Abstract
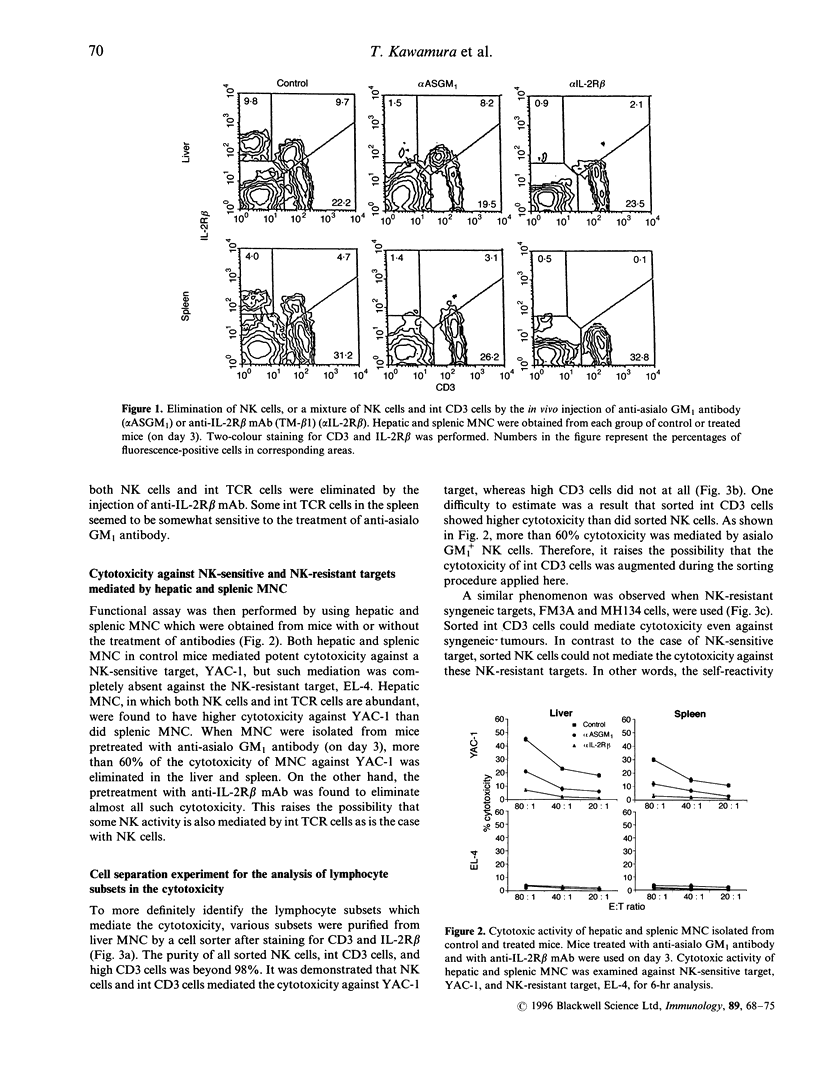
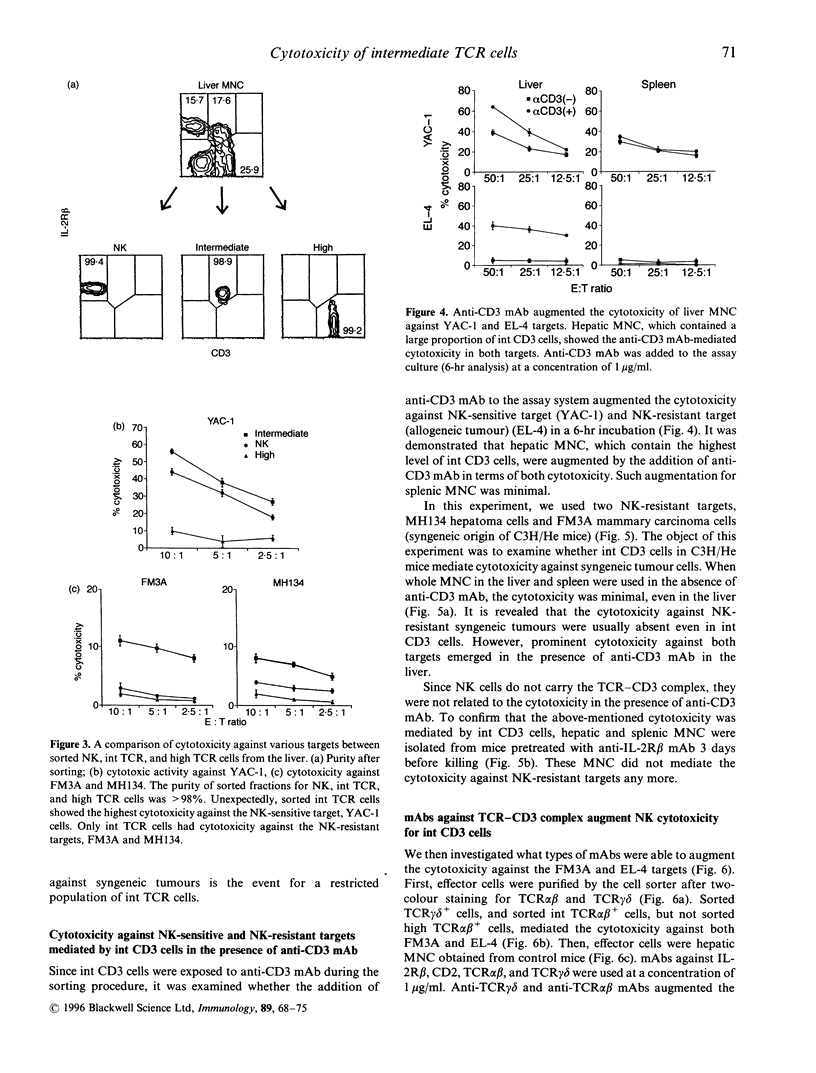
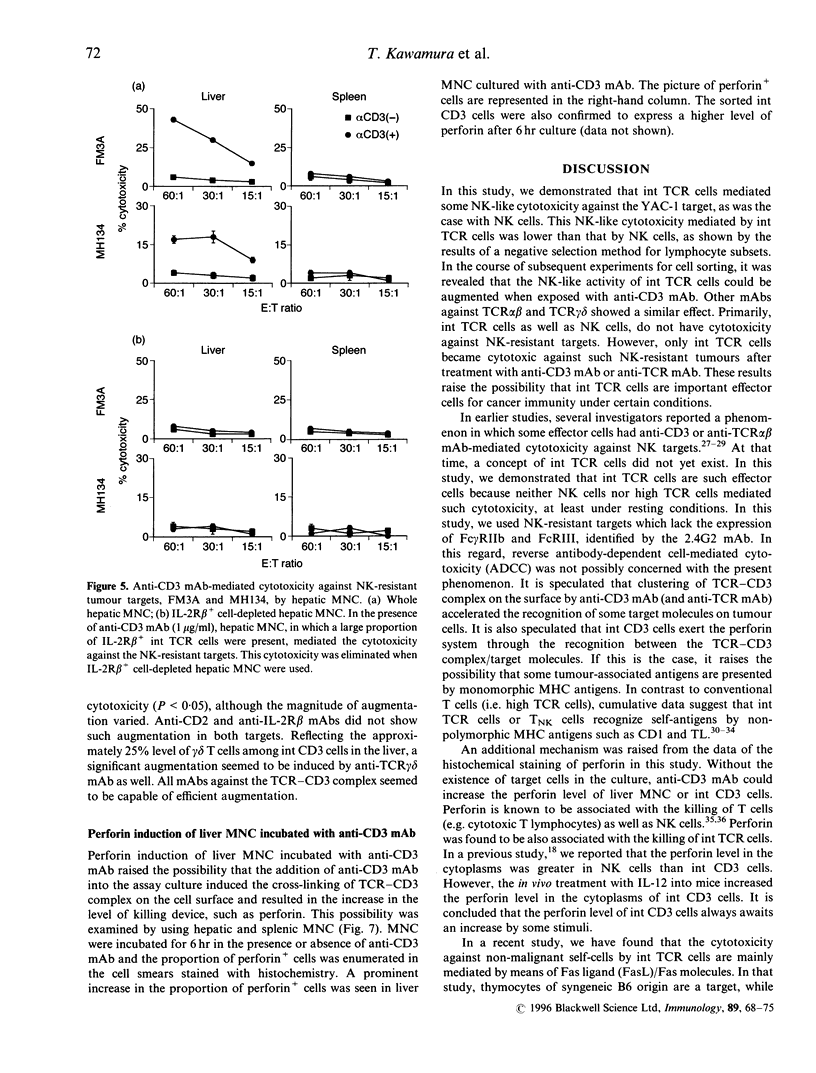
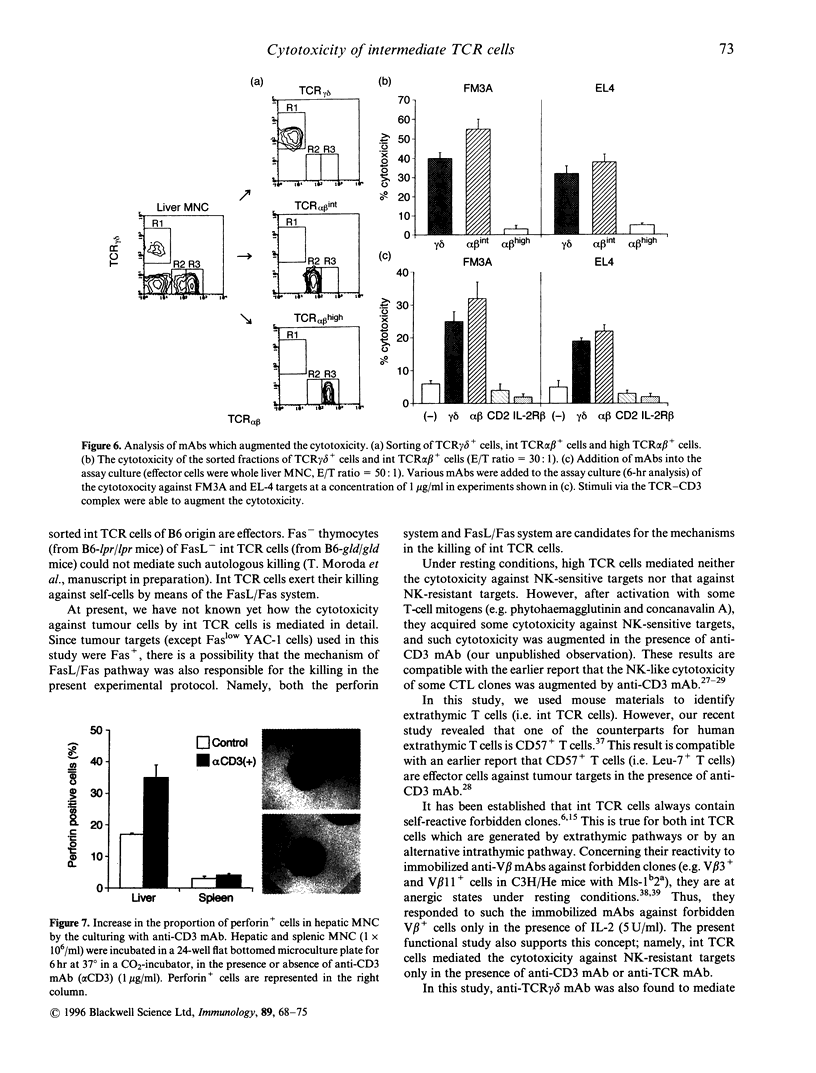
Morphological and phenotypic characterization in previous studies has indicated that intermediate (int) T-cell receptor (TCR) cells or T natural killer (TNK) cells may stand at an intermediate position between NK cells and high TCR cells of thymic origin in phylogenetic development. In this study, a functional study on cytotoxic activity against various tumour targets was performed in each purified subset. When a negative selection method entailing in vivo injection of anti-asialo GM, antibody or anti-interleukin (IL)-2R beta monoclonal antibody (mAb) was applied, IL-2R beta 1 CD3 NK cells were found to have the highest NK activity while IL-2R beta 1 int CD3 (or TCR) cells had a lower level of the NK activity. High CD3 cells (freshly isolated) did not have any such activity. Sorting experiments further revealed that the NK function mediated by int CD3 cells was augmented when they were exposed to anti-CD3 mAb. anti-TCR alpha beta, or anti-TCR-delta mAb. This phenomenon was not observed in NK cells and high CD3 cells. More importantly, when anti-CD3 mAb (or anti-TCR mAb) was added to the assay culture, int CD3 cells became cytotoxic against even NK-resistant tumour (Fc gamma R-. Fas+) targets. Liver mononuclear cells or int CD3 cells exposed to anti-CD3 mAb for 6 hr showed an elevated level of perforin in their cytoplasms. The present results suggest that int CD3 cells are usually non-cytotoxic against various tumours but become functional after being stimulated via the TCR CD3 complex.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo T., Ohteki T., Seki S., Koyamada N., Yoshikai Y., Masuda T., Rikiishi H., Kumagai K. The appearance of T cells bearing self-reactive T cell receptor in the livers of mice injected with bacteria. J Exp Med. 1991 Aug 1;174(2):417–424. doi: 10.1084/jem.174.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arase H., Arase N., Kobayashi Y., Nishimura Y., Yonehara S., Onoé K. Cytotoxicity of fresh NK1.1+ T cell receptor alpha/beta+ thymocytes against a CD4+8+ thymocyte population associated with intact Fas antigen expression on the target. J Exp Med. 1994 Aug 1;180(2):423–432. doi: 10.1084/jem.180.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bix M., Coles M., Raulet D. Positive selection of V beta 8+ CD4-8- thymocytes by class I molecules expressed by hematopoietic cells. J Exp Med. 1993 Sep 1;178(3):901–908. doi: 10.1084/jem.178.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budd R. C., Miescher G. C., Howe R. C., Lees R. K., Bron C., MacDonald H. R. Developmentally regulated expression of T cell receptor beta chain variable domains in immature thymocytes. J Exp Med. 1987 Aug 1;166(2):577–582. doi: 10.1084/jem.166.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles M. C., Raulet D. H. Class I dependence of the development of CD4+ CD8- NK1.1+ thymocytes. J Exp Med. 1994 Jul 1;180(1):395–399. doi: 10.1084/jem.180.1.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crispe I. N., Moore M. W., Husmann L. A., Smith L., Bevan M. J., Shimonkevitz R. P. Differentiation potential of subsets of CD4-8- thymocytes. Nature. 1987 Sep 24;329(6137):336–339. doi: 10.1038/329336a0. [DOI] [PubMed] [Google Scholar]
- Egerton M., Scollay R. Intrathymic selection of murine TCR alpha beta+CD4-CD8- thymocytes. Int Immunol. 1990;2(2):157–163. doi: 10.1093/intimm/2.2.157. [DOI] [PubMed] [Google Scholar]
- Finkel T. H., Cambier J. C., Kubo R. T., Born W. K., Marrack P., Kappler J. W. The thymus has two functionally distinct populations of immature alpha beta + T cells: one population is deleted by ligation of alpha beta TCR. Cell. 1989 Sep 22;58(6):1047–1054. doi: 10.1016/0092-8674(89)90503-5. [DOI] [PubMed] [Google Scholar]
- Fowlkes B. J., Kruisbeek A. M., Ton-That H., Weston M. A., Coligan J. E., Schwartz R. H., Pardoll D. M. A novel population of T-cell receptor alpha beta-bearing thymocytes which predominantly expresses a single V beta gene family. Nature. 1987 Sep 17;329(6136):251–254. doi: 10.1038/329251a0. [DOI] [PubMed] [Google Scholar]
- Goossens P. L., Jouin H., Marchal G., Milon G. Isolation and flow cytometric analysis of the free lymphomyeloid cells present in murine liver. J Immunol Methods. 1990 Aug 28;132(1):137–144. doi: 10.1016/0022-1759(90)90407-m. [DOI] [PubMed] [Google Scholar]
- Hashimoto W., Takeda K., Anzai R., Ogasawara K., Sakihara H., Sugiura K., Seki S., Kumagai K. Cytotoxic NK1.1 Ag+ alpha beta T cells with intermediate TCR induced in the liver of mice by IL-12. J Immunol. 1995 May 1;154(9):4333–4340. [PubMed] [Google Scholar]
- Iiai T., Watanabe H., Iwamoto T., Nakashima I., Abo T. Predominant activation of extrathymic T cells during melanoma development of metallothionein/ret transgenic mice. Cell Immunol. 1994 Feb;153(2):412–427. doi: 10.1006/cimm.1994.1039. [DOI] [PubMed] [Google Scholar]
- Iiai T., Watanabe H., Seki S., Sugiura K., Hirokawa K., Utsuyama M., Takahashi-Iwanaga H., Iwanaga T., Ohteki T., Abo T. Ontogeny and development of extrathymic T cells in mouse liver. Immunology. 1992 Dec;77(4):556–563. [PMC free article] [PubMed] [Google Scholar]
- Itoh H., Abo T., Sugawara S., Kanno A., Kumagai K. Age-related variation in the proportion and activity of murine liver natural killer cells and their cytotoxicity against regenerating hepatocytes. J Immunol. 1988 Jul 1;141(1):315–323. [PubMed] [Google Scholar]
- Kawachi Y., Watanabe H., Moroda T., Haga M., Iiai T., Hatakeyama K., Abo T. Self-reactive T cell clones in a restricted population of interleukin-2 receptor beta+ cells expressing intermediate levels of the T cell receptor in the liver and other immune organs. Eur J Immunol. 1995 Aug;25(8):2272–2278. doi: 10.1002/eji.1830250824. [DOI] [PubMed] [Google Scholar]
- Kawasaki A., Shinkai Y., Yagita H., Okumura K. Expression of perforin in murine natural killer cells and cytotoxic T lymphocytes in vivo. Eur J Immunol. 1992 May;22(5):1215–1219. doi: 10.1002/eji.1830220516. [DOI] [PubMed] [Google Scholar]
- Kisielow P., Blüthmann H., Staerz U. D., Steinmetz M., von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988 Jun 23;333(6175):742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- Kägi D., Ledermann B., Bürki K., Seiler P., Odermatt B., Olsen K. J., Podack E. R., Zinkernagel R. M., Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994 May 5;369(6475):31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- Kägi D., Vignaux F., Ledermann B., Bürki K., Depraetere V., Nagata S., Hengartner H., Golstein P. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science. 1994 Jul 22;265(5171):528–530. doi: 10.1126/science.7518614. [DOI] [PubMed] [Google Scholar]
- Levitsky H. I., Golumbek P. T., Pardoll D. M. The fate of CD4-8- T cell receptor-alpha beta+ thymocytes. J Immunol. 1991 Feb 15;146(4):1113–1117. [PubMed] [Google Scholar]
- Ohteki T., Okuyama R., Seki S., Abo T., Sugiura K., Kusumi A., Ohmori T., Watanabe H., Kumagai K. Age-dependent increase of extrathymic T cells in the liver and their appearance in the periphery of older mice. J Immunol. 1992 Sep 1;149(5):1562–1570. [PubMed] [Google Scholar]
- Ohteki T., Seki S., Abo T., Kumagai K. Liver is a possible site for the proliferation of abnormal CD3+4-8- double-negative lymphocytes in autoimmune MRL-lpr/lpr mice. J Exp Med. 1990 Jul 1;172(1):7–12. doi: 10.1084/jem.172.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka K., Iiai T., Watanabe H., Tanaka T., Miyasaka M., Sato K., Asakura H., Abo T. Similarities and differences between extrathymic T cells residing in mouse liver and intestine. Cell Immunol. 1994 Jan;153(1):52–66. doi: 10.1006/cimm.1994.1005. [DOI] [PubMed] [Google Scholar]
- Okada T., Iiai T., Kawachi Y., Moroda T., Takii Y., Hatakeyama K., Abo T. Origin of CD57+ T cells which increase at tumour sites in patients with colorectal cancer. Clin Exp Immunol. 1995 Oct;102(1):159–166. doi: 10.1111/j.1365-2249.1995.tb06650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papiernik M., Pontoux C. In vivo and in vitro repertoire of CD3+CD4-CD8- thymocytes. Int Immunol. 1990;2(5):407–412. doi: 10.1093/intimm/2.5.407. [DOI] [PubMed] [Google Scholar]
- Phillips J. H., Lanier L. L. Lectin-dependent and anti-CD3 induced cytotoxicity are preferentially mediated by peripheral blood cytotoxic T lymphocytes expressing Leu-7 antigen. J Immunol. 1986 Mar 1;136(5):1579–1585. [PubMed] [Google Scholar]
- Porcelli S., Morita C. T., Brenner M. B. CD1b restricts the response of human CD4-8- T lymphocytes to a microbial antigen. Nature. 1992 Dec 10;360(6404):593–597. doi: 10.1038/360593a0. [DOI] [PubMed] [Google Scholar]
- Sato K., Ohtsuka K., Watanabe H., Asakura H., Abo T. Detailed characterization of gamma delta T cells within the organs in mice: classification into three groups. Immunology. 1993 Nov;80(3):380–387. [PMC free article] [PubMed] [Google Scholar]
- Siliciano R. F., Pratt J. C., Schmidt R. E., Ritz J., Reinherz E. L. Activation of cytolytic T lymphocyte and natural killer cell function through the T11 sheep erythrocyte binding protein. Nature. 1985 Oct 3;317(6036):428–430. doi: 10.1038/317428a0. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Williams G. T., Kingston R., Jenkinson E. J., Owen J. J. Antibodies to CD3/T-cell receptor complex induce death by apoptosis in immature T cells in thymic cultures. Nature. 1989 Jan 12;337(6203):181–184. doi: 10.1038/337181a0. [DOI] [PubMed] [Google Scholar]
- Takahama Y., Kosugi A., Singer A. Phenotype, ontogeny, and repertoire of CD4-CD8- T cell receptor alpha beta + thymocytes. Variable influence of self-antigens on T cell receptor V beta usage. J Immunol. 1991 Feb 15;146(4):1134–1141. [PubMed] [Google Scholar]
- Tanaka T., Tsudo M., Karasuyama H., Kitamura F., Kono T., Hatakeyama M., Taniguchi T., Miyasaka M. A novel monoclonal antibody against murine IL-2 receptor beta-chain. Characterization of receptor expression in normal lymphoid cells and EL-4 cells. J Immunol. 1991 Oct 1;147(7):2222–2228. [PubMed] [Google Scholar]
- Teh H. S., Kisielow P., Scott B., Kishi H., Uematsu Y., Blüthmann H., von Boehmer H. Thymic major histocompatibility complex antigens and the alpha beta T-cell receptor determine the CD4/CD8 phenotype of T cells. Nature. 1988 Sep 15;335(6187):229–233. doi: 10.1038/335229a0. [DOI] [PubMed] [Google Scholar]
- Tsuchida M., Iiai T., Watanabe H., Abo T. Relative resistance of intermediate TCR cells to anti-CD3 mAb in mice in vivo and their partial functional characterization. Cell Immunol. 1992 Nov;145(1):78–90. doi: 10.1016/0008-8749(92)90314-f. [DOI] [PubMed] [Google Scholar]
- Watanabe H., Miyaji C., Kawachi Y., Iiai T., Ohtsuka K., Iwanage T., Takahashi-Iwanaga H., Abo T. Relationships between intermediate TCR cells and NK1.1+ T cells in various immune organs. NK1.1+ T cells are present within a population of intermediate TCR cells. J Immunol. 1995 Sep 15;155(6):2972–2983. [PubMed] [Google Scholar]
- Watanabe H., Ohtsuka K., Kimura M., Ikarashi Y., Ohmori K., Kusumi A., Ohteki T., Seki S., Abo T. Details of an isolation method for hepatic lymphocytes in mice. J Immunol Methods. 1992 Feb 5;146(2):145–154. doi: 10.1016/0022-1759(92)90223-g. [DOI] [PubMed] [Google Scholar]



