Y., Oka M., Himeno K. Role of L3T4+ and Lyt-2+ T cell subsets in protective immune responses of mice against infection with a low or high virulent strain of Toxoplasma gondii. Microbiol Immunol. 1991;35(3):215–222. doi: 10.1111/j.1348-0421.1991.tb01550.x. [DOI] [PubMed] [Google Scholar]
- Nagasawa H., Oka M., Maeda K., Jian-Guo C., Hisaeda H., Ito Y., Good R. A., Himeno K. Induction of heat shock protein closely correlates with protection against Toxoplasma gondii infection. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):3155–3158. doi: 10.1073/pnas.89.7.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien R. L., Fu Y. X., Cranfill R., Dallas A., Ellis C., Reardon C., Lang J., Carding S. R., Kubo R., Born W. Heat shock protein Hsp60-reactive gamma delta cells: a large, diversified T-lymphocyte subset with highly focused specificity. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4348–4352. doi: 10.1073/pnas.89.10.4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien R. L., Happ M. P., Dallas A., Palmer E., Kubo R., Born W. K. Stimulation of a major subset of lymphocytes expressing T cell receptor gamma delta by an antigen derived from Mycobacterium tuberculosis. Cell. 1989 May 19;57(4):667–674. doi: 10.1016/0092-8674(89)90135-9. [DOI] [PubMed] [Google Scholar]
- Penninger J. M., Wen T., Timms E., Potter J., Wallace V. A., Matsuyama T., Ferrick D., Sydora B., Kronenberg M., Mak T. W. Spontaneous resistance to acute T-cell leukaemias in TCRV gamma 1.1J gamma 4C gamma 4 transgenic mice. Nature. 1995 May 18;375(6528):241–244. doi: 10.1038/375241a0. [DOI] [PubMed] [Google Scholar]
- Reilly E. B., Kranz D. M., Tonegawa S., Eisen H. N. A functional gamma gene formed from known gamma-gene segments is not necessary for antigen-specific responses of murine cytotoxic T lymphocytes. 1986 Jun 26-Jul 2Nature. 321(6073):878–880. doi: 10.1038/321878a0. [DOI] [PubMed] [Google Scholar]
- Rocha B., Vassalli P., Guy-Grand D. The extrathymic T-cell development pathway. Immunol Today. 1992 Nov;13(11):449–454. doi: 10.1016/0167-5699(92)90074-H. [DOI] [PubMed] [Google Scholar]
- Rosat J. P., MacDonald H. R., Louis J. A. A role for gamma delta + T cells during experimental infection of mice with Leishmania major. J Immunol. 1993 Jan 15;150(2):550–555. [PubMed] [Google Scholar]
- Sandor M., Sperling A. I., Cook G. A., Weinstock J. V., Lynch R. G., Bluestone J. A. Two waves of gamma delta T cells expressing different V delta genes are recruited into schistosome-induced liver granulomas. J Immunol. 1995 Jul 1;155(1):275–284. [PubMed] [Google Scholar]
- Scalise F., Gerli R., Castellucci G., Spinozzi F., Fabietti G. M., Crupi S., Sensi L., Britta R., Vaccaro R., Bertotto A. Lymphocytes bearing the gamma delta T-cell receptor in acute toxoplasmosis. Immunology. 1992 Aug;76(4):668–670. [PMC free article] [PubMed] [Google Scholar]
- Sher A., Oswald I. P., Hieny S., Gazzinelli R. T. Toxoplasma gondii induces a T-independent IFN-gamma response in natural killer cells that requires both adherent accessory cells and tumor necrosis factor-alpha. J Immunol. 1993 May 1;150(9):3982–3989. [PubMed] [Google Scholar]
- Speiser D. E., Stübi U., Zinkernagel R. M. Extrathymic positive selection of alpha beta T-cell precursors in nude mice. Nature. 1992 Jan 9;355(6356):170–172. doi: 10.1038/355170a0. [DOI] [PubMed] [Google Scholar]
- Strominger J. L. The gamma delta T cell receptor and class Ib MHC-related proteins: enigmatic molecules of immune recognition. Cell. 1989 Jun 16;57(6):895–898. doi: 10.1016/0092-8674(89)90326-7. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Morita C. T., Tanaka Y., Nieves E., Brenner M. B., Bloom B. R. Natural and synthetic non-peptide antigens recognized by human gamma delta T cells. Nature. 1995 May 11;375(6527):155–158. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- Yasutomo K., Maeda K., Nagata S., Nagasawa H., Okada K., Good R. A., Kuroda Y., Himeno K. Defective T cells from gld mice play a pivotal role in development of Thy-1.2+B220+ cells and autoimmunity. J Immunol. 1994 Dec 15;153(12):5855–5864. [PubMed] [Google Scholar]





