Abstract
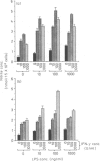
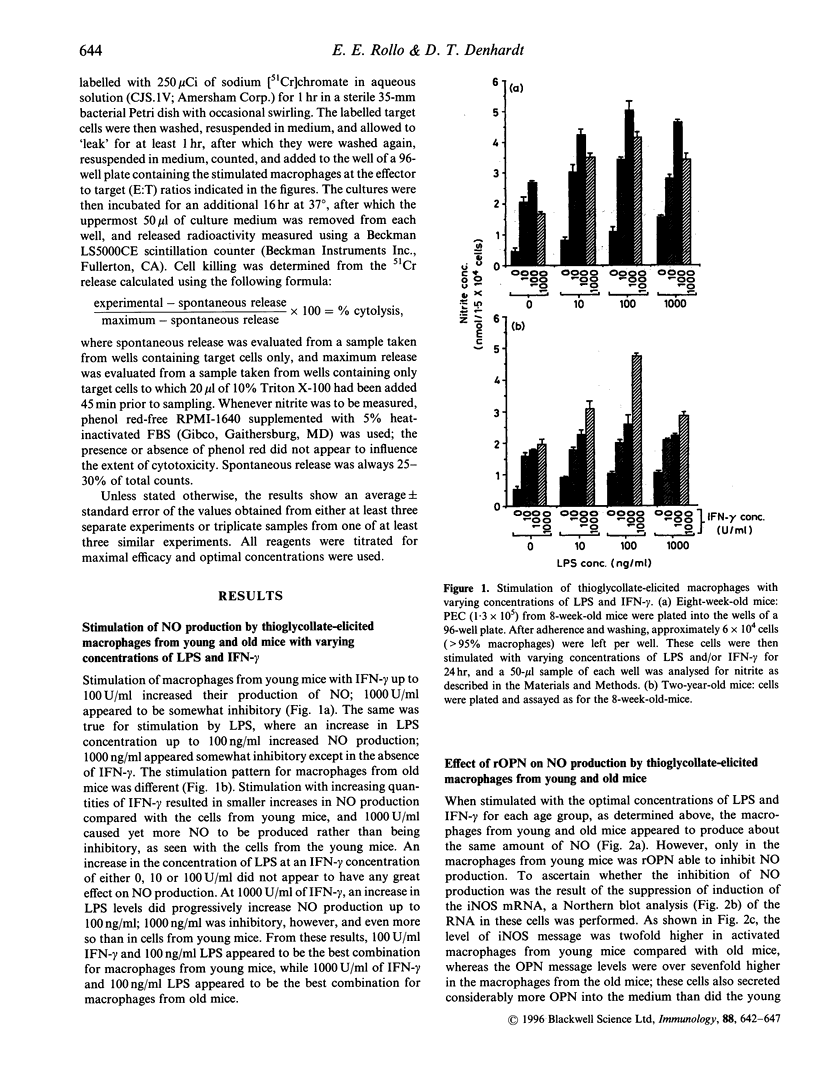
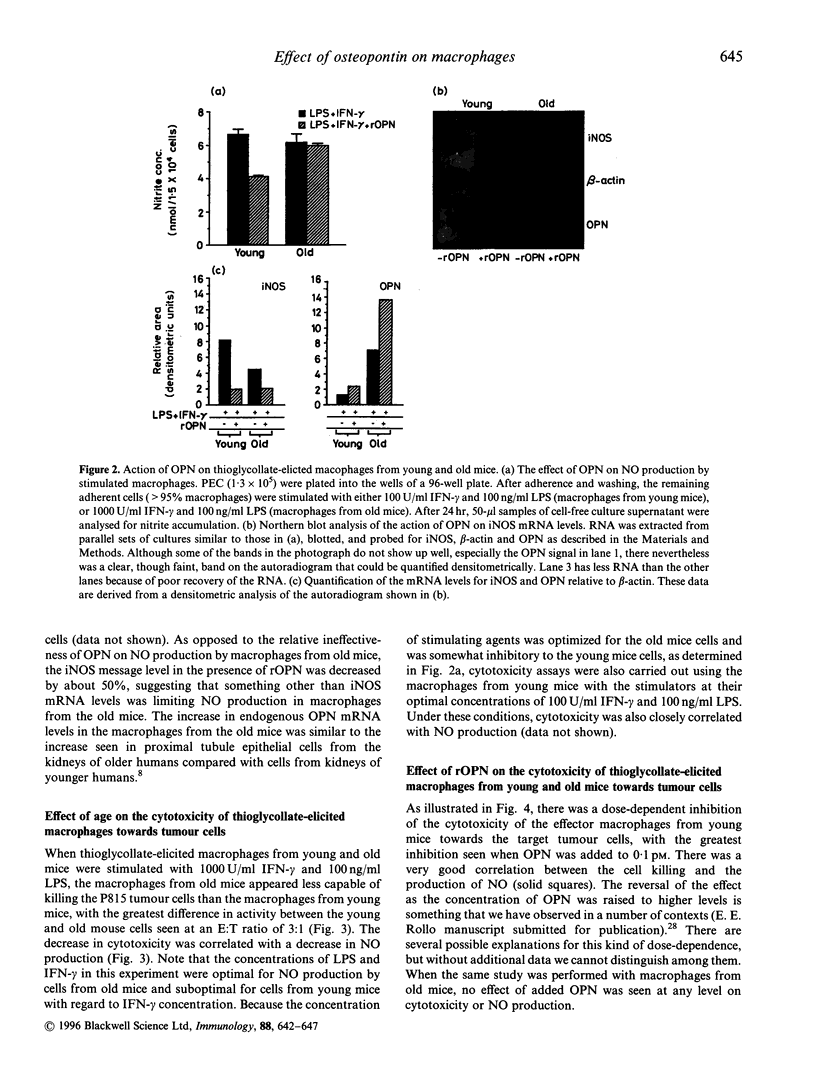
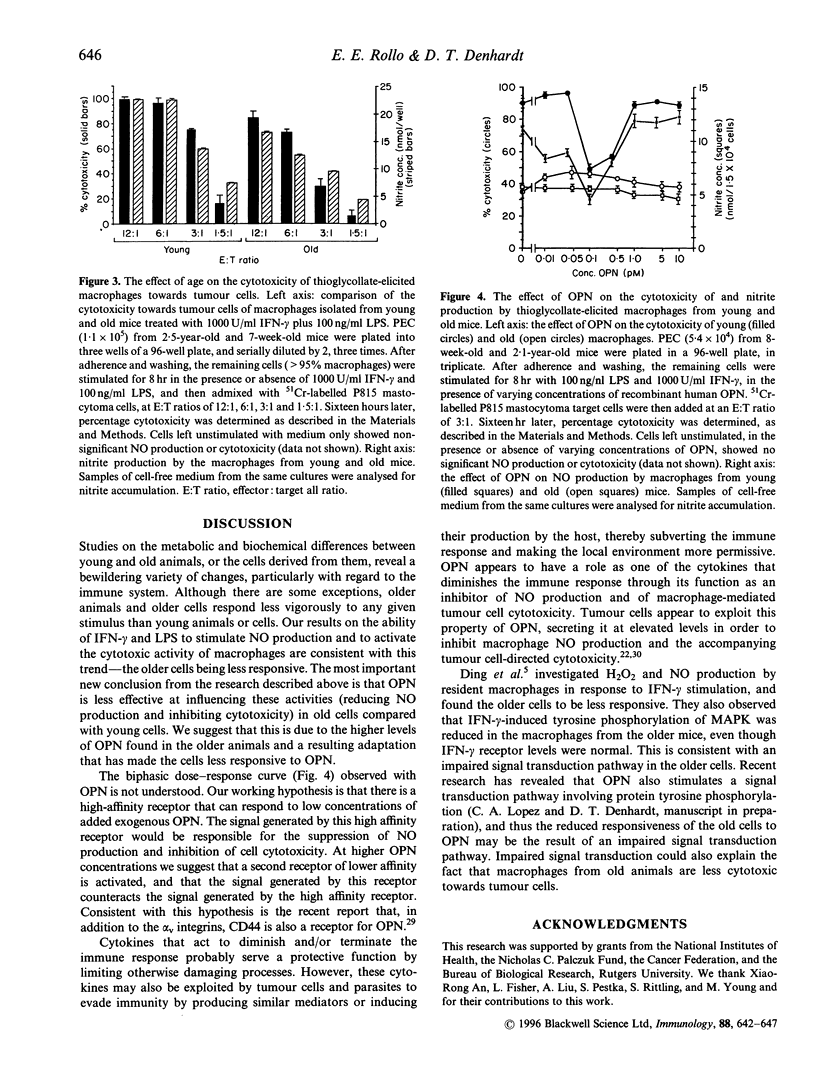
Osteopontin (OPN) is a secreted phosphoprotein found in body fluids (e.g. plasma, urine, milk) and in mineralized tissues. Its expression is increased in many transformed cells and in normal cells exposed to various cytokines. When stimulated with the inflammatory mediators lipopolysaccharide and interferon-gamma, mouse macrophages secrete nitric oxide (NO) as a cytotoxic agent effective against microbial invaders and tumour cells. This report documents (1) that thioglycollate-elicited peritoneal macrophages, activated with the inflammatory mediators, produced less NO and exhibited reduced cytotoxicity towards target cells when they were obtained from old animals than when they were obtained from young animals; and (2) that OPN was able to inhibit both the induced NO synthesis and cytotoxicity, but more effectively in macrophages from the young animals than those from the old animals. This may be due to the observed higher level of OPN expression in macrophages from old animals.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O. Macrophages. Methods Enzymol. 1979;58:494–505. doi: 10.1016/s0076-6879(79)58164-6. [DOI] [PubMed] [Google Scholar]
- Ames B. N., Shigenaga M. K., Hagen T. M. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrend E. I., Craig A. M., Wilson S. M., Denhardt D. T., Chambers A. F. Reduced malignancy of ras-transformed NIH 3T3 cells expressing antisense osteopontin RNA. Cancer Res. 1994 Feb 1;54(3):832–837. [PubMed] [Google Scholar]
- Craig A. M., Bowden G. T., Chambers A. F., Spearman M. A., Greenberg A. H., Wright J. A., McLeod M., Denhardt D. T. Secreted phosphoprotein mRNA is induced during multi-stage carcinogenesis in mouse skin and correlates with the metastatic potential of murine fibroblasts. Int J Cancer. 1990 Jul 15;46(1):133–137. doi: 10.1002/ijc.2910460124. [DOI] [PubMed] [Google Scholar]
- Craig A. M., Smith J. H., Denhardt D. T. Osteopontin, a transformation-associated cell adhesion phosphoprotein, is induced by 12-O-tetradecanoylphorbol 13-acetate in mouse epidermis. J Biol Chem. 1989 Jun 5;264(16):9682–9689. [PubMed] [Google Scholar]
- Denhardt D. T., Chambers A. F. Overcoming obstacles to metastasis--defenses against host defenses: osteopontin (OPN) as a shield against attack by cytotoxic host cells. J Cell Biochem. 1994 Sep;56(1):48–51. doi: 10.1002/jcb.240560109. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T., Guo X. Osteopontin: a protein with diverse functions. FASEB J. 1993 Dec;7(15):1475–1482. [PubMed] [Google Scholar]
- Denhardt D. T., Lopez C. A., Rollo E. E., Hwang S. M., An X. R., Walther S. E. Osteopontin-induced modifications of cellular functions. Ann N Y Acad Sci. 1995 Apr 21;760:127–142. doi: 10.1111/j.1749-6632.1995.tb44625.x. [DOI] [PubMed] [Google Scholar]
- Ding A., Hwang S., Schwab R. Effect of aging on murine macrophages. Diminished response to IFN-gamma for enhanced oxidative metabolism. J Immunol. 1994 Sep 1;153(5):2146–2152. [PubMed] [Google Scholar]
- Feng B., Rollo E. E., Denhardt D. T. Osteopontin (OPN) may facilitate metastasis by protecting cells from macrophage NO-mediated cytotoxicity: evidence from cell lines down-regulated for OPN expression by a targeted ribozyme. Clin Exp Metastasis. 1995 Nov;13(6):453–462. doi: 10.1007/BF00118184. [DOI] [PubMed] [Google Scholar]
- Flurkey K., Stadecker M., Miller R. A. Memory T lymphocyte hyporesponsiveness to non-cognate stimuli: a key factor in age-related immunodeficiency. Eur J Immunol. 1992 Apr;22(4):931–935. doi: 10.1002/eji.1830220408. [DOI] [PubMed] [Google Scholar]
- Giachelli C., Bae N., Lombardi D., Majesky M., Schwartz S. Molecular cloning and characterization of 2B7, a rat mRNA which distinguishes smooth muscle cell phenotypes in vitro and is identical to osteopontin (secreted phosphoprotein I, 2aR). Biochem Biophys Res Commun. 1991 Jun 14;177(2):867–873. doi: 10.1016/0006-291x(91)91870-i. [DOI] [PubMed] [Google Scholar]
- Hobbs M. V., Ernst D. N., Torbett B. E., Glasebrook A. L., Rehse M. A., McQuitty D. N., Thoman M. L., Bottomly K., Rothermel A. L., Noonan D. J. Cell proliferation and cytokine production by CD4+ cells from old mice. J Cell Biochem. 1991 Aug;46(4):312–320. doi: 10.1002/jcb.240460406. [DOI] [PubMed] [Google Scholar]
- Hobbs M. V., Weigle W. O., Noonan D. J., Torbett B. E., McEvilly R. J., Koch R. J., Cardenas G. J., Ernst D. N. Patterns of cytokine gene expression by CD4+ T cells from young and old mice. J Immunol. 1993 Apr 15;150(8 Pt 1):3602–3614. [PubMed] [Google Scholar]
- Hwang S. M., Wilson P. D., Laskin J. D., Denhardt D. T. Age and development-related changes in osteopontin and nitric oxide synthase mRNA levels in human kidney proximal tubule epithelial cells: contrasting responses to hypoxia and reoxygenation. J Cell Physiol. 1994 Jul;160(1):61–68. doi: 10.1002/jcp.1041600108. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Fukuto J. M., Griscavage J. M., Rogers N. E., Byrns R. E. Oxidation of nitric oxide in aqueous solution to nitrite but not nitrate: comparison with enzymatically formed nitric oxide from L-arginine. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8103–8107. doi: 10.1073/pnas.90.17.8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez C. A., Hoyer J. R., Wilson P. D., Waterhouse P., Denhardt D. T. Heterogeneity of osteopontin expression among nephrons in mouse kidneys and enhanced expression in sclerotic glomeruli. Lab Invest. 1993 Sep;69(3):355–363. [PubMed] [Google Scholar]
- Lorsbach R. B., Murphy W. J., Lowenstein C. J., Snyder S. H., Russell S. W. Expression of the nitric oxide synthase gene in mouse macrophages activated for tumor cell killing. Molecular basis for the synergy between interferon-gamma and lipopolysaccharide. J Biol Chem. 1993 Jan 25;268(3):1908–1913. [PubMed] [Google Scholar]
- Lyons C. R., Orloff G. J., Cunningham J. M. Molecular cloning and functional expression of an inducible nitric oxide synthase from a murine macrophage cell line. J Biol Chem. 1992 Mar 25;267(9):6370–6374. [PubMed] [Google Scholar]
- Miyazaki Y., Setoguchi M., Yoshida S., Higuchi Y., Akizuki S., Yamamoto S. The mouse osteopontin gene. Expression in monocytic lineages and complete nucleotide sequence. J Biol Chem. 1990 Aug 25;265(24):14432–14438. [PubMed] [Google Scholar]
- Nemir M., DeVouge M. W., Mukherjee B. B. Normal rat kidney cells secrete both phosphorylated and nonphosphorylated forms of osteopontin showing different physiological properties. J Biol Chem. 1989 Oct 25;264(30):18202–18208. [PubMed] [Google Scholar]
- Patarca R., Freeman G. J., Singh R. P., Wei F. Y., Durfee T., Blattner F., Regnier D. C., Kozak C. A., Mock B. A., Morse H. C., 3rd Structural and functional studies of the early T lymphocyte activation 1 (Eta-1) gene. Definition of a novel T cell-dependent response associated with genetic resistance to bacterial infection. J Exp Med. 1989 Jul 1;170(1):145–161. doi: 10.1084/jem.170.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack S. B., Linnemeyer P. A., Gill S. Induction of osteopontin mRNA expression during activation of murine NK cells. J Leukoc Biol. 1994 Mar;55(3):398–400. doi: 10.1002/jlb.55.3.398. [DOI] [PubMed] [Google Scholar]
- Senger D. R., Perruzzi C. A., Gracey C. F., Papadopoulos A., Tenen D. G. Secreted phosphoproteins associated with neoplastic transformation: close homology with plasma proteins cleaved during blood coagulation. Cancer Res. 1988 Oct 15;48(20):5770–5774. [PubMed] [Google Scholar]
- Senger D. R., Perruzzi C. A., Papadopoulos A. Elevated expression of secreted phosphoprotein I (osteopontin, 2ar) as a consequence of neoplastic transformation. Anticancer Res. 1989 Sep-Oct;9(5):1291–1299. [PubMed] [Google Scholar]
- Shigenaga M. K., Hagen T. M., Ames B. N. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci U S A. 1994 Nov 8;91(23):10771–10778. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R. P., Patarca R., Schwartz J., Singh P., Cantor H. Definition of a specific interaction between the early T lymphocyte activation 1 (Eta-1) protein and murine macrophages in vitro and its effect upon macrophages in vivo. J Exp Med. 1990 Jun 1;171(6):1931–1942. doi: 10.1084/jem.171.6.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber G. F., Ashkar S., Glimcher M. J., Cantor H. Receptor-ligand interaction between CD44 and osteopontin (Eta-1). Science. 1996 Jan 26;271(5248):509–512. doi: 10.1126/science.271.5248.509. [DOI] [PubMed] [Google Scholar]