Abstract
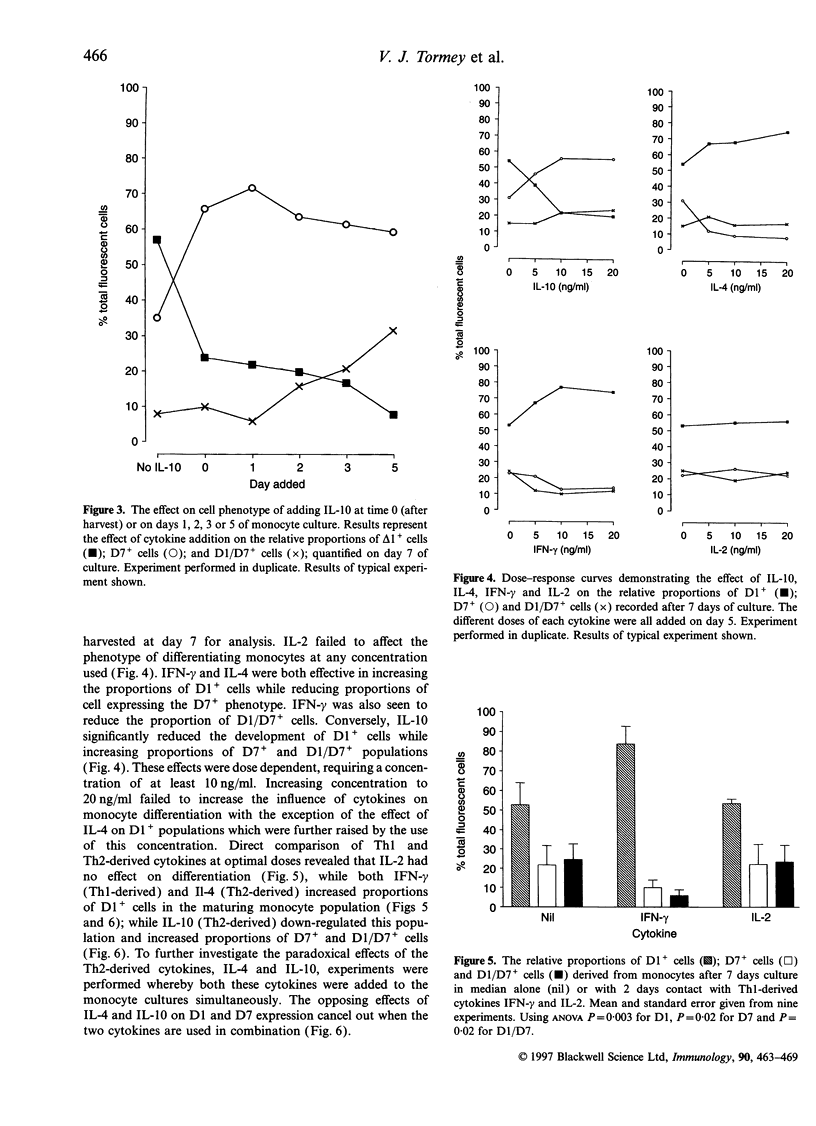
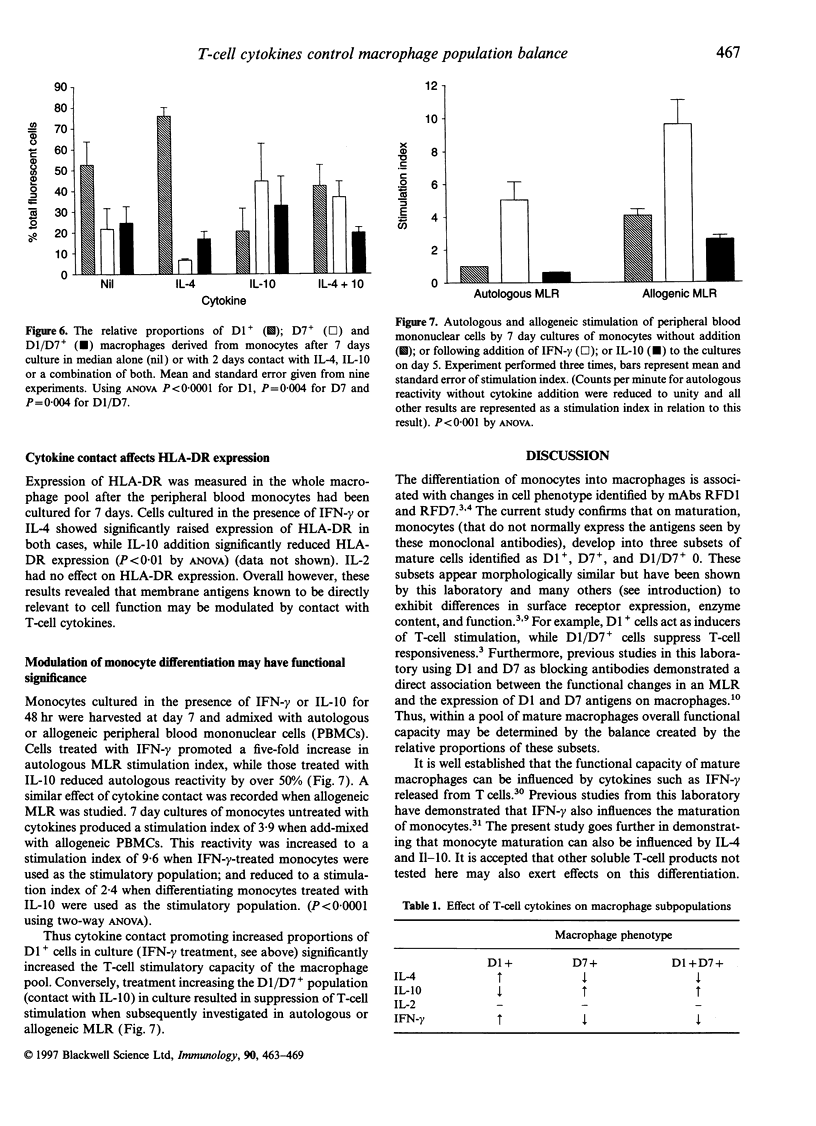
As monocytes differentiate into mature macrophages, subsets emerge that exhibit stimulatory, suppressive or phagocytic potential. These functionally distinct subsets can be discriminated using monoclonal antibodies RFD1 and RFD7. As examples of all these subsets have been repeatedly identified within the macrophage pool in a variety of organs the overall functional capacity of this pool will depend on the relative balance of these subpopulations. This study investigates whether this balance present in mature macrophage populations can be regulated by the local influence of T-cell-derived cytokines. The dose-dependent effect of cytokines interferon-gamma (IFN-gamma), interleukins (IL) IL-2, IL-4 and IL-10 on the phenotype and function of monocyte-derived macrophages was determined. Subsets of mature cells were quantified by identifying RFD1- RFD7- stimulatory cells (D1+); RFD1- RFD7+ phagocytes (D7+) and RFD1+ RFD7+ suppressive cells (D1 D7+). IFN-gamma and IL-4 increased the relative proportions of D1+ stimulatory cells and upregulated HLA-DR expression. IFN-gamma also increased the capacity of the mature macrophage pool to stimulate T-cell proliferation. IL-10 reduced the proportions of D1+ stimulatory cells while promoting the differentiation of D7+ phagocytes and D1/D7+ suppressive cells. IL-10 induced changes also reduced the functional capacity of the mature populations to stimulate T cells in auto and allogenic mixed lymphocyte reactions (MLR). IL-2 had no effect on differentiation of monocytes. Thus IL-4 and IFN-gamma are seen to induce the development of stimulatory macrophages while IL-10 promotes differentiation of monocytes to mature phagocytes and suppressive macrophages. It is concluded that mature macrophage phenotype is 'plastic' and under the control of T-cell-derived mediators. Furthermore, within the differentiating monocytes, phenotypic change appears to carry with it functional change, thus retaining the relationship between antigen expression and activity in the mature macrophage populations.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison M. C., Poulter L. W. Changes in phenotypically distinct mucosal macrophage populations may be a prerequisite for the development of inflammatory bowel disease. Clin Exp Immunol. 1991 Sep;85(3):504–509. doi: 10.1111/j.1365-2249.1991.tb05757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belardelli F. Role of interferons and other cytokines in the regulation of the immune response. APMIS. 1995 Mar;103(3):161–179. doi: 10.1111/j.1699-0463.1995.tb01092.x. [DOI] [PubMed] [Google Scholar]
- Campbell D. A., du Bois R. M., Butcher R. G., Poulter L. W. The density of HLA-DR antigen expression on alveolar macrophages is increased in pulmonary sarcoidosis. Clin Exp Immunol. 1986 Jul;65(1):165–171. [PMC free article] [PubMed] [Google Scholar]
- Campbell P. A. Macrophage-Listeria interactions. Immunol Ser. 1994;60:313–328. [PubMed] [Google Scholar]
- Ding L., Linsley P. S., Huang L. Y., Germain R. N., Shevach E. M. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J Immunol. 1993 Aug 1;151(3):1224–1234. [PubMed] [Google Scholar]
- Doherty T. M. T-cell regulation of macrophage function. Curr Opin Immunol. 1995 Jun;7(3):400–404. doi: 10.1016/0952-7915(95)80117-0. [DOI] [PubMed] [Google Scholar]
- Fernandes D. M., Baldwin C. L. Interleukin-10 downregulates protective immunity to Brucella abortus. Infect Immun. 1995 Mar;63(3):1130–1133. doi: 10.1128/iai.63.3.1130-1133.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino D. F., Zlotnik A., Mosmann T. R., Howard M., O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991 Dec 1;147(11):3815–3822. [PubMed] [Google Scholar]
- Flores Villanueva P. O., Reiser H., Stadecker M. J. Regulation of T helper cell responses in experimental murine schistosomiasis by IL-10. Effect on expression of B7 and B7-2 costimulatory molecules by macrophages. J Immunol. 1994 Dec 1;153(11):5190–5199. [PubMed] [Google Scholar]
- Hart P. H., Ahern M. J., Smith M. D., Finlay-Jones J. J. Comparison of the suppressive effects of interleukin-10 and interleukin-4 on synovial fluid macrophages and blood monocytes from patients with inflammatory arthritis. Immunology. 1995 Apr;84(4):536–542. [PMC free article] [PubMed] [Google Scholar]
- Hogg N., MacDonald S., Slusarenko M., Beverley P. C. Monoclonal antibodies specific for human monocytes, granulocytes and endothelium. Immunology. 1984 Dec;53(4):753–767. [PMC free article] [PubMed] [Google Scholar]
- Holt P. G., Oliver J., Bilyk N., McMenamin C., McMenamin P. G., Kraal G., Thepen T. Downregulation of the antigen presenting cell function(s) of pulmonary dendritic cells in vivo by resident alveolar macrophages. J Exp Med. 1993 Feb 1;177(2):397–407. doi: 10.1084/jem.177.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter C., Poulter L. W. The balance of macrophage subsets may be customised at mucosal surfaces. FEMS Microbiol Immunol. 1992 Dec;5(5-6):309–315. doi: 10.1111/j.1574-6968.1992.tb05915.x. [DOI] [PubMed] [Google Scholar]
- Kelly P. M., Bliss E., Morton J. A., Burns J., McGee J. O. Monoclonal antibody EBM/11: high cellular specificity for human macrophages. J Clin Pathol. 1988 May;41(5):510–515. doi: 10.1136/jcp.41.5.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz A., Heine M., Schuler G., Romani N. Human and murine dermis contain dendritic cells. Isolation by means of a novel method and phenotypical and functional characterization. J Clin Invest. 1993 Dec;92(6):2587–2596. doi: 10.1172/JCI116873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macatonia S. E., Doherty T. M., Knight S. C., O'Garra A. Differential effect of IL-10 on dendritic cell-induced T cell proliferation and IFN-gamma production. J Immunol. 1993 May 1;150(9):3755–3765. [PubMed] [Google Scholar]
- Molina I. J., Huber B. T. Regulation of macrophage activation markers by IL-4 and IFN-gamma is subpopulation-specific. Cell Immunol. 1991 Apr 15;134(1):241–248. doi: 10.1016/0008-8749(91)90347-e. [DOI] [PubMed] [Google Scholar]
- Poulter L. W., Burke C. M. Macrophages and allergic lung disease. Immunobiology. 1996 Oct;195(4-5):574–587. doi: 10.1016/S0171-2985(96)80023-4. [DOI] [PubMed] [Google Scholar]
- Poulter L. W., Campbell D. A., Munro C., Janossy G. Discrimination of human macrophages and dendritic cells by means of monoclonal antibodies. Scand J Immunol. 1986 Sep;24(3):351–357. doi: 10.1111/j.1365-3083.1986.tb02104.x. [DOI] [PubMed] [Google Scholar]
- Poulter L. W., Janossy G., Power C., Sreenan S., Burke C. Immunological/physiological relationships in asthma: potential regulation by lung macrophages. Immunol Today. 1994 Jun;15(6):258–261. doi: 10.1016/0167-5699(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Poulter L. W., Power C., Burke C. The relationship between bronchial immunopathology and hyperresponsiveness in asthma. Eur Respir J. 1990 Jul;3(7):792–799. [PubMed] [Google Scholar]
- Poulter L. W., Rook G. A., Steele J., Condez A. Influence of 1,25-(OH)2 vitamin D3 and gamma interferon on the phenotype of human peripheral blood monocyte-derived macrophages. Infect Immun. 1987 Sep;55(9):2017–2020. doi: 10.1128/iai.55.9.2017-2020.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruppert J., Friedrichs D., Xu H., Peters J. H. IL-4 decreases the expression of the monocyte differentiation marker CD14, paralleled by an increasing accessory potency. Immunobiology. 1991 Aug;182(5):449–464. doi: 10.1016/S0171-2985(11)80209-3. [DOI] [PubMed] [Google Scholar]
- Seldenrijk C. A., Drexhage H. A., Meuwissen S. G., Pals S. T., Meijer C. J. Dendritic cells and scavenger macrophages in chronic inflammatory bowel disease. Gut. 1989 Apr;30(4):484–491. doi: 10.1136/gut.30.4.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeen M. J., Ziegler H. K. Activation of gamma delta T cells for production of IFN-gamma is mediated by bacteria via macrophage-derived cytokines IL-1 and IL-12. J Immunol. 1995 Jun 1;154(11):5832–5841. [PubMed] [Google Scholar]
- Spiteri M. A., Clarke S. W., Poulter L. W. Alveolar macrophages that suppress T-cell responses may be crucial to the pathogenetic outcome of pulmonary sarcoidosis. Eur Respir J. 1992 Apr;5(4):394–403. [PubMed] [Google Scholar]
- Spiteri M. A., Clarke S. W., Poulter L. W. Isolation of phenotypically and functionally distinct macrophage subpopulations from human bronchoalveolar lavage. Eur Respir J. 1992 Jun;5(6):717–726. [PubMed] [Google Scholar]
- Spiteri M. A., Poulter L. W. Characterization of immune inducer and suppressor macrophages from the normal human lung. Clin Exp Immunol. 1991 Jan;83(1):157–162. doi: 10.1111/j.1365-2249.1991.tb05607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teunissen M. B., Wormmeester J., Krieg S. R., Peters P. J., Vogels I. M., Kapsenberg M. L., Bos J. D. Human epidermal Langerhans cells undergo profound morphologic and phenotypical changes during in vitro culture. J Invest Dermatol. 1990 Feb;94(2):166–173. doi: 10.1111/1523-1747.ep12874439. [DOI] [PubMed] [Google Scholar]
- Thepen T., Kraal G., Holt P. G. The role of alveolar macrophages in regulation of lung inflammation. Ann N Y Acad Sci. 1994 May 28;725:200–206. doi: 10.1111/j.1749-6632.1994.tb39802.x. [DOI] [PubMed] [Google Scholar]
- Toews G. B., Vial W. C., Dunn M. M., Guzzetta P., Nunez G., Stastny P., Lipscomb M. F. The accessory cell function of human alveolar macrophages in specific T cell proliferation. J Immunol. 1984 Jan;132(1):181–186. [PubMed] [Google Scholar]
- Weissman D., Poli G., Fauci A. S. Interleukin 10 blocks HIV replication in macrophages by inhibiting the autocrine loop of tumor necrosis factor alpha and interleukin 6 induction of virus. AIDS Res Hum Retroviruses. 1994 Oct;10(10):1199–1206. doi: 10.1089/aid.1994.10.1199. [DOI] [PubMed] [Google Scholar]
- Young H. A., Hardy K. J. Role of interferon-gamma in immune cell regulation. J Leukoc Biol. 1995 Oct;58(4):373–381. [PubMed] [Google Scholar]
- Zheng L., Teschler H., Guzman J., Hübner K., Striz I., Costabel U. Alveolar macrophage TNF-alpha release and BAL cell phenotypes in sarcoidosis. Am J Respir Crit Care Med. 1995 Sep;152(3):1061–1066. doi: 10.1164/ajrccm.152.3.7663784. [DOI] [PubMed] [Google Scholar]
- Zúiga-Pflücker J. C., Jiang D., Lenardo M. J. Requirement for TNF-alpha and IL-1 alpha in fetal thymocyte commitment and differentiation. Science. 1995 Jun 30;268(5219):1906–1909. doi: 10.1126/science.7541554. [DOI] [PubMed] [Google Scholar]
- de Waal Malefyt R., Abrams J., Bennett B., Figdor C. G., de Vries J. E. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991 Nov 1;174(5):1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]


