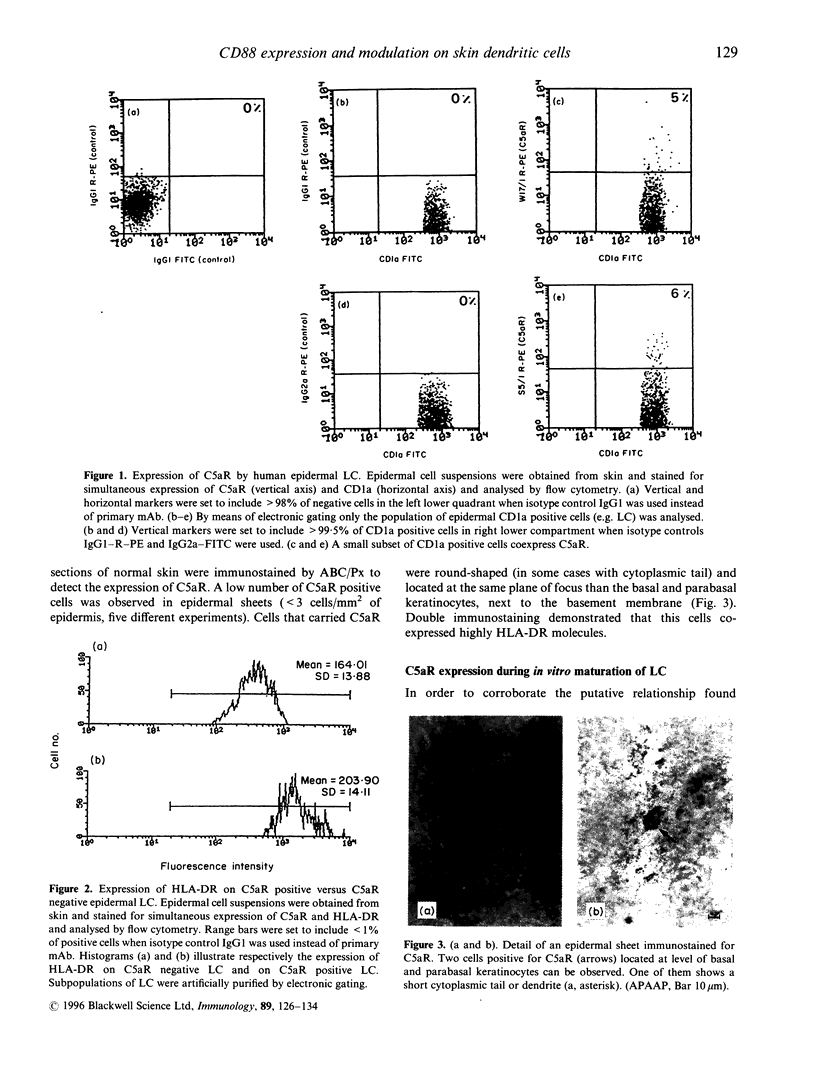
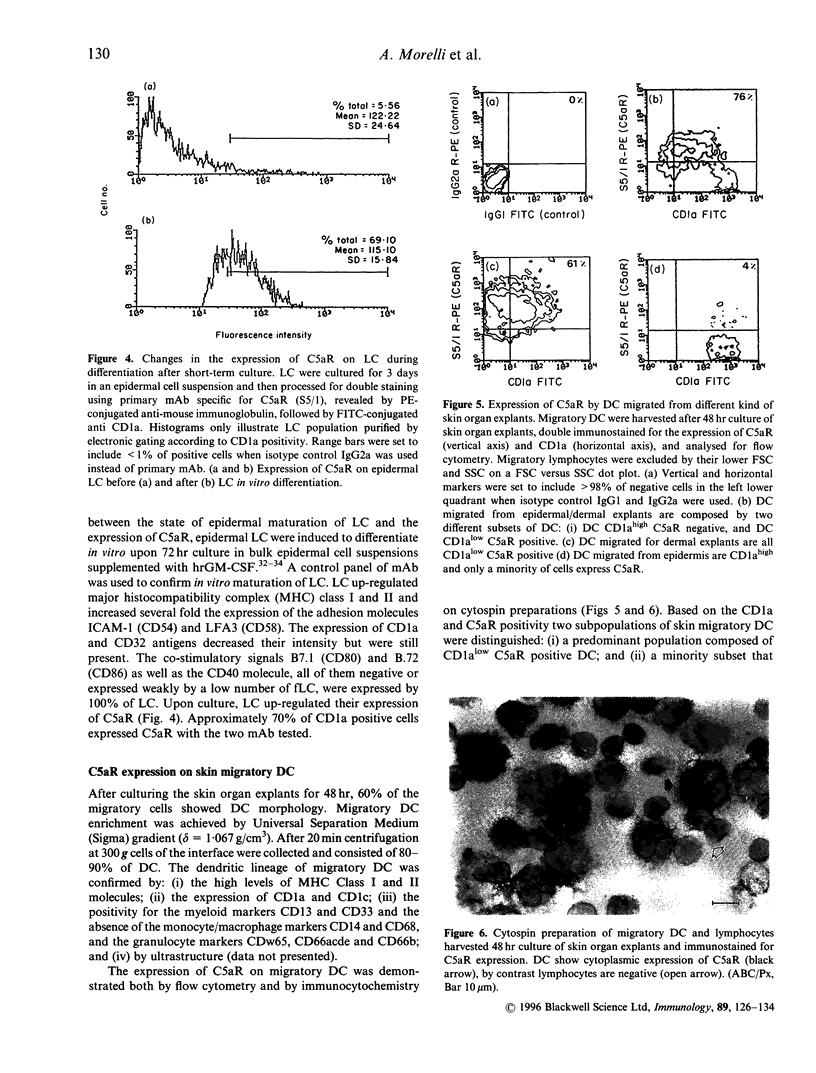
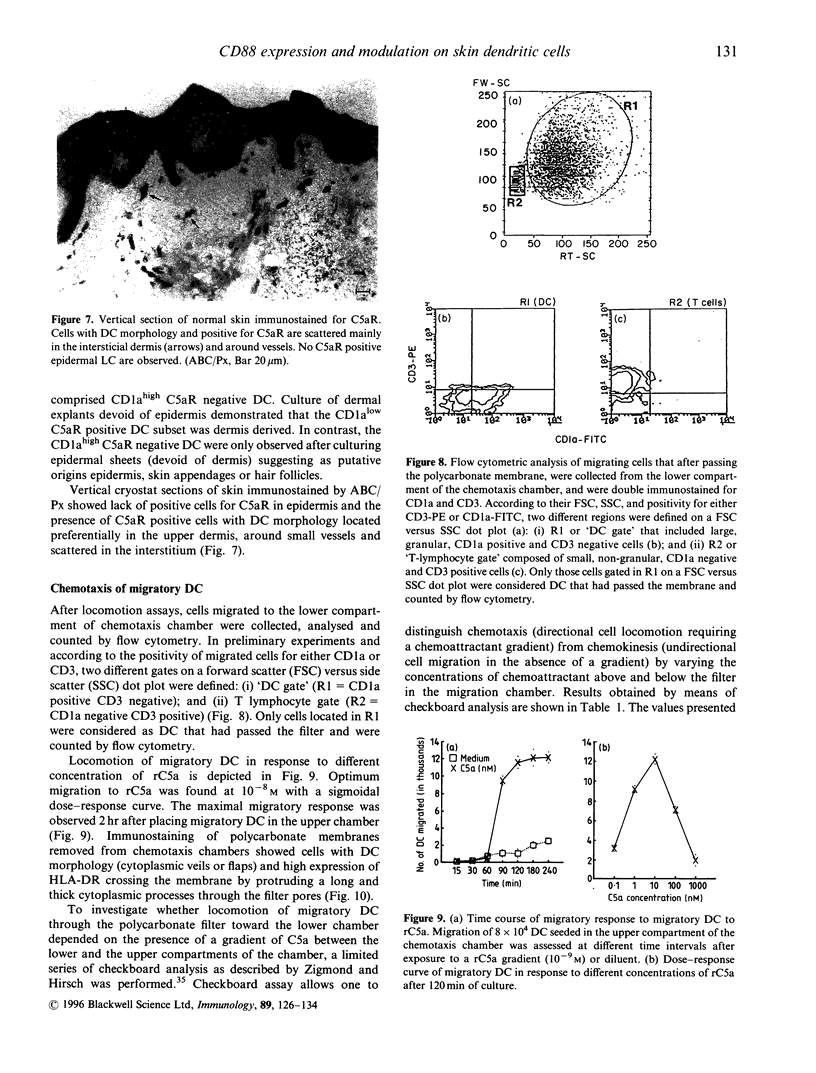
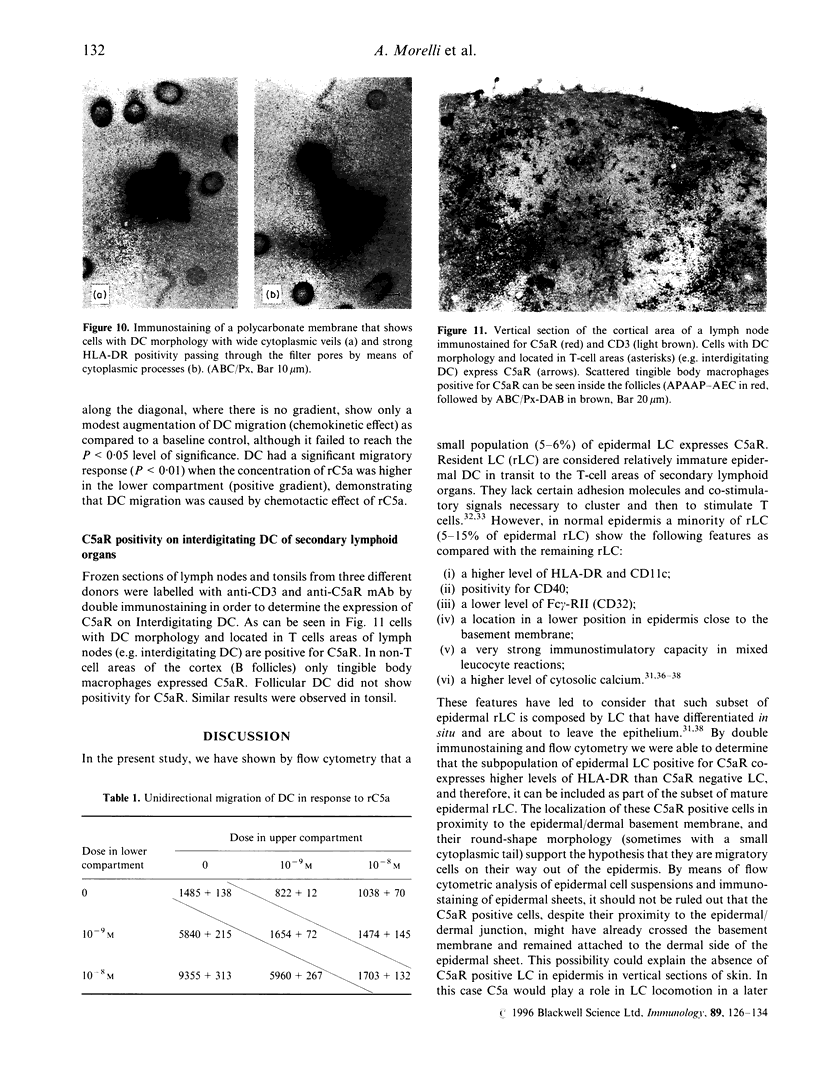
Abstract
Although it is known that dendritic cells (DC) migrate in response to inflammatory stimuli. There is little information about the expression of receptors for chemotactic factors on DC. The present study has demonstrated by double immunostaining and flow cytometry of Langerhan's cell (LC)-enriched epidermal cell suspensions that a small subpopulation (5-6%) of epidermal resident DC (rLC) expresses receptors for C5a (C5aR). Epidermal rLC positive for C5aR show a round-shape morphology, were located next to the basement membrane and express HLA-DR molecules higher than C5aR negative rLC. These observations suggest that rLC would express C5aR as part of their process of maturation during tissue trafficking. To investigate whether epidermal LC up-regulate C5aR along their differentiation pathway. LC were differentiated in vitro after culture in epidermal cell suspensions supplemented with granulocyte macrophage colony-stimulating factor (GM-CSF). As a result, in vitro differentiated LC increased the expression of C5aR up to 69% of the DC population. In accordance with this observation, interdigitating DC of secondary lymphoid organs (lymph node and tonsil) also expressed (5aR. Migratory CD1a positive DC that spontaneously migrated out of dermal or split-skin organ explants were also positive for C5aR and were used for chemotaxis and chemokinesis assays in response to human recombinant C5a (rC5a). Optimum migration to rC5a was observed at 10(-8)M with a sigmoidal dose response curve. Checkboard analysis demonstrated that locomotion in response to rC5a was chemotaxis and not chemokinesis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bottazzi B., Introna M., Allavena P., Villa A., Mantovani A. In vitro migration of human large granular lymphocytes. J Immunol. 1985 Apr;134(4):2316–2321. [PubMed] [Google Scholar]
- Cumberbatch M., Fielding I., Kimber I. Modulation of epidermal Langerhans' cell frequency by tumour necrosis factor-alpha. Immunology. 1994 Mar;81(3):395–401. [PMC free article] [PubMed] [Google Scholar]
- Cumberbatch M., Kimber I. Dermal tumour necrosis factor-alpha induces dendritic cell migration to draining lymph nodes, and possibly provides one stimulus for Langerhans' cell migration. Immunology. 1992 Feb;75(2):257–263. [PMC free article] [PubMed] [Google Scholar]
- Cumberbatch M., Kimber I. Phenotypic characteristics of antigen-bearing cells in the draining lymph nodes of contact sensitized mice. Immunology. 1990 Nov;71(3):404–410. [PMC free article] [PubMed] [Google Scholar]
- Cumberbatch M., Kimber I. Tumour necrosis factor-alpha is required for accumulation of dendritic cells in draining lymph nodes and for optimal contact sensitization. Immunology. 1995 Jan;84(1):31–35. [PMC free article] [PubMed] [Google Scholar]
- Frank M. M., Fries L. F. The role of complement in inflammation and phagocytosis. Immunol Today. 1991 Sep;12(9):322–326. doi: 10.1016/0167-5699(91)90009-I. [DOI] [PubMed] [Google Scholar]
- Gerard N. P., Gerard C. The chemotactic receptor for human C5a anaphylatoxin. Nature. 1991 Feb 14;349(6310):614–617. doi: 10.1038/349614a0. [DOI] [PubMed] [Google Scholar]
- Heufler C., Koch F., Schuler G. Granulocyte/macrophage colony-stimulating factor and interleukin 1 mediate the maturation of murine epidermal Langerhans cells into potent immunostimulatory dendritic cells. J Exp Med. 1988 Feb 1;167(2):700–705. doi: 10.1084/jem.167.2.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim M. A., Chain B. M., Katz D. R. The injured cell: the role of the dendritic cell system as a sentinel receptor pathway. Immunol Today. 1995 Apr;16(4):181–186. doi: 10.1016/0167-5699(95)80118-9. [DOI] [PubMed] [Google Scholar]
- Knight S. C., Stagg A. J. Antigen-presenting cell types. Curr Opin Immunol. 1993 Jun;5(3):374–382. doi: 10.1016/0952-7915(93)90056-x. [DOI] [PubMed] [Google Scholar]
- Kupp L. I., Kosco M. H., Schenkein H. A., Tew J. G. Chemotaxis of germinal center B cells in response to C5a. Eur J Immunol. 1991 Nov;21(11):2697–2701. doi: 10.1002/eji.1830211108. [DOI] [PubMed] [Google Scholar]
- Larregina A., Morelli A., Kolkowski E., Fainboim L. Flow cytometric analysis of cytokine receptors on human Langerhans' cells. Changes observed after short-term culture. Immunology. 1996 Feb;87(2):317–325. doi: 10.1046/j.1365-2567.1996.451513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen C. P., Steinman R. M., Witmer-Pack M., Hankins D. F., Morris P. J., Austyn J. M. Migration and maturation of Langerhans cells in skin transplants and explants. J Exp Med. 1990 Nov 1;172(5):1483–1493. doi: 10.1084/jem.172.5.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz A., Heine M., Schuler G., Romani N. Human and murine dermis contain dendritic cells. Isolation by means of a novel method and phenotypical and functional characterization. J Clin Invest. 1993 Dec;92(6):2587–2596. doi: 10.1172/JCI116873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist E. N., Bäck O. Interleukin-1 decreases the number of Ia+ epidermal dendritic cells but increases their expression of Ia antigen. Acta Derm Venereol. 1990;70(5):391–394. [PubMed] [Google Scholar]
- MacPherson G. G., Jenkins C. D., Stein M. J., Edwards C. Endotoxin-mediated dendritic cell release from the intestine. Characterization of released dendritic cells and TNF dependence. J Immunol. 1995 Feb 1;154(3):1317–1322. [PubMed] [Google Scholar]
- MacPherson G. G. Properties of lymph-borne (veiled) dendritic cells in culture. I. Modulation of phenotype, survival and function: partial dependence on GM-CSF. Immunology. 1989 Sep;68(1):102–107. [PMC free article] [PubMed] [Google Scholar]
- Macatonia S. E., Edwards A. J., Knight S. C. Dendritic cells and the initiation of contact sensitivity to fluorescein isothiocyanate. Immunology. 1986 Dec;59(4):509–514. [PMC free article] [PubMed] [Google Scholar]
- Macatonia S. E., Knight S. C., Edwards A. J., Griffiths S., Fryer P. Localization of antigen on lymph node dendritic cells after exposure to the contact sensitizer fluorescein isothiocyanate. Functional and morphological studies. J Exp Med. 1987 Dec 1;166(6):1654–1667. doi: 10.1084/jem.166.6.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWilliam A. S., Nelson D., Thomas J. A., Holt P. G. Rapid dendritic cell recruitment is a hallmark of the acute inflammatory response at mucosal surfaces. J Exp Med. 1994 Apr 1;179(4):1331–1336. doi: 10.1084/jem.179.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier L., Gonzalez-Ramos A., Cooper K. D. Heterogeneous populations of class II MHC+ cells in human dermal cell suspensions. Identification of a small subset responsible for potent dermal antigen-presenting cell activity with features analogous to Langerhans cells. J Immunol. 1993 Oct 15;151(8):4067–4080. [PubMed] [Google Scholar]
- Nestle F. O., Zheng X. G., Thompson C. B., Turka L. A., Nickoloff B. J. Characterization of dermal dendritic cells obtained from normal human skin reveals phenotypic and functionally distinctive subsets. J Immunol. 1993 Dec 1;151(11):6535–6545. [PubMed] [Google Scholar]
- Pope M., Betjes M. G., Hirmand H., Hoffman L., Steinman R. M. Both dendritic cells and memory T lymphocytes emigrate from organ cultures of human skin and form distinctive dendritic-T-cell conjugates. J Invest Dermatol. 1995 Jan;104(1):11–17. doi: 10.1111/1523-1747.ep12613452. [DOI] [PubMed] [Google Scholar]
- Richters C. D., Hoekstra M. J., van Baare J., Du Pont J. S., Hoefsmit E. C., Kamperdijk E. W. Isolation and characterization of migratory human skin dendritic cells. Clin Exp Immunol. 1994 Nov;98(2):330–336. doi: 10.1111/j.1365-2249.1994.tb06146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roake J. A., Rao A. S., Morris P. J., Larsen C. P., Hankins D. F., Austyn J. M. Dendritic cell loss from nonlymphoid tissues after systemic administration of lipopolysaccharide, tumor necrosis factor, and interleukin 1. J Exp Med. 1995 Jun 1;181(6):2237–2247. doi: 10.1084/jem.181.6.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani N., Lenz A., Glassel H., Stössel H., Stanzl U., Majdic O., Fritsch P., Schuler G. Cultured human Langerhans cells resemble lymphoid dendritic cells in phenotype and function. J Invest Dermatol. 1989 Nov;93(5):600–609. doi: 10.1111/1523-1747.ep12319727. [DOI] [PubMed] [Google Scholar]
- Shibaki A., Meunier L., Ra C., Shimada S., Ohkawara A., Cooper K. D. Differential responsiveness of Langerhans cell subsets of varying phenotypic states in normal human epidermis. J Invest Dermatol. 1995 Jan;104(1):42–46. doi: 10.1111/1523-1747.ep12613476. [DOI] [PubMed] [Google Scholar]
- Sozzani S., Sallusto F., Luini W., Zhou D., Piemonti L., Allavena P., Van Damme J., Valitutti S., Lanzavecchia A., Mantovani A. Migration of dendritic cells in response to formyl peptides, C5a, and a distinct set of chemokines. J Immunol. 1995 Oct 1;155(7):3292–3295. [PubMed] [Google Scholar]
- Teunissen M. B., Wormmeester J., Krieg S. R., Peters P. J., Vogels I. M., Kapsenberg M. L., Bos J. D. Human epidermal Langerhans cells undergo profound morphologic and phenotypical changes during in vitro culture. J Invest Dermatol. 1990 Feb;94(2):166–173. doi: 10.1111/1523-1747.ep12874439. [DOI] [PubMed] [Google Scholar]
- Werfel T., Oppermann M., Schulze M., Krieger G., Weber M., Götze O. Binding of fluorescein-labeled anaphylatoxin C5a to human peripheral blood, spleen, and bone marrow leukocytes. Blood. 1992 Jan 1;79(1):152–160. [PubMed] [Google Scholar]
- Wetsel R. A. Structure, function and cellular expression of complement anaphylatoxin receptors. Curr Opin Immunol. 1995 Feb;7(1):48–53. doi: 10.1016/0952-7915(95)80028-x. [DOI] [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973 Feb 1;137(2):387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]