Abstract
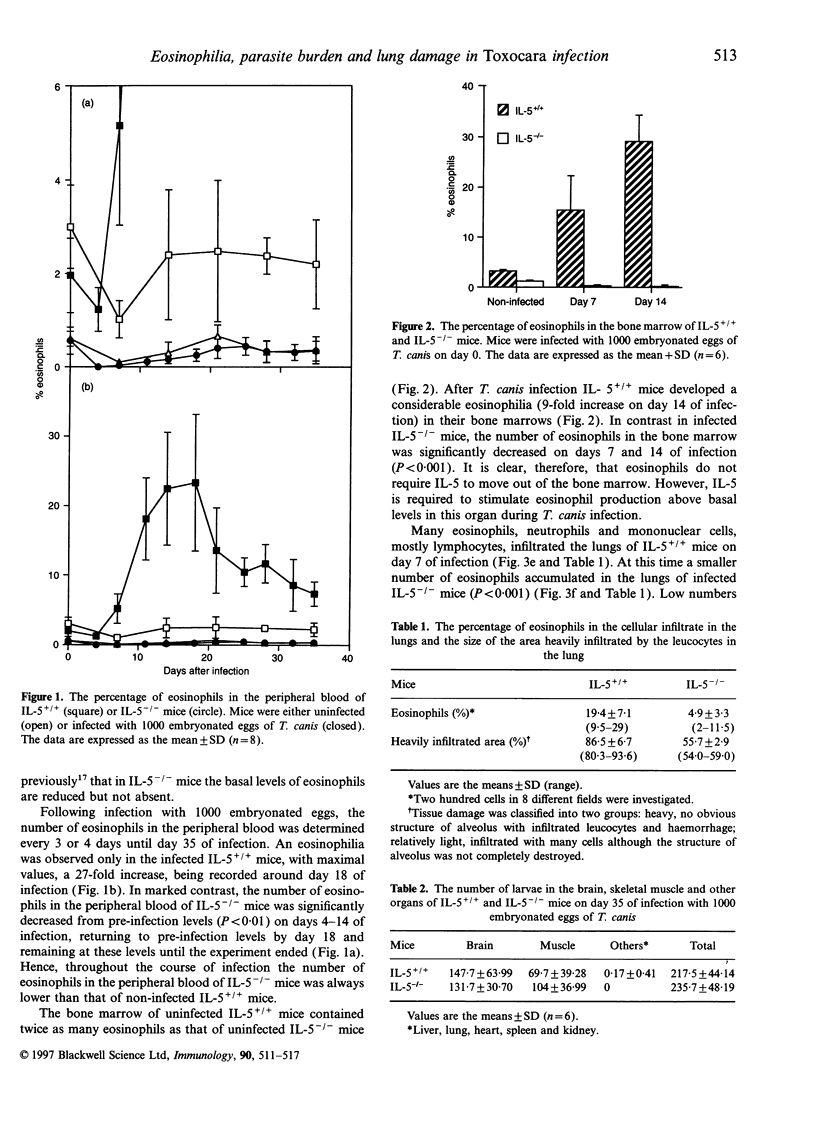
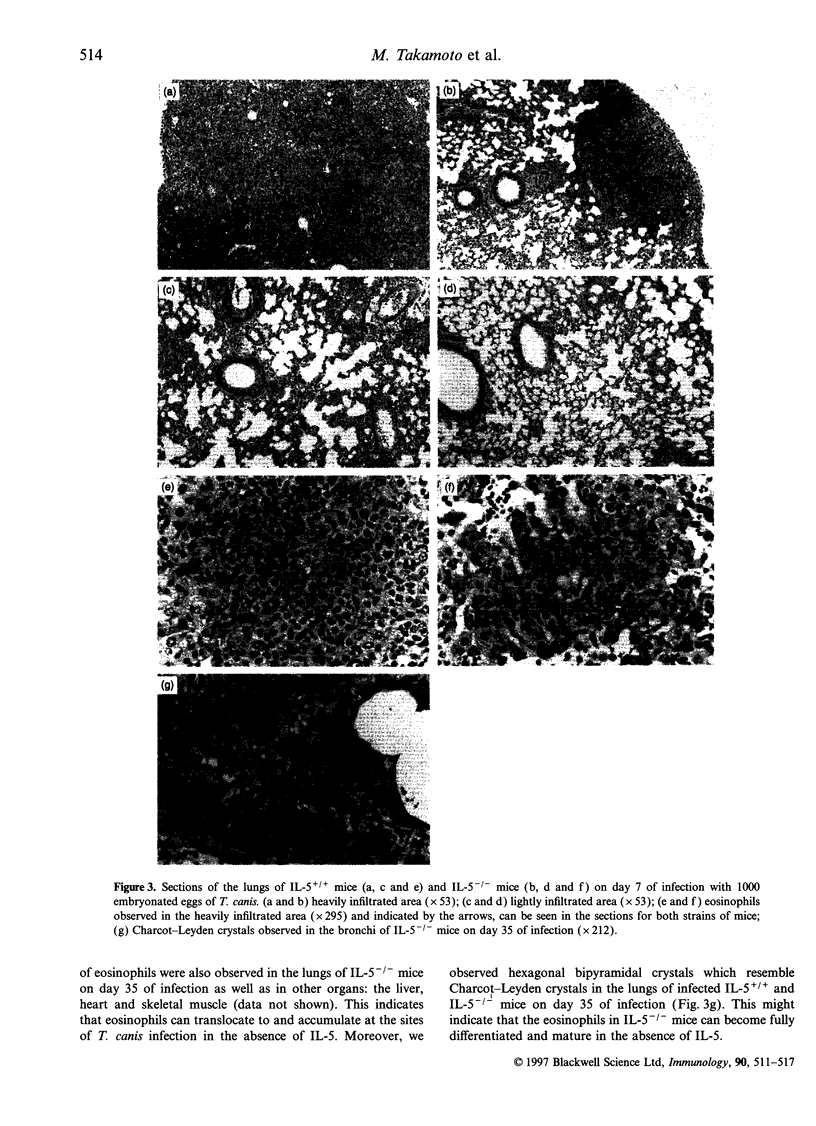
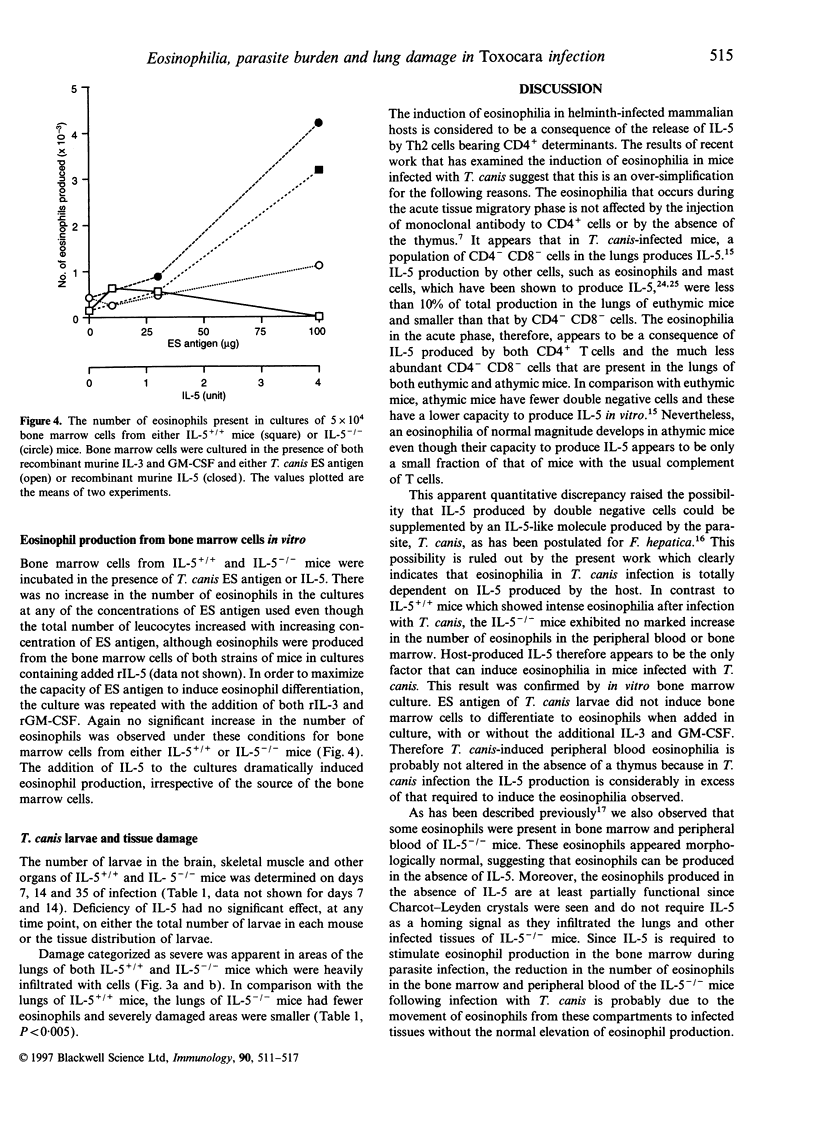
C57Bl/6 mice genetically deficient in interleukin (IL)-5 (IL-5-/-) and mice with the normal IL-5 gene (IL-5+/+) were infected with embryonated eggs of Toxocara canis. IL-5+/+ mice developed a marked eosinophilia in their peripheral bloods and bone marrows after infection. In contrast, the number of eosinophils at these sites actually decreased during the acute phase of infection in IL-5-/- mice. A smaller number of eosinophils infiltrated the lung, liver, heart and skeletal muscle of infected IL-5-/- mice than those of infected IL-5+/+ mice. Eosinophils were not produced in cultures of bone marrow cells from either IL-5+/+ or IL-5-/- mice which were stimulated with excretory secretory antigen of T. canis larvae. The capacity of cells from the bone marrow to differentiate into eosinophils when stimulated in vitro with recombinant murine IL-5 was the same whether the cells were from IL-5+/+ or IL-5-/- mice. Taken together, these results show that an IL-5-like molecule is not produced by the T. canis larvae and that IL-5 produced by host cells is solely responsible for the eosinophilia in mice infected with this nematode. The number and location of T. canis larvae were not altered in the absence of IL-5. In contrast, lung damage in infected IL-5-/- mice was less extensive than that in infected IL-5+/+ mice, although structures resembling Charcot-Leyden crystals were seen in the lungs of both IL-5+/+ and IL-5-/- mice. These results suggest that eosinophils play a role in the pathology in mice infected with T. canis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansari A., Williams J. F. The eosinophilic response of the rat to infection with Taenia taeniaeformis. J Parasitol. 1976 Oct;62(5):728–736. [PubMed] [Google Scholar]
- Basten A., Boyer M. H., Beeson P. B. Mechanism of eosinophilia. I. Factors affecting the eosinophil response of rats to Trichinella spiralis. J Exp Med. 1970 Jun 1;131(6):1271–1287. doi: 10.1084/jem.131.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruickshank J. K., Price K. M., Mackenzie C. D., Spry C. J., Denham D. A. Infection of inbred and nude (athymic) rats with Brugia spp. Parasite Immunol. 1983 Nov;5(6):527–537. doi: 10.1111/j.1365-3024.1983.tb00769.x. [DOI] [PubMed] [Google Scholar]
- Dubucquoi S., Desreumaux P., Janin A., Klein O., Goldman M., Tavernier J., Capron A., Capron M. Interleukin 5 synthesis by eosinophils: association with granules and immunoglobulin-dependent secretion. J Exp Med. 1994 Feb 1;179(2):703–708. doi: 10.1084/jem.179.2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelman F. D., Pearce E. J., Urban J. F., Jr, Sher A. Regulation and biological function of helminth-induced cytokine responses. Immunol Today. 1991 Mar;12(3):A62–A66. doi: 10.1016/S0167-5699(05)80018-0. [DOI] [PubMed] [Google Scholar]
- Foster P. S., Hogan S. P., Ramsay A. J., Matthaei K. I., Young I. G. Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model. J Exp Med. 1996 Jan 1;183(1):195–201. doi: 10.1084/jem.183.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingley E., Cutler R. L., Fung M. C., Sanderson C. J., Young I. G. Production and purification of recombinant human interleukin-5 from yeast and baculovirus expression systems. Eur J Biochem. 1991 Mar 28;196(3):623–629. doi: 10.1111/j.1432-1033.1991.tb15858.x. [DOI] [PubMed] [Google Scholar]
- Ishida K., Yoshimura K. Eosinophil responses of permissive and nonpermissive hosts to the young adult worms of Angiostrongylus cantonensis. Z Parasitenkd. 1986;72(5):661–671. doi: 10.1007/BF00925488. [DOI] [PubMed] [Google Scholar]
- Kennedy J. D., Pierce C. W., Lake J. P. Extrathymic T cell maturation. Phenotypic analysis of T cell subsets in nude mice as a function of age. J Immunol. 1992 Mar 15;148(6):1620–1629. [PubMed] [Google Scholar]
- Kopf M., Brombacher F., Hodgkin P. D., Ramsay A. J., Milbourne E. A., Dai W. J., Ovington K. S., Behm C. A., Köhler G., Young I. G. IL-5-deficient mice have a developmental defect in CD5+ B-1 cells and lack eosinophilia but have normal antibody and cytotoxic T cell responses. Immunity. 1996 Jan;4(1):15–24. doi: 10.1016/s1074-7613(00)80294-0. [DOI] [PubMed] [Google Scholar]
- Korenaga M., Hitoshi Y., Yamaguchi N., Sato Y., Takatsu K., Tada I. The role of interleukin-5 in protective immunity to Strongyloides venezuelensis infection in mice. Immunology. 1991 Apr;72(4):502–507. [PMC free article] [PubMed] [Google Scholar]
- Kusama Y., Takamoto M., Kasahara T., Takatsu K., Nariuchi H., Sugane K. Mechanisms of eosinophilia in BALB/c-nu/+ and congenitally athymic BALB/c-nu/nu mice infected with Toxocara canis. Immunology. 1995 Mar;84(3):461–468. [PMC free article] [PubMed] [Google Scholar]
- Milbourne E. A., Howell M. J. Eosinophil differentiation in response to Fasciola hepatica and its excretory/secretory antigens. Int J Parasitol. 1993 Dec;23(8):1005–1009. doi: 10.1016/0020-7519(93)90120-n. [DOI] [PubMed] [Google Scholar]
- O'Garra A., Barbis D., Wu J., Hodgkin P. D., Abrams J., Howard M. The BCL1 B lymphoma responds to IL-4, IL-5, and GM-CSF. Cell Immunol. 1989 Oct 1;123(1):189–200. doi: 10.1016/0008-8749(89)90279-7. [DOI] [PubMed] [Google Scholar]
- Okayama Y., Petit-Frére C., Kassel O., Semper A., Quint D., Tunon-de-Lara M. J., Bradding P., Holgate S. T., Church M. K. IgE-dependent expression of mRNA for IL-4 and IL-5 in human lung mast cells. J Immunol. 1995 Aug 15;155(4):1796–1808. [PubMed] [Google Scholar]
- Sasaki O., Sugaya H., Ishida K., Yoshimura K. Ablation of eosinophils with anti-IL-5 antibody enhances the survival of intracranial worms of Angiostrongylus cantonensis in the mouse. Parasite Immunol. 1993 Jun;15(6):349–354. doi: 10.1111/j.1365-3024.1993.tb00619.x. [DOI] [PubMed] [Google Scholar]
- Savigny D. H. In vitro maintenance of Toxocara canis larvae and a simple method for the production of Toxocara ES antigen for use in serodiagnostic tests for visceral larva migrans. J Parasitol. 1975 Aug;61(4):781–782. [PubMed] [Google Scholar]
- Sugane K., Oshima T. Eosinophilia, granuloma formation and migratory behaviour of larvae in the congenitally athymic mouse infected with Toxocara canis. Parasite Immunol. 1982 Sep;4(5):307–318. doi: 10.1111/j.1365-3024.1982.tb00442.x. [DOI] [PubMed] [Google Scholar]
- Sweeney J. F., Nguyen P. K., Omann G. M., Hinshaw D. B. Ultraviolet irradiation accelerates apoptosis in human polymorphonuclear leukocytes: protection by LPS and GM-CSF. J Leukoc Biol. 1997 Oct;62(4):517–523. doi: 10.1002/jlb.62.4.517. [DOI] [PubMed] [Google Scholar]
- Takamoto M., Kusama Y., Takatsu K., Nariuchi H., Sugane K. Occurrence of interleukin-5 production by CD4- CD8- (double-negative) T cells in lungs of both normal and congenitally athymic nude mice infected with Toxocara canis. Immunology. 1995 Jun;85(2):285–291. [PMC free article] [PubMed] [Google Scholar]
- Takamoto M., Sugane K. Mechanisms of eosinophilia in Toxocara canis infected mice: in vitro production of interleukin 5 by lung cells of both normal and congenitally athymic nude mice. Parasite Immunol. 1993 Sep;15(9):493–500. doi: 10.1111/j.1365-3024.1993.tb00636.x. [DOI] [PubMed] [Google Scholar]
- Tchernitchin A., Roorijck J., Tchernitchin X., Vandenhende J., Galand F. Dramatic early increase in uterine eosinophils after oestrogen administration. Nature. 1974 Mar 8;248(5444):142–143. doi: 10.1038/248142a0. [DOI] [PubMed] [Google Scholar]
- Tsuda S., Fukuyama K., Epstein W. L. Induction of T cell-independent eosinophilia in mice with polymyxin B and schistosome infection. Lab Invest. 1980 Dec;43(6):495–499. [PubMed] [Google Scholar]
- Urban J. F., Jr, Katona I. M., Paul W. E., Finkelman F. D. Interleukin 4 is important in protective immunity to a gastrointestinal nematode infection in mice. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5513–5517. doi: 10.1073/pnas.88.13.5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y., Matsui T., Kasahara T., Etoh S., Tominaga A., Takatsu K., Miura Y., Suda T. In vivo changes of hemopoietic progenitors and the expression of the interleukin 5 gene in eosinophilic mice infected with Toxocara canis. Exp Hematol. 1990 Dec;18(11):1152–1157. [PubMed] [Google Scholar]




