Abstract
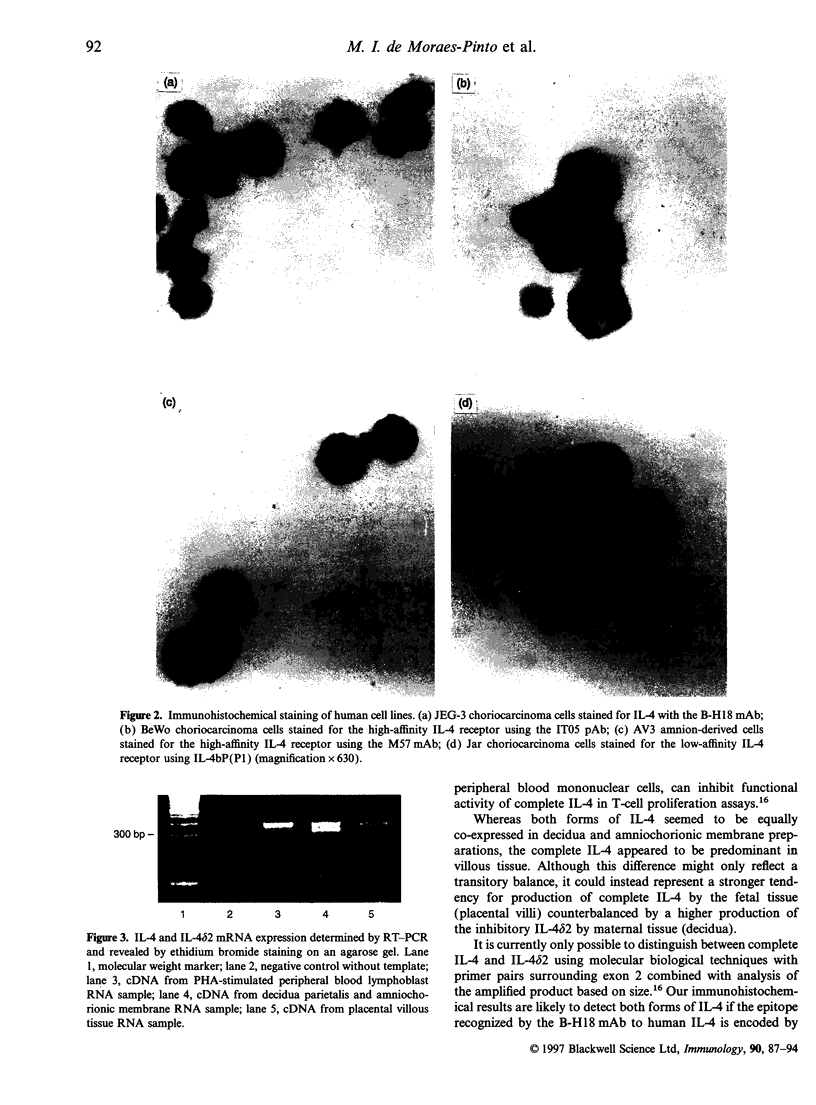
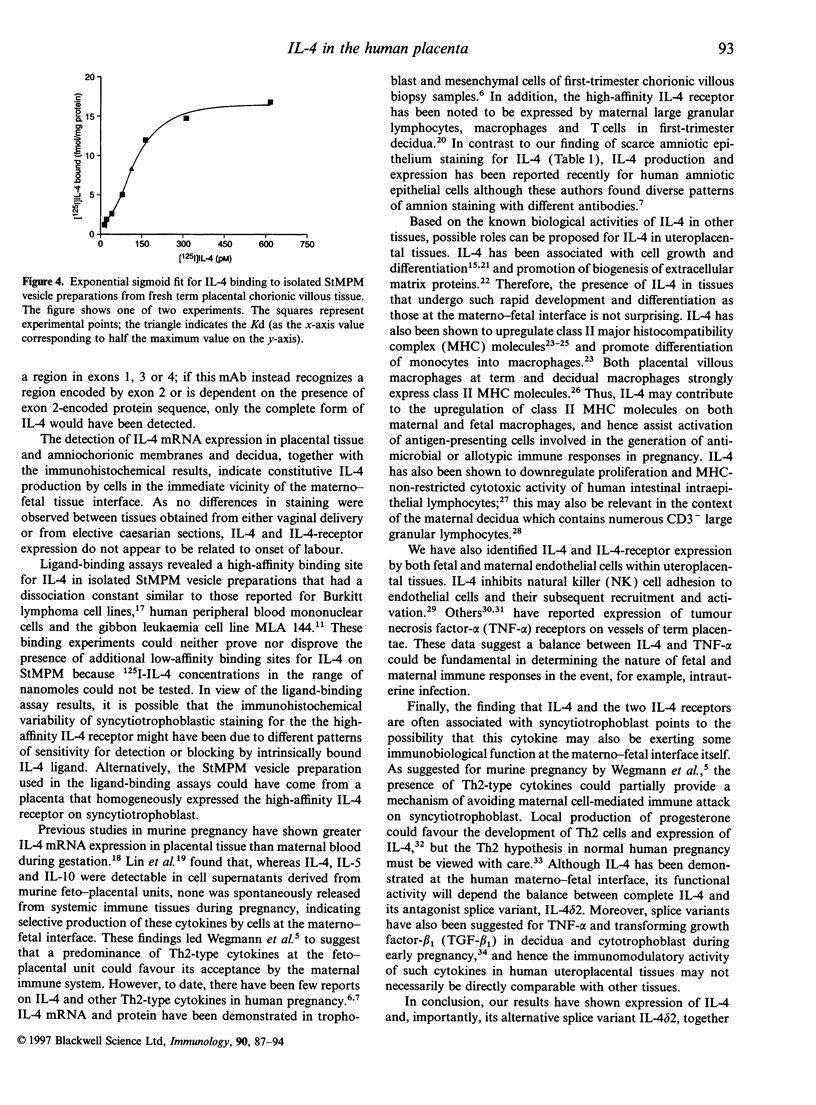
There has been much recent interest in cytokine expression at the materno-fetal interface. Although T-helper 2 (Th2)-type cytokines have been described in the murine feto-placental unit, few studies have as yet been performed in human pregnancy. We have examined the production of interleukin-4 (IL-4) and expression of IL-4 receptors in the human term placenta, decidua and amniochorionic membranes. Immunohistochemical analyses revealed that cytotrophoblast, decidual macrophages and both maternal and fetal endothelial cells consistently expressed IL-4, whereas syncytiotrophoblast and placental macrophages showed an inconsistent pattern between specimens. High- and low-affinity IL-4 receptors were demonstrated by immunohistochemistry at the same cellular sites as stained for IL-4, and detection of IL-4 receptors was also variable in syncytiotrophoblast. Reverse-transcribed-polymerase chain reaction (RT-PCR) analysis showed that both IL-4 and its alternative splice variant, IL-482, are produced both in placental villi and in amniochorionic and decidual tissue. Ligand-binding assays identified the presence, on isolated term syncytiotrophoblast microvillous plasma membrane vesicle preparations, of functional high-affinity binding sites for IL-4 with a Kd in the range 102-112 pM and an apparent receptor density in the range 99-102 x 10(8) sites/mg protein. Three human choriocarcinoma (BeWo, JEG-3 and Jar) and one amnion-derived (AV3) cell lines expressed IL-4 and both high- and low-affinity IL-4 receptors. The constitutive expression of both IL-4 and IL-4 receptors, together with the novel finding of the alternative splice variant IL-482 in the immediate tissues at the materno fetal interface suggest an immunobiological role for IL-4 in human pregnancy.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armitage R. J., Beckmann M. P., Idzerda R. L., Alpert A., Fanslow W. C. Regulation of interleukin 4 receptors on human T cells. Int Immunol. 1990;2(11):1039–1045. doi: 10.1093/intimm/2.11.1039. [DOI] [PubMed] [Google Scholar]
- Atamas S. P., Choi J., Yurovsky V. V., White B. An alternative splice variant of human IL-4, IL-4 delta 2, inhibits IL-4-stimulated T cell proliferation. J Immunol. 1996 Jan 15;156(2):435–441. [PubMed] [Google Scholar]
- Austgulen R., Espevik T., Mecsei R., Scott H. Expression of receptors for tumor necrosis factor in human placenta at term. Acta Obstet Gynecol Scand. 1992 Aug;71(6):417–424. doi: 10.3109/00016349209021090. [DOI] [PubMed] [Google Scholar]
- Bulmer J. N., Longfellow M., Ritson A. Leukocytes and resident blood cells in endometrium. Ann N Y Acad Sci. 1991;622:57–68. doi: 10.1111/j.1749-6632.1991.tb37850.x. [DOI] [PubMed] [Google Scholar]
- Cabrillat H., Galizzi J. P., Djossou O., Arai N., Yokota T., Arai K., Banchereau J. High affinity binding of human interleukin 4 to cell lines. Biochem Biophys Res Commun. 1987 Dec 31;149(3):995–1001. doi: 10.1016/0006-291x(87)90507-9. [DOI] [PubMed] [Google Scholar]
- Delassus S., Coutinho G. C., Saucier C., Darche S., Kourilsky P. Differential cytokine expression in maternal blood and placenta during murine gestation. J Immunol. 1994 Mar 1;152(5):2411–2420. [PubMed] [Google Scholar]
- Ebert E. C., Roberts A. I. IL-4 down-regulates the responsiveness of human intraepithelial lymphocytes. Clin Exp Immunol. 1996 Sep;105(3):556–560. doi: 10.1046/j.1365-2249.1996.d01-782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanslow W. C., Spriggs M. K., Rauch C. T., Clifford K. N., Macduff B. M., Ziegler S. F., Schooley K. A., Mohler K. M., March C. J., Armitage R. J. Identification of a distinct low-affinity receptor for human interleukin-4 on pre-B cells. Blood. 1993 Jun 1;81(11):2998–3005. [PubMed] [Google Scholar]
- Foxwell B. M., Woerly G., Ryffel B. Identification of interleukin 4 receptor-associated proteins and expression of both high- and low-affinity binding on human lymphoid cells. Eur J Immunol. 1989 Sep;19(9):1637–1641. doi: 10.1002/eji.1830190918. [DOI] [PubMed] [Google Scholar]
- Haynes M. K., Jackson L. G., Tuan R. S., Shepley K. J., Smith J. B. Cytokine production in first trimester chorionic villi: detection of mRNAs and protein products in situ. Cell Immunol. 1993 Oct 15;151(2):300–308. doi: 10.1006/cimm.1993.1240. [DOI] [PubMed] [Google Scholar]
- Hunt J. S. Cytokine networks in the uteroplacental unit: macrophages as pivotal regulatory cells. J Reprod Immunol. 1989 Sep;16(1):1–17. doi: 10.1016/0165-0378(89)90002-8. [DOI] [PubMed] [Google Scholar]
- Jones C. A., Williams K. A., Finlay-Jones J. J., Hart P. H. Interleukin 4 production by human amnion epithelial cells and regulation of its activity by glycosaminoglycan binding. Biol Reprod. 1995 Apr;52(4):839–847. doi: 10.1095/biolreprod52.4.839. [DOI] [PubMed] [Google Scholar]
- Kesson A. M., Fear W. R., Williams L., Chang J., King N. J., Cunningham A. L. HIV infection of placental macrophages: their potential role in vertical transmission. J Leukoc Biol. 1994 Sep;56(3):241–246. doi: 10.1002/jlb.56.3.241. [DOI] [PubMed] [Google Scholar]
- King A., Jokhi P. P., Smith S. K., Sharkey A. M., Loke Y. W. Screening for cytokine mRNA in human villous and extravillous trophoblasts using the reverse-transcriptase polymerase chain reaction (RT-PCR). Cytokine. 1995 May;7(4):364–371. doi: 10.1006/cyto.1995.0046. [DOI] [PubMed] [Google Scholar]
- Kishimoto T., Taga T., Akira S. Cytokine signal transduction. Cell. 1994 Jan 28;76(2):253–262. doi: 10.1016/0092-8674(94)90333-6. [DOI] [PubMed] [Google Scholar]
- Lin H., Mosmann T. R., Guilbert L., Tuntipopipat S., Wegmann T. G. Synthesis of T helper 2-type cytokines at the maternal-fetal interface. J Immunol. 1993 Nov 1;151(9):4562–4573. [PubMed] [Google Scholar]
- Morisaki T., Yuzuki D. H., Lin R. T., Foshag L. J., Morton D. L., Hoon D. S. Interleukin 4 receptor expression and growth inhibition of gastric carcinoma cells by interleukin 4. Cancer Res. 1992 Nov 1;52(21):6059–6065. [PubMed] [Google Scholar]
- Obiri N. I., Siegel J. P., Varricchio F., Puri R. K. Expression of high-affinity IL-4 receptors on human melanoma, ovarian and breast carcinoma cells. Clin Exp Immunol. 1994 Jan;95(1):148–155. doi: 10.1111/j.1365-2249.1994.tb06029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogbimi A. O., Johnson P. M., Brown P. J., Fox H. Characterisation of the soluble fraction of human syncytiotrophoblast microvillous plasma membrane-associated proteins. J Reprod Immunol. 1979 Jul;1(2):127–140. doi: 10.1016/0165-0378(79)90013-5. [DOI] [PubMed] [Google Scholar]
- Paganin C., Matteucci C., Cenzuales S., Mantovani A., Allavena P. IL-4 inhibits binding and cytotoxicity of NK cells to vascular endothelium. Cytokine. 1994 Mar;6(2):135–140. doi: 10.1016/1043-4666(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Piccinni M. P., Giudizi M. G., Biagiotti R., Beloni L., Giannarini L., Sampognaro S., Parronchi P., Manetti R., Annunziato F., Livi C. Progesterone favors the development of human T helper cells producing Th2-type cytokines and promotes both IL-4 production and membrane CD30 expression in established Th1 cell clones. J Immunol. 1995 Jul 1;155(1):128–133. [PubMed] [Google Scholar]
- Pollard J. W. Lymphohematopoietic cytokines in the female reproductive tract. Curr Opin Immunol. 1991 Oct;3(5):772–777. doi: 10.1016/0952-7915(91)90112-e. [DOI] [PubMed] [Google Scholar]
- Postlethwaite A. E., Holness M. A., Katai H., Raghow R. Human fibroblasts synthesize elevated levels of extracellular matrix proteins in response to interleukin 4. J Clin Invest. 1992 Oct;90(4):1479–1485. doi: 10.1172/JCI116015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigley K. P., Thurstan S. M., Callard R. E. Independent regulation of interleukin 4 (IL-4)-induced expression of human B cell surface CD23 and IgM: functional evidence for two IL-4 receptors. Int Immunol. 1991 Feb;3(2):197–203. doi: 10.1093/intimm/3.2.197. [DOI] [PubMed] [Google Scholar]
- Robertson S. A., Seamark R. F., Guilbert L. J., Wegmann T. G. The role of cytokines in gestation. Crit Rev Immunol. 1994;14(3-4):239–292. doi: 10.1615/critrevimmunol.v14.i3-4.30. [DOI] [PubMed] [Google Scholar]
- Ruppert J., Friedrichs D., Xu H., Peters J. H. IL-4 decreases the expression of the monocyte differentiation marker CD14, paralleled by an increasing accessory potency. Immunobiology. 1991 Aug;182(5):449–464. doi: 10.1016/S0171-2985(11)80209-3. [DOI] [PubMed] [Google Scholar]
- Ruppert J., Schütt C., Ostermeier D., Peters J. H. Down-regulation and release of CD14 on human monocytes by IL-4 depends on the presence of serum or GM-CSF. Adv Exp Med Biol. 1993;329:281–286. doi: 10.1007/978-1-4615-2930-9_47. [DOI] [PubMed] [Google Scholar]
- Starkey P. M. Expression on cells of early human pregnancy decidua, of the p75, IL-2 and p145, IL-4 receptor proteins. Immunology. 1991 May;73(1):64–70. [PMC free article] [PubMed] [Google Scholar]
- Vince G. S., Johnson P. M. Immunobiology of human uteroplacental macrophages--friend and foe? Placenta. 1996 May;17(4):191–199. doi: 10.1016/s0143-4004(96)90038-7. [DOI] [PubMed] [Google Scholar]
- Wegmann T. G., Lin H., Guilbert L., Mosmann T. R. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993 Jul;14(7):353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- te Velde A. A., Klomp J. P., Yard B. A., de Vries J. E., Figdor C. G. Modulation of phenotypic and functional properties of human peripheral blood monocytes by IL-4. J Immunol. 1988 Mar 1;140(5):1548–1554. [PubMed] [Google Scholar]





