Abstract
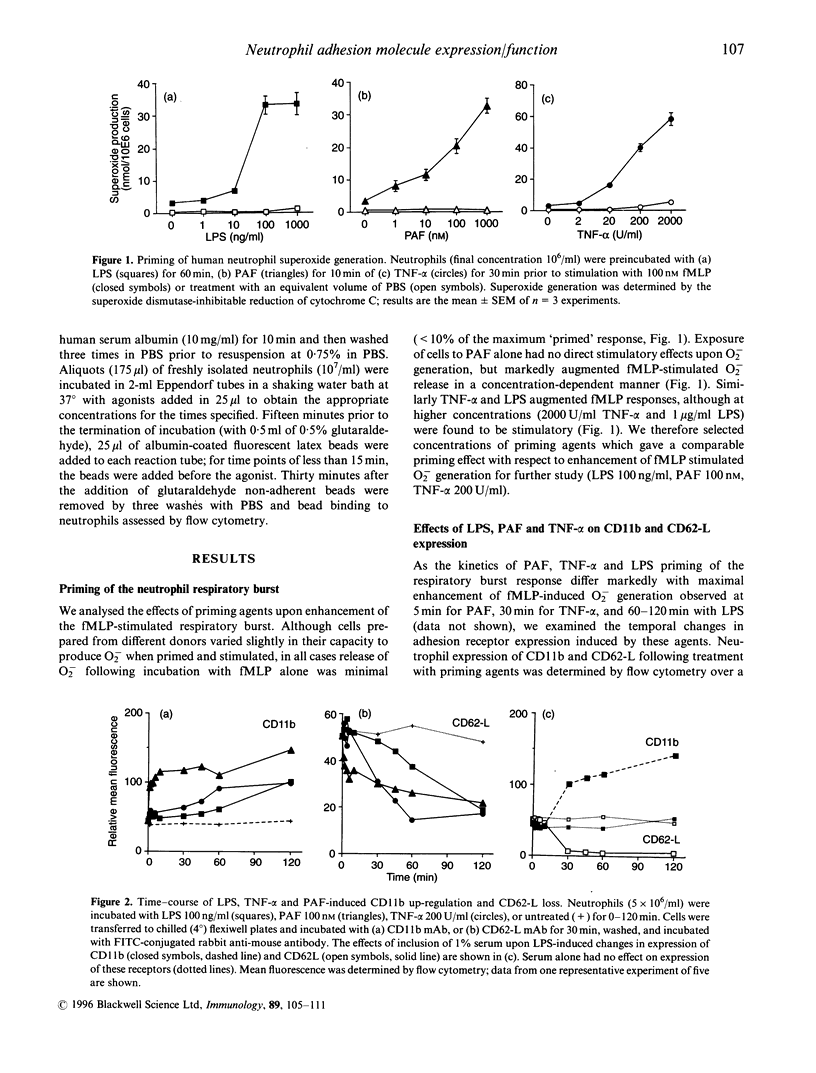
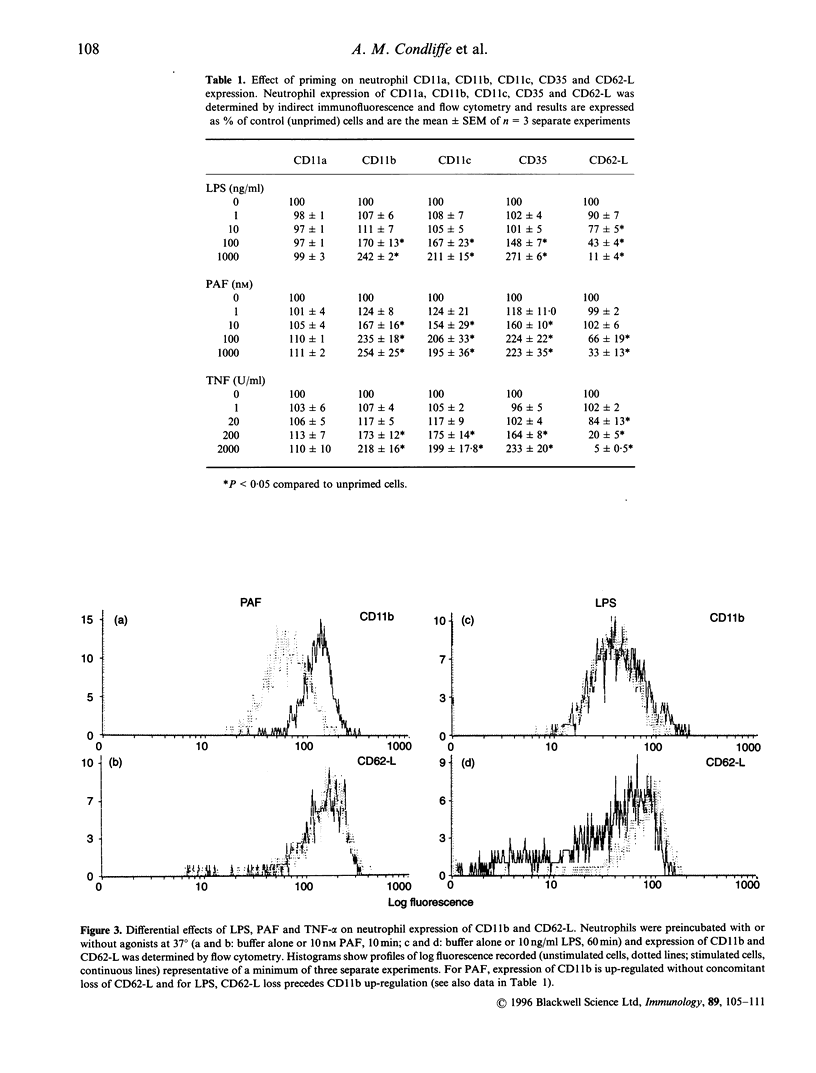
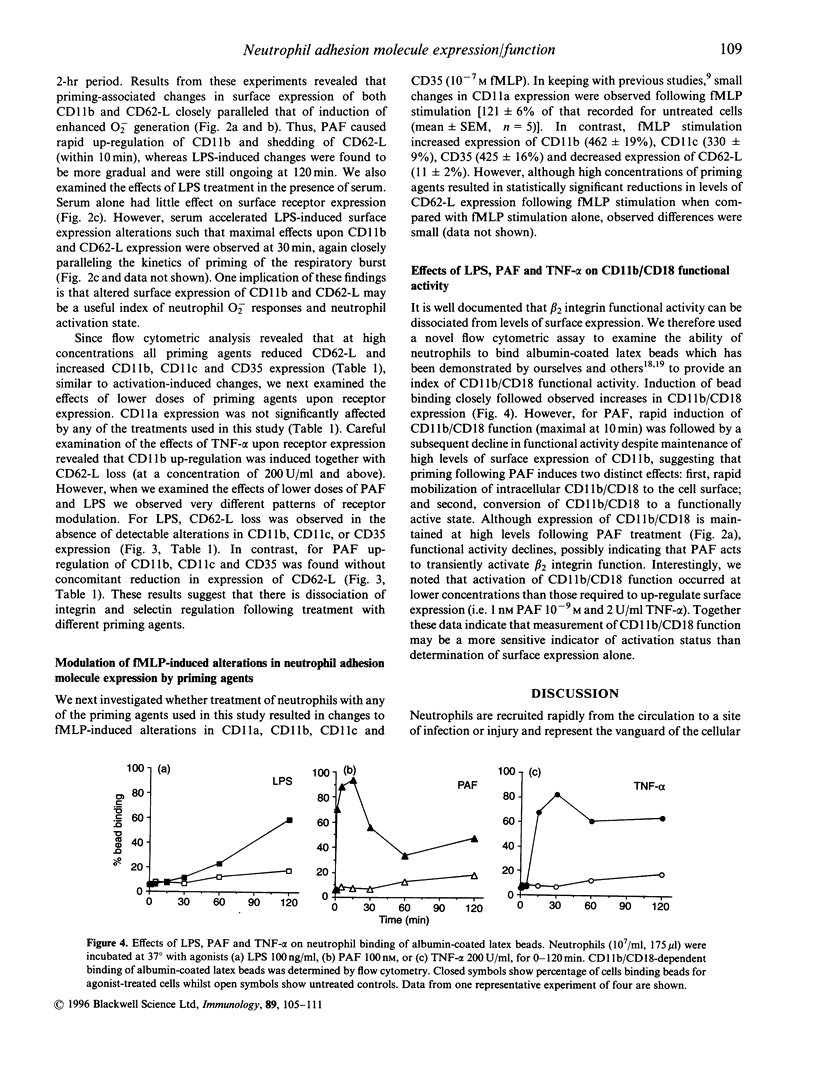
Lung injury in a variety of disease states is critically dependent on neutrophil-mediated inflammatory responses. Neutrophil recruitment to sites of infection or tissue damage requires co-ordinated regulation of neutrophil adhesion and activation status. We have examined the effects of treatment of human peripheral blood neutrophils with priming agents [lipopolysaccharide (LPS). tumor necrosis factor-alpha (TNF-alpha) and platelet-activating factor (PAF)] upon expression of CD11a. CD11b. CD11c. CD35 and CD62-1 and CD11b function to assess whether subtle regulation of neutrophil adhesion potential accompanies augmented formyl-methionyl-leucyl-phenylalanine-stimulated superoxide production. We have found that there are differential effects of priming concentrations of these agents. For LPS. CD62L loss occurs in the absence of changes in CD11b, whereas for PAF. CD11b up-regulation occurs in the absence of detectable loss of CD62-L. However, for TNF-2, decreased expression of CD62-L occurs concomitantly with increased expression of CD11b. In addition, we have shown that priming agents augment CD11b functional activity in a manner that parallels the priming of the respiratory burst. Thus, priming agents may differentially regulate neutrophil adhesive capacity and data presented in this manuscript suggest that the increased effector cell function observed in primed cells may be associated with a distinct repertoire of potential adhesive interactions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aida Y., Pabst M. J. Priming of neutrophils by lipopolysaccharide for enhanced release of superoxide. Requirement for plasma but not for tumor necrosis factor-alpha. J Immunol. 1990 Nov 1;145(9):3017–3025. [PubMed] [Google Scholar]
- Berkow R. L., Wang D., Larrick J. W., Dodson R. W., Howard T. H. Enhancement of neutrophil superoxide production by preincubation with recombinant human tumor necrosis factor. J Immunol. 1987 Dec 1;139(11):3783–3791. [PubMed] [Google Scholar]
- Donnelly S. C., Haslett C., Dransfield I., Robertson C. E., Carter D. C., Ross J. A., Grant I. S., Tedder T. F. Role of selectins in development of adult respiratory distress syndrome. Lancet. 1994 Jul 23;344(8917):215–219. doi: 10.1016/s0140-6736(94)92995-5. [DOI] [PubMed] [Google Scholar]
- Dransfield I., Cabañas C., Craig A., Hogg N. Divalent cation regulation of the function of the leukocyte integrin LFA-1. J Cell Biol. 1992 Jan;116(1):219–226. doi: 10.1083/jcb.116.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin J. D., Spertini O., Ernst T. J., Belvin M. P., Levine H. B., Kanakura Y., Tedder T. F. Granulocyte-macrophage colony-stimulating factor and other cytokines regulate surface expression of the leukocyte adhesion molecule-1 on human neutrophils, monocytes, and their precursors. J Immunol. 1990 Jul 15;145(2):576–584. [PubMed] [Google Scholar]
- Guthrie L. A., McPhail L. C., Henson P. M., Johnston R. B., Jr Priming of neutrophils for enhanced release of oxygen metabolites by bacterial lipopolysaccharide. Evidence for increased activity of the superoxide-producing enzyme. J Exp Med. 1984 Dec 1;160(6):1656–1671. doi: 10.1084/jem.160.6.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslett C., Guthrie L. A., Kopaniak M. M., Johnston R. B., Jr, Henson P. M. Modulation of multiple neutrophil functions by preparative methods or trace concentrations of bacterial lipopolysaccharide. Am J Pathol. 1985 Apr;119(1):101–110. [PMC free article] [PubMed] [Google Scholar]
- Haslett C., Savill J. S., Meagher L. The neutrophil. Curr Opin Immunol. 1989 Oct;2(1):10–18. doi: 10.1016/0952-7915(89)90091-5. [DOI] [PubMed] [Google Scholar]
- Heflin A. C., Jr, Brigham K. L. Prevention by granulocyte depletion of increased vascular permeability of sheep lung following endotoxemia. J Clin Invest. 1981 Nov;68(5):1253–1260. doi: 10.1172/JCI110371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T. K., Jutila M. A., Berg E. L., Butcher E. C. Neutrophil Mac-1 and MEL-14 adhesion proteins inversely regulated by chemotactic factors. Science. 1989 Sep 15;245(4923):1238–1241. doi: 10.1126/science.2551036. [DOI] [PubMed] [Google Scholar]
- Levin J., Poore T. E., Zauber N. P., Oser R. S. Detection of endotoxin in the blood of patients with sepsis due to gran-negative bacteria. N Engl J Med. 1970 Dec 10;283(24):1313–1316. doi: 10.1056/NEJM197012102832404. [DOI] [PubMed] [Google Scholar]
- Lien D. C., Wagner W. W., Jr, Capen R. L., Haslett C., Hanson W. L., Hofmeister S. E., Henson P. M., Worthen G. S. Physiological neutrophil sequestration in the lung: visual evidence for localization in capillaries. J Appl Physiol (1985) 1987 Mar;62(3):1236–1243. doi: 10.1152/jappl.1987.62.3.1236. [DOI] [PubMed] [Google Scholar]
- Molad Y., Haines K. A., Anderson D. C., Buyon J. P., Cronstein B. N. Immunocomplexes stimulate different signalling events to chemoattractants in the neutrophil and regulate L-selectin and beta 2-integrin expression differently. Biochem J. 1994 May 1;299(Pt 3):881–887. doi: 10.1042/bj2990881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C., Srimal S., Farber C., Sanchez E., Kabbash L., Asch A., Gailit J., Wright S. D. Cytokine-induced respiratory burst of human neutrophils: dependence on extracellular matrix proteins and CD11/CD18 integrins. J Cell Biol. 1989 Sep;109(3):1341–1349. doi: 10.1083/jcb.109.3.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea J. J., Brown E. J., Seligmann B. E., Metcalf J. A., Frank M. M., Gallin J. I. Evidence for distinct intracellular pools of receptors for C3b and C3bi in human neutrophils. J Immunol. 1985 Apr;134(4):2580–2587. [PubMed] [Google Scholar]
- Parsons P. E., Worthen G. S., Moore E. E., Tate R. M., Henson P. M. The association of circulating endotoxin with the development of the adult respiratory distress syndrome. Am Rev Respir Dis. 1989 Aug;140(2):294–301. doi: 10.1164/ajrccm/140.2.294. [DOI] [PubMed] [Google Scholar]
- Saverymuttu S. H., Peters A. M., Danpure H. J., Reavy H. J., Osman S., Lavender J. P. Lung transit of 111Indium-labelled granulocytes. Relationship to labelling techniques. Scand J Haematol. 1983 Feb;30(2):151–160. doi: 10.1111/j.1600-0609.1983.tb01463.x. [DOI] [PubMed] [Google Scholar]
- Smith C. W., Marlin S. D., Rothlein R., Toman C., Anderson D. C. Cooperative interactions of LFA-1 and Mac-1 with intercellular adhesion molecule-1 in facilitating adherence and transendothelial migration of human neutrophils in vitro. J Clin Invest. 1989 Jun;83(6):2008–2017. doi: 10.1172/JCI114111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocks S. C., Kerr M. A., Haslett C., Dransfield I. CD66-dependent neutrophil activation: a possible mechanism for vascular selectin-mediated regulation of neutrophil adhesion. J Leukoc Biol. 1995 Jul;58(1):40–48. doi: 10.1002/jlb.58.1.40. [DOI] [PubMed] [Google Scholar]
- Tedder T. F. Cell-surface receptor shedding: a means of regulating function. Am J Respir Cell Mol Biol. 1991 Oct;5(4):305–306. doi: 10.1165/ajrcmb/5.4.305. [DOI] [PubMed] [Google Scholar]
- Terashita Z., Imura Y., Nishikawa K., Sumida S. Is platelet activating factor (PAF) a mediator of endotoxin shock? Eur J Pharmacol. 1985 Feb 26;109(2):257–261. doi: 10.1016/0014-2999(85)90427-3. [DOI] [PubMed] [Google Scholar]
- Till G. O., Johnson K. J., Kunkel R., Ward P. A. Intravascular activation of complement and acute lung injury. Dependency on neutrophils and toxic oxygen metabolites. J Clin Invest. 1982 May;69(5):1126–1135. doi: 10.1172/JCI110548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercellotti G. M., Yin H. Q., Gustafson K. S., Nelson R. D., Jacob H. S. Platelet-activating factor primes neutrophil responses to agonists: role in promoting neutrophil-mediated endothelial damage. Blood. 1988 Apr;71(4):1100–1107. [PubMed] [Google Scholar]
- Weiss S. J. Tissue destruction by neutrophils. N Engl J Med. 1989 Feb 9;320(6):365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- Worthen G. S., Haslett C., Rees A. J., Gumbay R. S., Henson J. E., Henson P. M. Neutrophil-mediated pulmonary vascular injury. Synergistic effect of trace amounts of lipopolysaccharide and neutrophil stimuli on vascular permeability and neutrophil sequestration in the lung. Am Rev Respir Dis. 1987 Jul;136(1):19–28. doi: 10.1164/ajrccm/136.1.19. [DOI] [PubMed] [Google Scholar]
- Worthen G. S., Seccombe J. F., Clay K. L., Guthrie L. A., Johnston R. B., Jr The priming of neutrophils by lipopolysaccharide for production of intracellular platelet-activating factor. Potential role in mediation of enhanced superoxide secretion. J Immunol. 1988 May 15;140(10):3553–3559. [PubMed] [Google Scholar]
- Young S. K., Worthen G. S., Haslett C., Tonnesen M. G., Henson P. M. Interaction between chemoattractants and bacterial lipopolysaccharide in the induction and enhancement of neutrophil adhesion. Am J Respir Cell Mol Biol. 1990 Jun;2(6):523–532. doi: 10.1165/ajrcmb/2.6.523. [DOI] [PubMed] [Google Scholar]



