Abstract
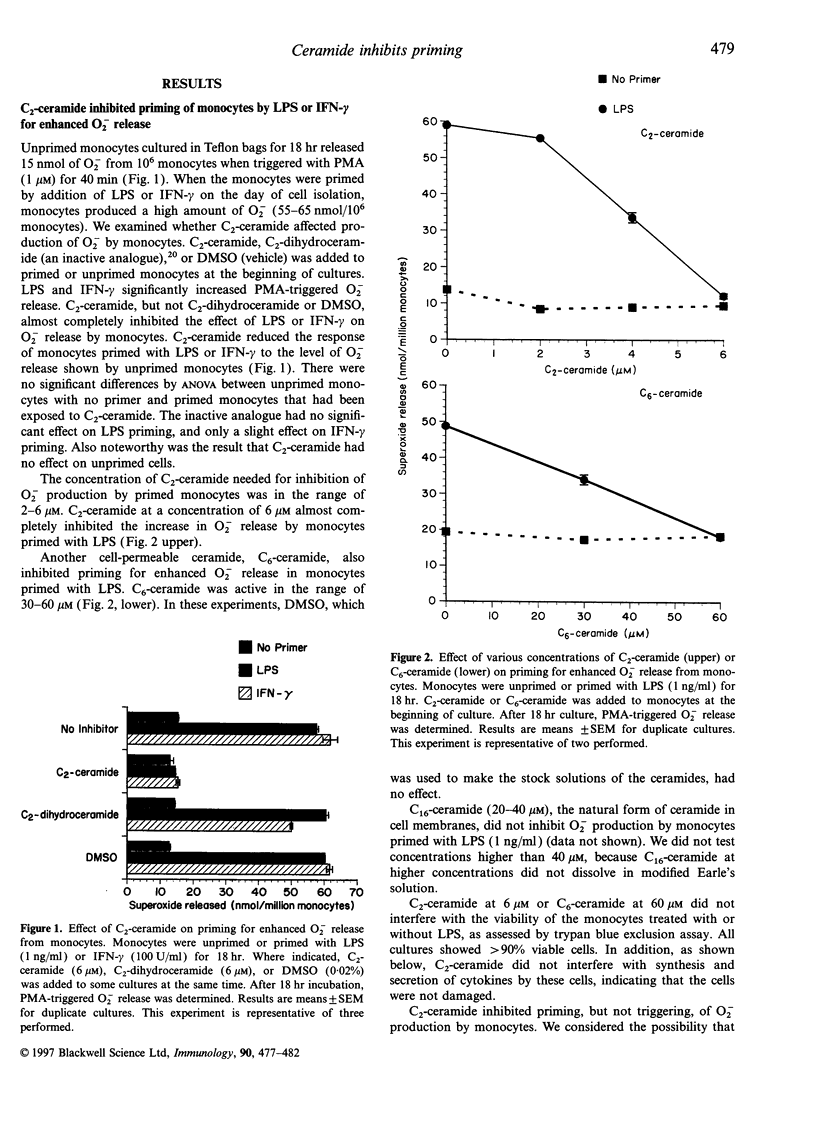
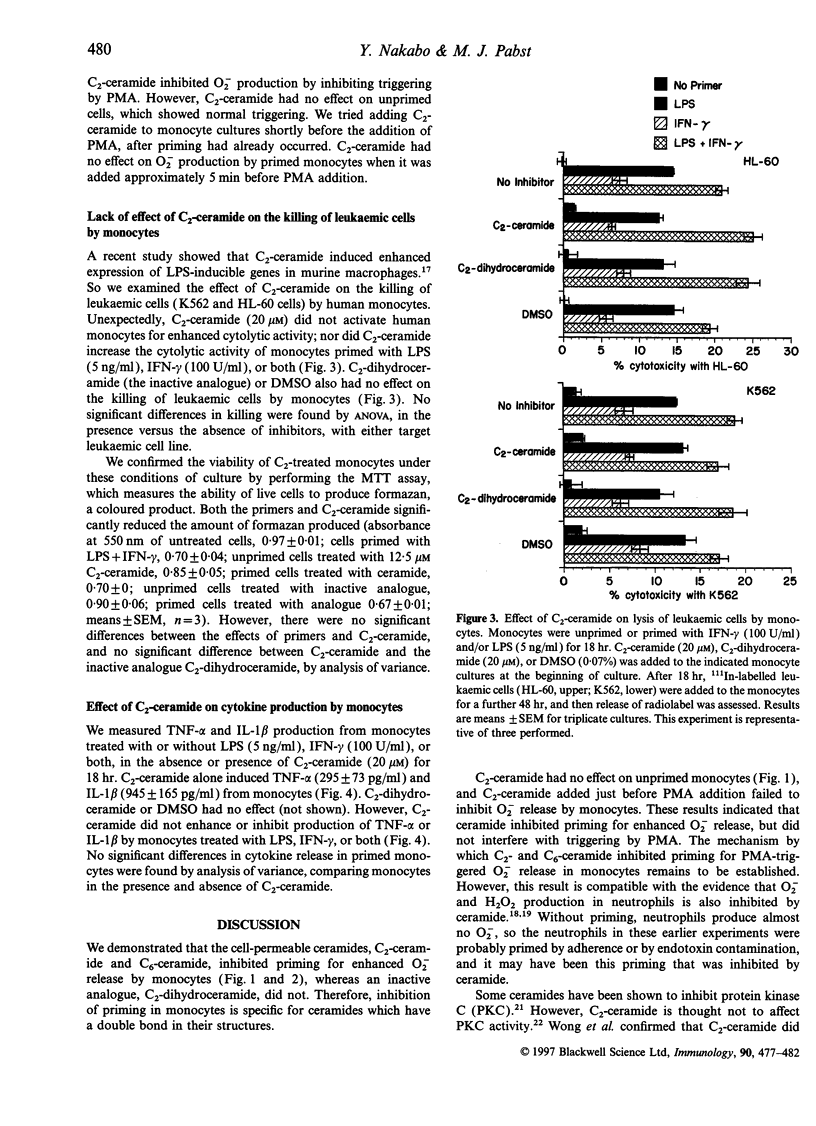
Ceramide acts as an intracellular second messenger in cellular signal transduction. We examined the effects of two cell-permeable ceramides, C2-ceramide and C6-ceramide, on human monocyte functions. After monocytes were primed with lipopolysaccharide (LPS) or interferon-gamma (IFN-gamma) for 18 hr in suspension culture, they produced a high amount of superoxide (O2-) when triggered by phorbol myristate acetate. C2- or C6-ceramide inhibited O2- release from monocytes primed with LPS (1 ng/ml) or IFN-gamma (100 U/ml), but did not affect unprimed monocytes. An analogue, C2-dihydroceramide, was inactive. C2-ceramide was most effective at 6 microM, and C6-ceramide at 60 microM. C2- or C6-ceramide at these concentrations was not toxic for monocytes, as assessed by trypan blue exclusion and by the 3-[4, 5-dimethylthiazol-2-y1]-2,5 diphenyl tetrazolium bromide (MTT) assay which measures the ability of live cells to produce formazan. C2-ceramide (20 microM) had no effect on the killing of leukaemic cells (HL-60 and K562 cells) by monocytes treated with IFN-gamma, LPS, or both for 18 hr, with killing assessed by an 111 Indium-releasing assay. C2-ceramide (20 microM) induced secretion of low amounts of tumour necrosis factor-alpha (TNF-alpha) and interleukin-1 beta (IL-1 beta) from the monocytes. But C2-ceramide did not alter the higher secretion of TNF-alpha or IL-1 beta from monocytes treated with IFN-gamma or LPS. Thus the cell-permeable ceramides acted like antagonists of LPS, rather than analogues of LPS, as has been proposed. The results here showed that the signal transduction pathway for O2- release by monocytes differed from that for the cytolysis of leukaemic cells, and confirmed that oxygen radicals are not involved in cytolysis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aida Y., Kusumoto K., Nakatomi K., Takada H., Pabst M. J., Maeda K. An analogue of lipid A and LPS from Rhodobacter sphaeroides inhibits neutrophil responses to LPS by blocking receptor recognition of LPS and by depleting LPS-binding protein in plasma. J Leukoc Biol. 1995 Dec;58(6):675–682. doi: 10.1002/jlb.58.6.675. [DOI] [PubMed] [Google Scholar]
- Aida Y., Pabst M. J. Priming of neutrophils by lipopolysaccharide for enhanced release of superoxide. Requirement for plasma but not for tumor necrosis factor-alpha. J Immunol. 1990 Nov 1;145(9):3017–3025. [PubMed] [Google Scholar]
- Barber S. A., Perera P. Y., Vogel S. N. Defective ceramide response in C3H/HeJ (Lpsd) macrophages. J Immunol. 1995 Sep 1;155(5):2303–2305. [PubMed] [Google Scholar]
- Braun D. P., Ahn M. C., Harris J. E., Chu E., Casey L., Wilbanks G., Siziopikou K. P. Sensitivity of tumoricidal function in macrophages from different anatomical sites of cancer patients to modulation of arachidonic acid metabolism. Cancer Res. 1993 Jul 15;53(14):3362–3368. [PubMed] [Google Scholar]
- Fidler I. J., Schroit A. J. Recognition and destruction of neoplastic cells by activated macrophages: discrimination of altered self. Biochim Biophys Acta. 1988 Nov 15;948(2):151–173. doi: 10.1016/0304-419x(88)90009-1. [DOI] [PubMed] [Google Scholar]
- Forehand J. R., Pabst M. J., Phillips W. A., Johnston R. B., Jr Lipopolysaccharide priming of human neutrophils for an enhanced respiratory burst. Role of intracellular free calcium. J Clin Invest. 1989 Jan;83(1):74–83. doi: 10.1172/JCI113887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golenbock D. T., Hampton R. Y., Qureshi N., Takayama K., Raetz C. R. Lipid A-like molecules that antagonize the effects of endotoxins on human monocytes. J Biol Chem. 1991 Oct 15;266(29):19490–19498. [PubMed] [Google Scholar]
- Hannun Y. A., Bell R. M. Lysosphingolipids inhibit protein kinase C: implications for the sphingolipidoses. Science. 1987 Feb 6;235(4789):670–674. doi: 10.1126/science.3101176. [DOI] [PubMed] [Google Scholar]
- Hannun Y. A., Obeid L. M., Wolff R. A. The novel second messenger ceramide: identification, mechanism of action, and cellular activity. Adv Lipid Res. 1993;25:43–64. [PubMed] [Google Scholar]
- Koff W. C., Fidler I. J., Showalter S. D., Chakrabarty M. K., Hampar B., Ceccorulli L. M., Kleinerman E. S. Human monocytes activated by immunomodulators in liposomes lyse herpesvirus-infected but not normal cells. Science. 1984 Jun 1;224(4652):1007–1009. doi: 10.1126/science.6426057. [DOI] [PubMed] [Google Scholar]
- Kolesnick R., Golde D. W. The sphingomyelin pathway in tumor necrosis factor and interleukin-1 signaling. Cell. 1994 May 6;77(3):325–328. doi: 10.1016/0092-8674(94)90147-3. [DOI] [PubMed] [Google Scholar]
- Mangan D. F., Robertson B., Wahl S. M. IL-4 enhances programmed cell death (apoptosis) in stimulated human monocytes. J Immunol. 1992 Mar 15;148(6):1812–1816. [PubMed] [Google Scholar]
- Manthey C. L., Brandes M. E., Perera P. Y., Vogel S. N. Taxol increases steady-state levels of lipopolysaccharide-inducible genes and protein-tyrosine phosphorylation in murine macrophages. J Immunol. 1992 Oct 1;149(7):2459–2465. [PubMed] [Google Scholar]
- Megyeri P., Pabst K. M., Pabst M. J. Serine protease inhibitors block priming of monocytes for enhanced release of superoxide. Immunology. 1995 Dec;86(4):629–635. [PMC free article] [PubMed] [Google Scholar]
- Nakabo Y., Pabst M. J. Lysis of leukemic cells by human macrophages: inhibition by 4-(2-aminoethyl)-benzenesulfonyl fluoride (AEBSF), a serine protease inhibitor. J Leukoc Biol. 1996 Sep;60(3):328–336. doi: 10.1002/jlb.60.3.328. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotney M., Chang Z. L., Uchiyama H., Suzuki T. Protein kinase C in tumoricidal activation of mouse macrophage cell lines. Biochemistry. 1991 Jun 4;30(22):5597–5604. doi: 10.1021/bi00236a037. [DOI] [PubMed] [Google Scholar]
- Obeid L. M., Linardic C. M., Karolak L. A., Hannun Y. A. Programmed cell death induced by ceramide. Science. 1993 Mar 19;259(5102):1769–1771. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- Pabst M. J., Johnston R. B., Jr Increased production of superoxide anion by macrophages exposed in vitro to muramyl dipeptide or lipopolysaccharide. J Exp Med. 1980 Jan 1;151(1):101–114. doi: 10.1084/jem.151.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasada M., Johnston R. B., Jr Macrophage microbicidal activity. Correlation between phagocytosis-associated oxidative metabolism and the killing of Candida by macrophages. J Exp Med. 1980 Jul 1;152(1):85–98. doi: 10.1084/jem.152.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein S. L., Gold M. R., DeFranco A. L. Bacterial lipopolysaccharide stimulates protein tyrosine phosphorylation in macrophages. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4148–4152. doi: 10.1073/pnas.88.10.4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K., Li X. B., Hunchuk N. N-acetylsphingosine (C2-ceramide) inhibited neutrophil superoxide formation and calcium influx. J Biol Chem. 1995 Feb 17;270(7):3056–3062. doi: 10.1074/jbc.270.7.3056. [DOI] [PubMed] [Google Scholar]
- Worthen G. S., Seccombe J. F., Clay K. L., Guthrie L. A., Johnston R. B., Jr The priming of neutrophils by lipopolysaccharide for production of intracellular platelet-activating factor. Potential role in mediation of enhanced superoxide secretion. J Immunol. 1988 May 15;140(10):3553–3559. [PubMed] [Google Scholar]
- Wright S. D., Kolesnick R. N. Does endotoxin stimulate cells by mimicking ceramide? Immunol Today. 1995 Jun;16(6):297–302. doi: 10.1016/0167-5699(95)80185-5. [DOI] [PubMed] [Google Scholar]






