Abstract
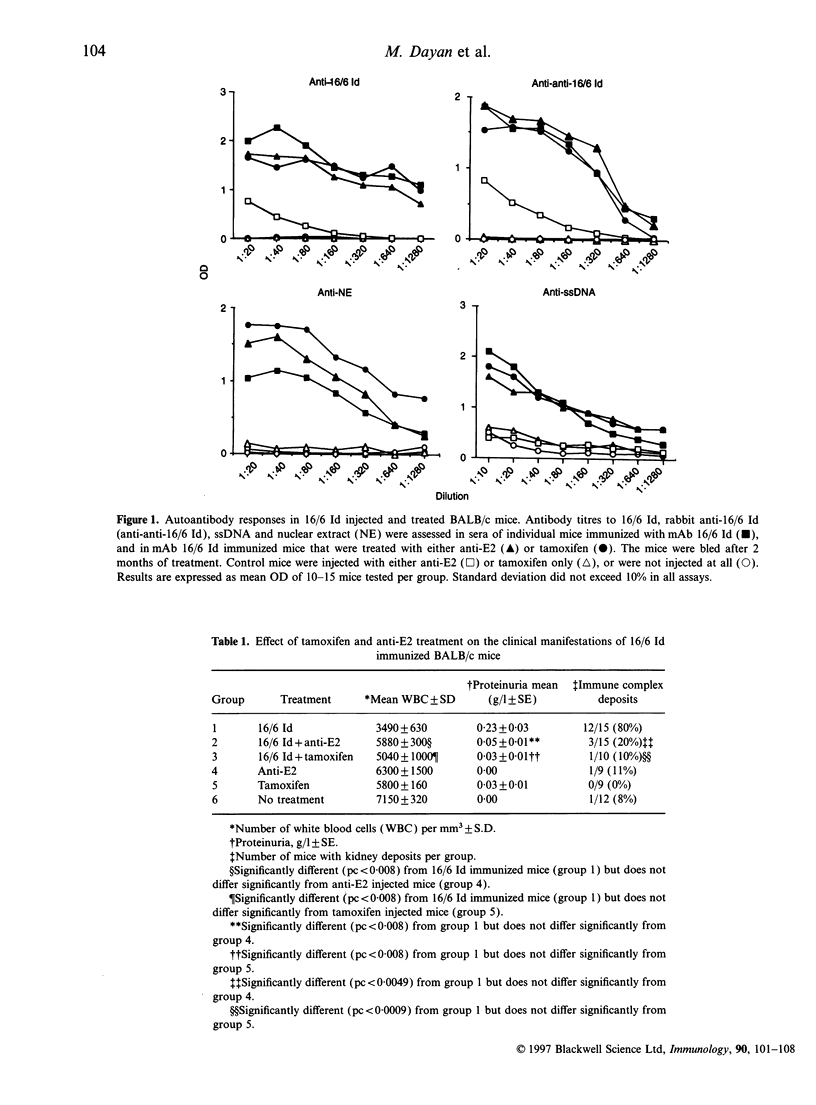
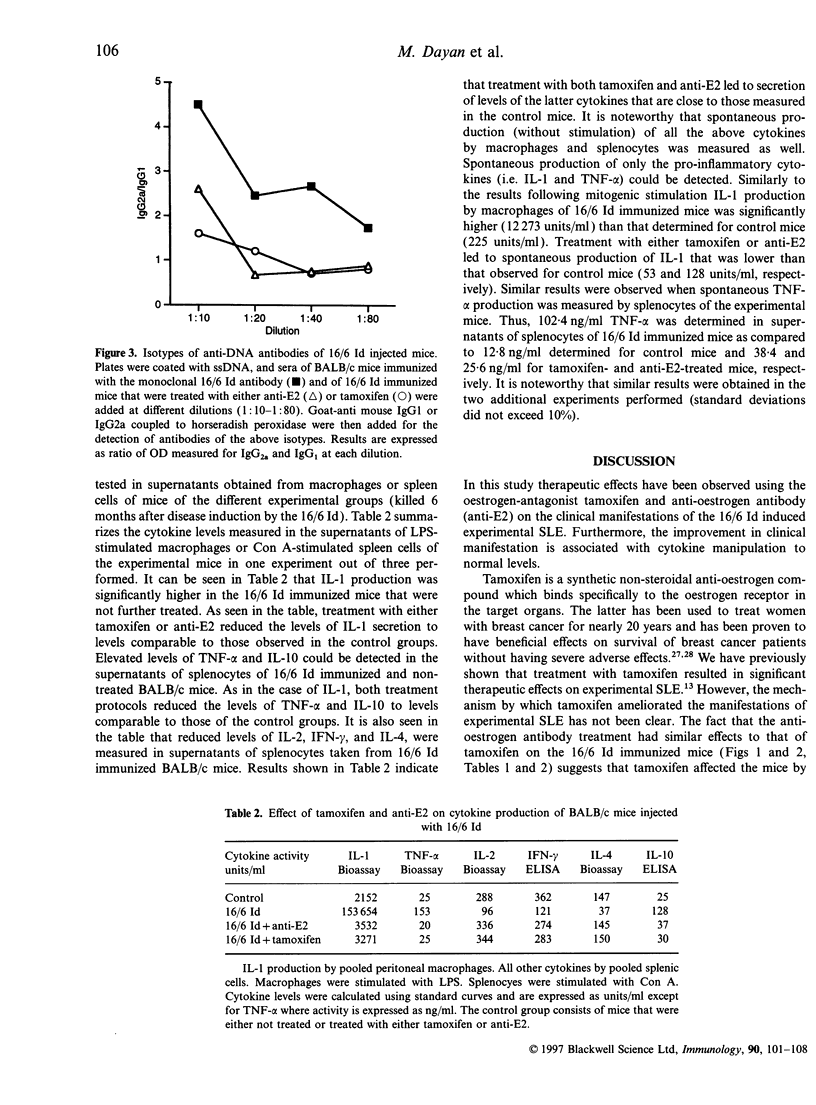
In an attempt to elucidate the role of oestrogens in systemic lupus erythematosus (SLE) we investigated the effects of treatment with an oestrogen antagonist-tamoxifen and a monoclonal anti-oestradiol (anti-E2) antibody on mice in which experimental systemic lupus erythematosus (SLE) was induced by a human monoclonal anti-DNA antibody bearing the 16/6 idiotype (16/6 Id). Thus, groups of BALB/c female mice were immunized with the 16/6 Id and 3 weeks following the booster injection, when antibody titres were elevated in the injected mice, treatment protocols with anti-oestradiol or tamoxifen were initiated. Control groups that were not immunized with the 16/6 Id but were similarly treated with the above agents were included in the study. The treatment with the above agents had no effect on the total autoantibody titres; however, a decrease in the immunoglobulin G (IgG)2a/IgG1 ratio of the anti-DNA antibodies was determined in the 16/6 Id immunized and treated mice. Further both the anti-oestradiol and tamoxifen had beneficial effects on the clinical manifestations (white blood cell counts, levels of protein in the urine and immune complex deposits in the kidneys) of the 16/6 Id immunized and treated mice. We have previously observed a significant elevation in interleukin-1 (IL-1) and tumour necrosis factor-alpha (TNF-alpha) secretion in mice with experimental SLE and a reduction in IL-2, IL-4 and interferon-gamma (INF-gamma) levels as compared with the levels detected in healthy controls. Treatment with either the anti-oestradiol antibody or with tamoxifen restored the levels of all the above cytokines to the normal levels observed in the control mice. These findings suggest that cytokine modulation may be the basis for the therapeutic effects of both anti-oestrogens in experimental SLE.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arzt E., Buric R., Stelzer G., Stalla J., Sauer J., Renner U., Stalla G. K. Interleukin involvement in anterior pituitary cell growth regulation: effects of IL-2 and IL-6. Endocrinology. 1993 Jan;132(1):459–467. doi: 10.1210/endo.132.1.8419142. [DOI] [PubMed] [Google Scholar]
- Barañao R. I., Tenenbaum A., Sales M. E., Rumi L. S. Functional alterations of murine peritoneal macrophages during pregnancy. Am J Reprod Immunol. 1992 Jan-Mar;27(1-2):82–86. doi: 10.1111/j.1600-0897.1992.tb00728.x. [DOI] [PubMed] [Google Scholar]
- Bateman A., Singh A., Kral T., Solomon S. The immune-hypothalamic-pituitary-adrenal axis. Endocr Rev. 1989 Feb;10(1):92–112. doi: 10.1210/edrv-10-1-92. [DOI] [PubMed] [Google Scholar]
- Bernton E. W., Beach J. E., Holaday J. W., Smallridge R. C., Fein H. G. Release of multiple hormones by a direct action of interleukin-1 on pituitary cells. Science. 1987 Oct 23;238(4826):519–521. doi: 10.1126/science.2821620. [DOI] [PubMed] [Google Scholar]
- Besedovsky H. O., del Rey A. Feed-back interactions between immunological cells and the hypothalamus-pituitary-adrenal axis. Neth J Med. 1991 Oct;39(3-4):274–280. [PubMed] [Google Scholar]
- Blank M., Mendlovic S., Fricke H., Mozes E., Talal N., Shoenfeld Y. Sex hormone involvement in the induction of experimental systemic lupus erythematosus by a pathogenic anti-DNA idiotype in naive mice. J Rheumatol. 1990 Mar;17(3):311–317. [PubMed] [Google Scholar]
- Davidson N. E. Tamoxifen--panacea or Pandora's box? N Engl J Med. 1992 Mar 26;326(13):885–886. doi: 10.1056/NEJM199203263261308. [DOI] [PubMed] [Google Scholar]
- Daynes R. A., Dudley D. J., Araneo B. A. Regulation of murine lymphokine production in vivo. II. Dehydroepiandrosterone is a natural enhancer of interleukin 2 synthesis by helper T cells. Eur J Immunol. 1990 Apr;20(4):793–802. doi: 10.1002/eji.1830200413. [DOI] [PubMed] [Google Scholar]
- Daynes R. A., Meikle A. W., Araneo B. A. Locally active steroid hormones may facilitate compartmentalization of immunity by regulating the types of lymphokines produced by helper T cells. Res Immunol. 1991 Jan;142(1):40–45. doi: 10.1016/0923-2494(91)90010-g. [DOI] [PubMed] [Google Scholar]
- Denicoff K. D., Durkin T. M., Lotze M. T., Quinlan P. E., Davis C. L., Listwak S. J., Rosenberg S. A., Rubinow D. R. The neuroendocrine effects of interleukin-2 treatment. J Clin Endocrinol Metab. 1989 Aug;69(2):402–410. doi: 10.1210/jcem-69-2-402. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvic M., Steinberg A. D., Klassen L. W. Effect of the anti-estrogen, Nafoxidine, on NZB/W autoimmune disease. Arthritis Rheum. 1978 May;21(4):414–417. doi: 10.1002/art.1780210403. [DOI] [PubMed] [Google Scholar]
- Fisher B., Brown A., Wolmark N., Redmond C., Wickerham D. L., Wittliff J., Dimitrov N., Legault-Poisson S., Schipper H., Prager D. Prolonging tamoxifen therapy for primary breast cancer. Findings from the National Surgical Adjuvant Breast and Bowel Project clinical trial. Ann Intern Med. 1987 May;106(5):649–654. doi: 10.7326/0003-4819-106-5-649. [DOI] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Hermus A. R., Sweep C. G. Cytokines and the hypothalamic-pituitary-adrenal axis. J Steroid Biochem Mol Biol. 1990 Dec 20;37(6):867–871. doi: 10.1016/0960-0760(90)90434-m. [DOI] [PubMed] [Google Scholar]
- Hu-Li J., Ohara J., Watson C., Tsang W., Paul W. E. Derivation of a T cell line that is highly responsive to IL-4 and IL-2 (CT.4R) and of an IL-2 hyporesponsive mutant of that line (CT.4S). J Immunol. 1989 Feb 1;142(3):800–807. [PubMed] [Google Scholar]
- Isenberg D. A., Shoenfeld Y., Madaio M. P., Rauch J., Reichlin M., Stollar B. D., Schwartz R. S. Anti-DNA antibody idiotypes in systemic lupus erythematosus. Lancet. 1984 Aug 25;2(8400):417–422. doi: 10.1016/s0140-6736(84)92904-0. [DOI] [PubMed] [Google Scholar]
- Karanth S., McCann S. M. Anterior pituitary hormone control by interleukin 2. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2961–2965. doi: 10.1073/pnas.88.7.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahita R. G. The influence of sex hormones on the disease systemic lupus erythematosus. Springer Semin Immunopathol. 1986;9(2-3):305–314. doi: 10.1007/BF02099028. [DOI] [PubMed] [Google Scholar]
- Mendlovic S., Brocke S., Shoenfeld Y., Ben-Bassat M., Meshorer A., Bakimer R., Mozes E. Induction of a systemic lupus erythematosus-like disease in mice by a common human anti-DNA idiotype. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2260–2264. doi: 10.1073/pnas.85.7.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendlovic S., Fricke H., Shoenfeld Y., Mozes E. The role of anti-idiotypic antibodies in the induction of experimental systemic lupus erythematosus in mice. Eur J Immunol. 1989 Apr;19(4):729–734. doi: 10.1002/eji.1830190424. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Naito Y., Fukata J., Tominaga T., Masui Y., Hirai Y., Murakami N., Tamai S., Mori K., Imura H. Adrenocorticotropic hormone-releasing activities of interleukins in a homologous in vivo system. Biochem Biophys Res Commun. 1989 Nov 15;164(3):1262–1267. doi: 10.1016/0006-291x(89)91805-6. [DOI] [PubMed] [Google Scholar]
- Ohnishi K., Ebling F. M., Mitchell B., Singh R. R., Hahn B. H., Tsao B. P. Comparison of pathogenic and non-pathogenic murine antibodies to DNA: antigen binding and structural characteristics. Int Immunol. 1994 Jun;6(6):817–830. doi: 10.1093/intimm/6.6.817. [DOI] [PubMed] [Google Scholar]
- Rook G. A., Hernandez-Pando R., Lightman S. L. Hormones, peripherally activated prohormones and regulation of the Th1/Th2 balance. Immunol Today. 1994 Jul;15(7):301–303. doi: 10.1016/0167-5699(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Roubinian J. R., Talal N., Greenspan J. S., Goodman J. R., Siiteri P. K. Delayed androgen treatment prolongs survival in murine lupus. J Clin Invest. 1979 May;63(5):902–911. doi: 10.1172/JCI109390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roubinian J., Talal N., Siiteri P. K., Sadakian J. A. Sex hormone modulation of autoimmunity in NZB/NZW mice. Arthritis Rheum. 1979 Nov;22(11):1162–1169. doi: 10.1002/art.1780221102. [DOI] [PubMed] [Google Scholar]
- Sarvetnick N., Fox H. S. Interferon-gamma and the sexual dimorphism of autoimmunity. Mol Biol Med. 1990 Aug;7(4):323–331. [PubMed] [Google Scholar]
- Segal R., Dayan M., Zinger H., Mozes E. Methotrexate treatment in murine experimental systemic lupus erythematosus (SLE); clinical benefits associated with cytokine manipulation. Clin Exp Immunol. 1995 Jul;101(1):66–72. doi: 10.1111/j.1365-2249.1995.tb02278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoenfeld Y., Rauch J., Massicotte H., Datta S. K., André-Schwartz J., Stollar B. D., Schwartz R. S. Polyspecificity of monoclonal lupus autoantibodies produced by human-human hybridomas. N Engl J Med. 1983 Feb 24;308(8):414–420. doi: 10.1056/NEJM198302243080802. [DOI] [PubMed] [Google Scholar]
- Spangelo B. L., Judd A. M., Isakson P. C., MacLeod R. M. Interleukin-6 stimulates anterior pituitary hormone release in vitro. Endocrinology. 1989 Jul;125(1):575–577. doi: 10.1210/endo-125-1-575. [DOI] [PubMed] [Google Scholar]
- Steinberg A. D., Roths J. B., Murphy E. D., Steinberg R. T., Raveche E. S. Effects of thymectomy or androgen administration upon the autoimmune disease of MRL/Mp-lpr/lpr mice. J Immunol. 1980 Aug;125(2):871–873. [PubMed] [Google Scholar]
- Sthoeger Z. M., Bentwich Z., Zinger H., Mozes E. The beneficial effect of the estrogen antagonist, tamoxifen, on experimental systemic lupus erythematosus. J Rheumatol. 1994 Dec;21(12):2231–2238. [PubMed] [Google Scholar]
- Tan E. M. Antinuclear antibodies: diagnostic markers for autoimmune diseases and probes for cell biology. Adv Immunol. 1989;44:93–151. doi: 10.1016/s0065-2776(08)60641-0. [DOI] [PubMed] [Google Scholar]
- Tominaga T., Fukata J., Naito Y., Usui T., Murakami N., Fukushima M., Nakai Y., Hirai Y., Imura H. Prostaglandin-dependent in vitro stimulation of adrenocortical steroidogenesis by interleukins. Endocrinology. 1991 Jan;128(1):526–531. doi: 10.1210/endo-128-1-526. [DOI] [PubMed] [Google Scholar]
- van Vollenhoven R. F., Engleman E. G., McGuire J. L. An open study of dehydroepiandrosterone in systemic lupus erythematosus. Arthritis Rheum. 1994 Sep;37(9):1305–1310. doi: 10.1002/art.1780370906. [DOI] [PubMed] [Google Scholar]



