Abstract
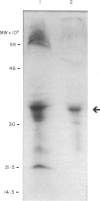
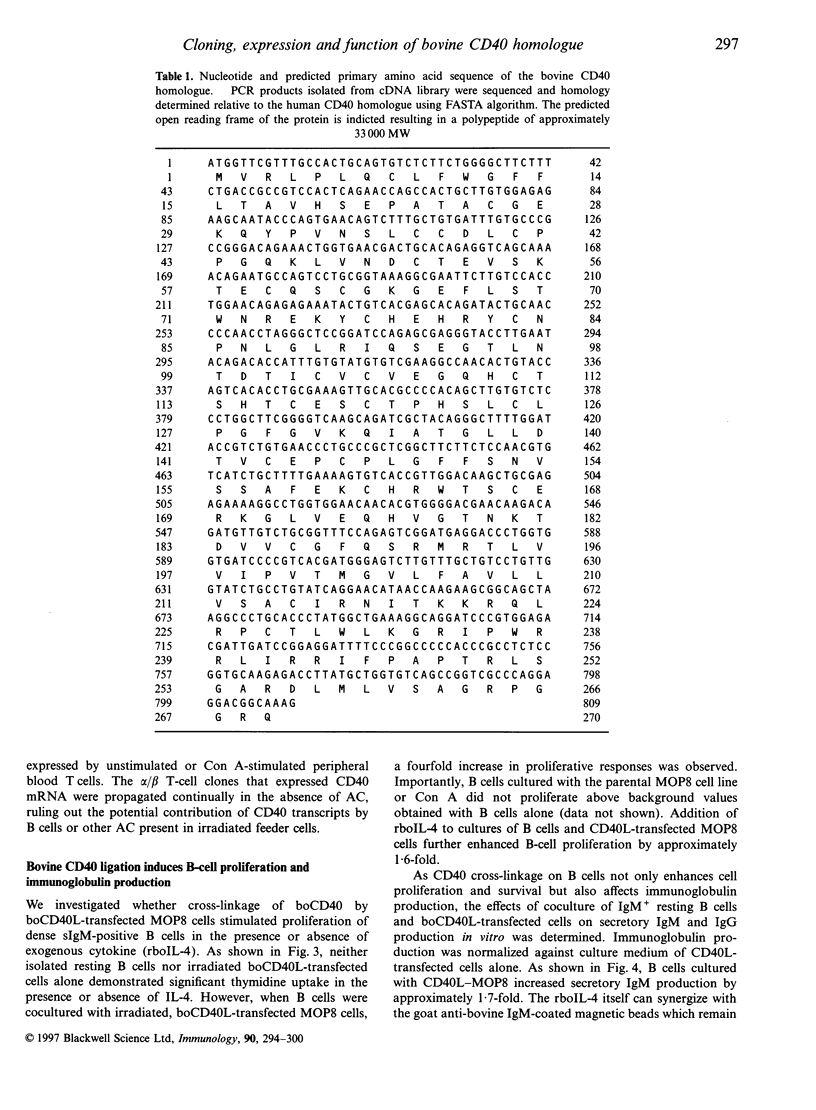
Members of the tumour necrosis factor receptor superfamily play a key role in B-lymphocyte survival, proliferation, differentiation and programmed cell death. A member of this superfamily, the CD40 molecule plays an important role in the differentiation of B lymphocytes into effector cells and in early activation through cognate interaction with T lymphocytes. In this report, we describe a cDNA and its protein product identified in cattle with approximately 70% sequence conservation at the nucleic acid level with the human CD40 gene. Transcripts for the boCD40 molecule were identified in resting and activated B lymphocytes, some but not all CD4+ and CD8+ T lymphocyte clones, and peripheral blood-derived T lymphocytes. Coculture of resting B cells with simian virus 40 (SV40)-transformed NIH3T3 (MOP8) cells stably transfected with the ligand for CD40 (bovine CD40 ligand, boCD40L), resulted in proliferation which was enhanced by addition of rbo interleukin-4 (IL-4). Cross-linkage of CD40 on bovine B lymphocytes upon coculture with CD40L-transfected cells resulted in the increased production of secretory IgM and, to a lesser extent, of IgG. Addition of rboIL-4 to these cultures increased levels of IgM and IgG secretion approximately twofold over those induced by CD40L alone. Our results indicate that many of the functions described for human and mouse CD40 are also conserved in the bovine but that differences in subset distribution of expression of the CD40 molecule in lymphoid cell types in cattle may impact on regulation of the early activation steps in the acquired immune response.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C., Armitage R. J., Conley M. E., Rosenblatt H., Jenkins N. A., Copeland N. G., Bedell M. A., Edelhoff S., Disteche C. M., Simoneaux D. K. CD40 ligand gene defects responsible for X-linked hyper-IgM syndrome. Science. 1993 Feb 12;259(5097):990–993. doi: 10.1126/science.7679801. [DOI] [PubMed] [Google Scholar]
- Armitage R. J., Fanslow W. C., Strockbine L., Sato T. A., Clifford K. N., Macduff B. M., Anderson D. M., Gimpel S. D., Davis-Smith T., Maliszewski C. R. Molecular and biological characterization of a murine ligand for CD40. Nature. 1992 May 7;357(6373):80–82. doi: 10.1038/357080a0. [DOI] [PubMed] [Google Scholar]
- Armitage R. J., Macduff B. M., Spriggs M. K., Fanslow W. C. Human B cell proliferation and Ig secretion induced by recombinant CD40 ligand are modulated by soluble cytokines. J Immunol. 1993 May 1;150(9):3671–3680. [PubMed] [Google Scholar]
- Aruffo A., Farrington M., Hollenbaugh D., Li X., Milatovich A., Nonoyama S., Bajorath J., Grosmaire L. S., Stenkamp R., Neubauer M. The CD40 ligand, gp39, is defective in activated T cells from patients with X-linked hyper-IgM syndrome. Cell. 1993 Jan 29;72(2):291–300. doi: 10.1016/0092-8674(93)90668-g. [DOI] [PubMed] [Google Scholar]
- Aversa G., Punnonen J., Cocks B. G., de Waal Malefyt R., Vega F., Jr, Zurawski S. M., Zurawski G., de Vries J. E. An interleukin 4 (IL-4) mutant protein inhibits both IL-4 or IL-13-induced human immunoglobulin G4 (IgG4) and IgE synthesis and B cell proliferation: support for a common component shared by IL-4 and IL-13 receptors. J Exp Med. 1993 Dec 1;178(6):2213–2218. doi: 10.1084/jem.178.6.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J., Bazan F., Blanchard D., Brière F., Galizzi J. P., van Kooten C., Liu Y. J., Rousset F., Saeland S. The CD40 antigen and its ligand. Annu Rev Immunol. 1994;12:881–922. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- Banchereau J., Rousset F. Growing human B lymphocytes in the CD40 system. Nature. 1991 Oct 17;353(6345):678–679. doi: 10.1038/353678a0. [DOI] [PubMed] [Google Scholar]
- Banchereau J., de Paoli P., Vallé A., Garcia E., Rousset F. Long-term human B cell lines dependent on interleukin-4 and antibody to CD40. Science. 1991 Jan 4;251(4989):70–72. doi: 10.1126/science.1702555. [DOI] [PubMed] [Google Scholar]
- Brown W. C., Davis W. C., Dobbelaere D. A., Rice-Ficht A. C. CD4+ T-cell clones obtained from cattle chronically infected with Fasciola hepatica and specific for adult worm antigen express both unrestricted and Th2 cytokine profiles. Infect Immun. 1994 Mar;62(3):818–827. doi: 10.1128/iai.62.3.818-827.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. C., Woods V. M., Chitko-McKown C. G., Hash S. M., Rice-Ficht A. C. Interleukin-10 is expressed by bovine type 1 helper, type 2 helper, and unrestricted parasite-specific T-cell clones and inhibits proliferation of all three subsets in an accessory-cell-dependent manner. Infect Immun. 1994 Nov;62(11):4697–4708. doi: 10.1128/iai.62.11.4697-4708.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark E. A., Ledbetter J. A. Activation of human B cells mediated through two distinct cell surface differentiation antigens, Bp35 and Bp50. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4494–4498. doi: 10.1073/pnas.83.12.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemons D. J., Besch-Williford C., Steffen E. K., Riley L. K., Moore D. H. Evaluation of a subcutaneously implanted chamber for antibody production in rabbits. Lab Anim Sci. 1992 Jun;42(3):307–311. [PubMed] [Google Scholar]
- Cocks B. G., de Waal Malefyt R., Galizzi J. P., de Vries J. E., Aversa G. IL-13 induces proliferation and differentiation of human B cells activated by the CD40 ligand. Int Immunol. 1993 Jun;5(6):657–663. doi: 10.1093/intimm/5.6.657. [DOI] [PubMed] [Google Scholar]
- Defrance T., Vanbervliet B., Brière F., Durand I., Rousset F., Banchereau J. Interleukin 10 and transforming growth factor beta cooperate to induce anti-CD40-activated naive human B cells to secrete immunoglobulin A. J Exp Med. 1992 Mar 1;175(3):671–682. doi: 10.1084/jem.175.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiSanto J. P., Bonnefoy J. Y., Gauchat J. F., Fischer A., de Saint Basile G. CD40 ligand mutations in x-linked immunodeficiency with hyper-IgM. Nature. 1993 Feb 11;361(6412):541–543. doi: 10.1038/361541a0. [DOI] [PubMed] [Google Scholar]
- Estes D. M., Closser N. M., Allen G. K. IFN-gamma stimulates IgG2 production from bovine B cells costimulated with anti-mu and mitogen. Cell Immunol. 1994 Apr 1;154(1):287–295. doi: 10.1006/cimm.1994.1078. [DOI] [PubMed] [Google Scholar]
- Estes D. M., Hirano A., Heussler V. T., Dobbelaere D. A., Brown W. C. Expression and biological activities of bovine interleukin 4: effects of recombinant bovine interleukin 4 on T cell proliferation and B cell differentiation and proliferation in vitro. Cell Immunol. 1995 Jul;163(2):268–279. doi: 10.1006/cimm.1995.1126. [DOI] [PubMed] [Google Scholar]
- Estes D. M., Teale J. M. Biochemical and functional analysis of extracellular stress proteins of Mesocestoides corti. J Immunol. 1991 Dec 1;147(11):3926–3934. [PubMed] [Google Scholar]
- Estes D. M., Turaga P. S., Sievers K. M., Teale J. M. Characterization of an unusual cell type (CD4+ CD3-) expanded by helminth infection and related to the parasite stress response. J Immunol. 1993 Mar 1;150(5):1846–1856. [PubMed] [Google Scholar]
- Foy T. M., Aruffo A., Bajorath J., Buhlmann J. E., Noelle R. J. Immune regulation by CD40 and its ligand GP39. Annu Rev Immunol. 1996;14:591–617. doi: 10.1146/annurev.immunol.14.1.591. [DOI] [PubMed] [Google Scholar]
- Kimata H., Fujimoto M. Induction of IgA1 and IgA2 production in immature human fetal B cells and pre-B cells by vasoactive intestinal peptide. Blood. 1995 Apr 15;85(8):2098–2104. [PubMed] [Google Scholar]
- Korthäuer U., Graf D., Mages H. W., Brière F., Padayachee M., Malcolm S., Ugazio A. G., Notarangelo L. D., Levinsky R. J., Kroczek R. A. Defective expression of T-cell CD40 ligand causes X-linked immunodeficiency with hyper-IgM. Nature. 1993 Feb 11;361(6412):539–541. doi: 10.1038/361539a0. [DOI] [PubMed] [Google Scholar]
- Layton J. E., Krammer P. H., Hamaoka T., Uhr J. W., Vitetta E. S. Small and large B cells respond differently to T cell-derived B cell growth and differentiation factors. J Mol Cell Immunol. 1985;2(3):155–167. [PubMed] [Google Scholar]
- Noelle R. J., Roy M., Shepherd D. M., Stamenkovic I., Ledbetter J. A., Aruffo A. A 39-kDa protein on activated helper T cells binds CD40 and transduces the signal for cognate activation of B cells. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6550–6554. doi: 10.1073/pnas.89.14.6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punnonen J., de Vries J. E. IL-13 induces proliferation, Ig isotype switching, and Ig synthesis by immature human fetal B cells. J Immunol. 1994 Feb 1;152(3):1094–1102. [PubMed] [Google Scholar]
- Roy M., Aruffo A., Ledbetter J., Linsley P., Kehry M., Noelle R. Studies on the interdependence of gp39 and B7 expression and function during antigen-specific immune responses. Eur J Immunol. 1995 Feb;25(2):596–603. doi: 10.1002/eji.1830250243. [DOI] [PubMed] [Google Scholar]
- Stamenkovic I., Clark E. A., Seed B. A B-lymphocyte activation molecule related to the nerve growth factor receptor and induced by cytokines in carcinomas. EMBO J. 1989 May;8(5):1403–1410. doi: 10.1002/j.1460-2075.1989.tb03521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren W. D., Berton M. T. Induction of germ-line gamma 1 and epsilon Ig gene expression in murine B cells. IL-4 and the CD40 ligand-CD40 interaction provide distinct but synergistic signals. J Immunol. 1995 Dec 15;155(12):5637–5646. [PubMed] [Google Scholar]