Abstract
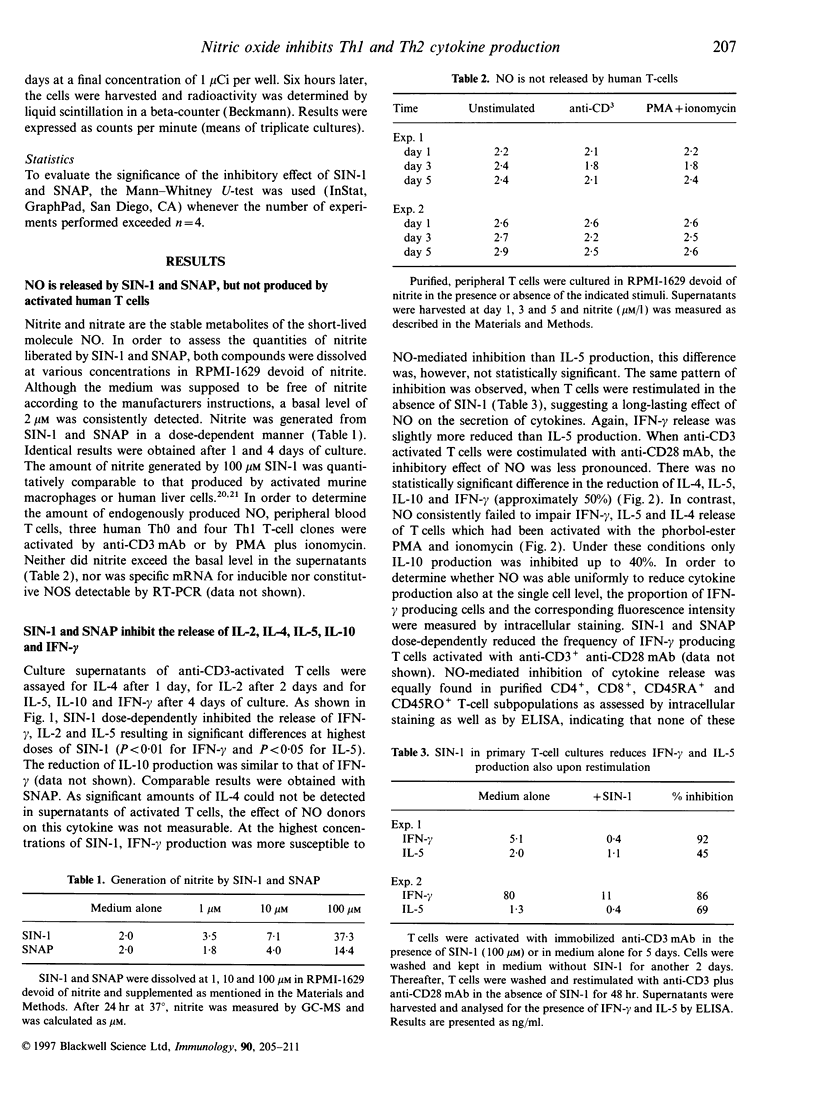
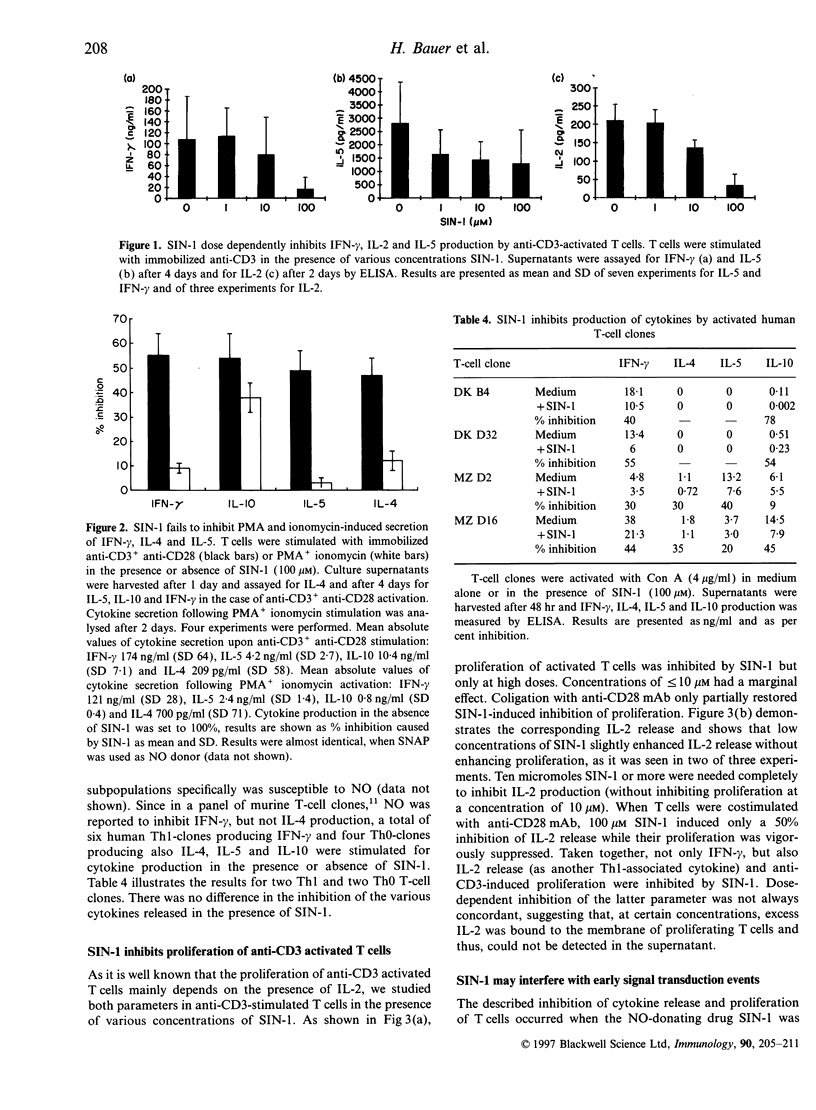
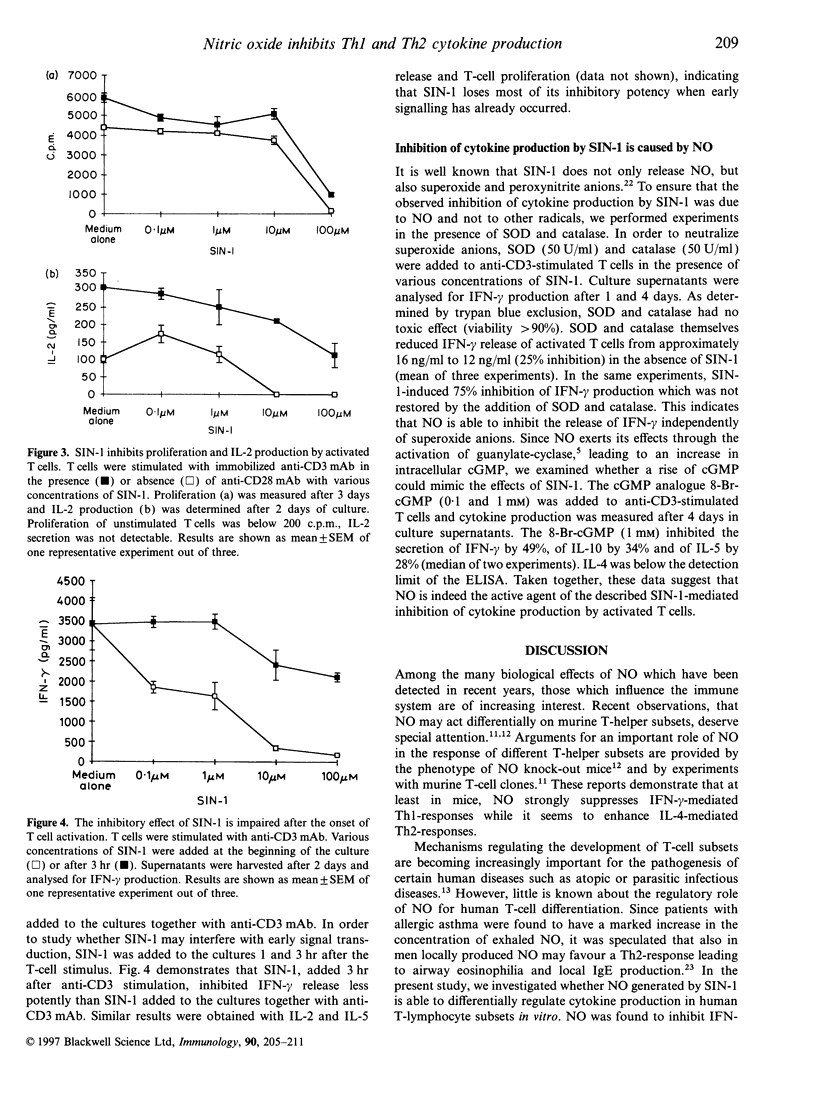
Mechanisms regulating the balance of T-helper 1 (Th1) and T-helper 2 (Th2) immune responses are of great interest as they may determine the outcome of allergic and infectious diseases. Recently, in mice, nitric oxide (NO), a powerful modulator of inflammation, has been reported to preferentially down-regulate Th1-mediated immune responses. In the present study, we investigated the effect of NO on the production of Th1- and Th2-associated cytokines by activated human T cells and human T-cell clones. Cytokine secretion was measured in the presence of the NO-donating agents 3-morpholinosydnonimine (SIN-1) and S-nitroso-N-acetylpenicillamine (SNAP). Both NO-donors markedly inhibited the release of interferon-gamma (IFN-gamma), interleukin-2 (IL-2), IL-5, IL-10 and IL-4 by anti-CD3 activated T cells. A preferential inhibition of Th1-associated cytokines was not observed. Neither was nitrite found in the supernatants of activated T cells, nor was specific mRNA for inducible and constitutive NO synthase detectable, indicating that T cells themselves did not contribute to the observed effect of the NO donors. Costimulation with anti-CD28 monoclonal antibodies (mAb) prevented SIN-1/SNAP-mediated down-regulation of cytokine production only in part. In contrast, when T cells were stimulated by phorbol-ester and ionomycin, they were refractory to SIN-1-induced inhibition of cytokine production. When SIN-1 was added after the onset of anti-CD3 stimulation, the inhibitory effect was found to be less pronounced, indicating that SIN-1 may interfere with early signal transduction events. The addition of superoxide dismutase (SOD) and catalase did not restore the effects of SIN-1, demonstrating that the inhibition of cytokines was due to NO and not to oxygen intermediates. Furthermore, 8-Br-cGMP-mediated increase of intracellular cGMP caused the same pattern of cytokine inhibition as observed with SIN-1 and SNAP. Using a single cell assay, these agents were shown to reduce the frequency of IFN-gamma-producing T cells, suggesting that not all T cells are susceptible to SIN-1/SNAP. However, cytokine production by purified T-cell subpopulations (CD4+, CD8+, CD45RA+, and CD45RO+) was equally impaired by NO donors. In conclusion, in contrast to the murine system, our results do not provide evidence that NO preferentially inhibits Th1-cytokine secretion of activated human T cells in vitro.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes P. J., Liew F. Y. Nitric oxide and asthmatic inflammation. Immunol Today. 1995 Mar;16(3):128–130. doi: 10.1016/0167-5699(95)80128-6. [DOI] [PubMed] [Google Scholar]
- Chan J., Xing Y., Magliozzo R. S., Bloom B. R. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J Exp Med. 1992 Apr 1;175(4):1111–1122. doi: 10.1084/jem.175.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cui S., Reichner J. S., Mateo R. B., Albina J. E. Activated murine macrophages induce apoptosis in tumor cells through nitric oxide-dependent or -independent mechanisms. Cancer Res. 1994 May 1;54(9):2462–2467. [PubMed] [Google Scholar]
- Ding A. H., Nathan C. F., Stuehr D. J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988 Oct 1;141(7):2407–2412. [PubMed] [Google Scholar]
- Gajewski T. F., Schell S. R., Fitch F. W. Evidence implicating utilization of different T cell receptor-associated signaling pathways by TH1 and TH2 clones. J Immunol. 1990 Jun 1;144(11):4110–4120. [PubMed] [Google Scholar]
- Garthwaite J. Glutamate, nitric oxide and cell-cell signalling in the nervous system. Trends Neurosci. 1991 Feb;14(2):60–67. doi: 10.1016/0166-2236(91)90022-m. [DOI] [PubMed] [Google Scholar]
- Geiger J., Nolte C., Walter U. Regulation of calcium mobilization and entry in human platelets by endothelium-derived factors. Am J Physiol. 1994 Jul;267(1 Pt 1):C236–C244. doi: 10.1152/ajpcell.1994.267.1.C236. [DOI] [PubMed] [Google Scholar]
- Gutgesell C., Yssel H., Scheel D., Gerdes J., Neumann C. IL-10 secretion of allergen-specific skin-derived T cells correlates positively with that of the Th2 cytokines IL-4 and IL-5. Exp Dermatol. 1994 Dec;3(6):304–313. doi: 10.1111/j.1600-0625.1994.tb00293.x. [DOI] [PubMed] [Google Scholar]
- Jung T., Schauer U., Heusser C., Neumann C., Rieger C. Detection of intracellular cytokines by flow cytometry. J Immunol Methods. 1993 Feb 26;159(1-2):197–207. doi: 10.1016/0022-1759(93)90158-4. [DOI] [PubMed] [Google Scholar]
- Jung T., Schauer U., Rieger C., Wagner K., Einsle K., Neumann C., Heusser C. Interleukin-4 and interleukin-5 are rarely co-expressed by human T cells. Eur J Immunol. 1995 Aug;25(8):2413–2416. doi: 10.1002/eji.1830250843. [DOI] [PubMed] [Google Scholar]
- Kwon N. S., Stuehr D. J., Nathan C. F. Inhibition of tumor cell ribonucleotide reductase by macrophage-derived nitric oxide. J Exp Med. 1991 Oct 1;174(4):761–767. doi: 10.1084/jem.174.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Los M., Dröge W., Stricker K., Baeuerle P. A., Schulze-Osthoff K. Hydrogen peroxide as a potent activator of T lymphocyte functions. Eur J Immunol. 1995 Jan;25(1):159–165. doi: 10.1002/eji.1830250127. [DOI] [PubMed] [Google Scholar]
- MacNaul K. L., Hutchinson N. I. Differential expression of iNOS and cNOS mRNA in human vascular smooth muscle cells and endothelial cells under normal and inflammatory conditions. Biochem Biophys Res Commun. 1993 Nov 15;196(3):1330–1334. doi: 10.1006/bbrc.1993.2398. [DOI] [PubMed] [Google Scholar]
- Marcinkiewicz J., Chain B. M. Differential regulation of cytokine production by nitric oxide. Immunology. 1993 Sep;80(1):146–150. [PMC free article] [PubMed] [Google Scholar]
- Nakazawa M., Imai S. Rp-8-Br-guanosine-3',5'-cyclic monophosphorothioate inhibits relaxation elicited by nitroglycerin in rabbit aorta. Eur J Pharmacol. 1994 Feb 21;253(1-2):179–181. doi: 10.1016/0014-2999(94)90775-7. [DOI] [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Reiling N., Kröncke R., Ulmer A. J., Gerdes J., Flad H. D., Hauschildt S. Nitric oxide synthase: expression of the endothelial, Ca2+/calmodulin-dependent isoform in human B and T lymphocytes. Eur J Immunol. 1996 Mar;26(3):511–516. doi: 10.1002/eji.1830260302. [DOI] [PubMed] [Google Scholar]
- Reiling N., Ulmer A. J., Duchrow M., Ernst M., Flad H. D., Hauschildt S. Nitric oxide synthase: mRNA expression of different isoforms in human monocytes/macrophages. Eur J Immunol. 1994 Aug;24(8):1941–1944. doi: 10.1002/eji.1830240836. [DOI] [PubMed] [Google Scholar]
- Romagnani S. Lymphokine production by human T cells in disease states. Annu Rev Immunol. 1994;12:227–257. doi: 10.1146/annurev.iy.12.040194.001303. [DOI] [PubMed] [Google Scholar]
- Sager N., Feldmann A., Schilling G., Kreitsch P., Neumann C. House dust mite-specific T cells in the skin of subjects with atopic dermatitis: frequency and lymphokine profile in the allergen patch test. J Allergy Clin Immunol. 1992 Apr;89(4):801–810. doi: 10.1016/0091-6749(92)90434-4. [DOI] [PubMed] [Google Scholar]
- Taylor-Robinson A. W., Liew F. Y., Severn A., Xu D., McSorley S. J., Garside P., Padron J., Phillips R. S. Regulation of the immune response by nitric oxide differentially produced by T helper type 1 and T helper type 2 cells. Eur J Immunol. 1994 Apr;24(4):980–984. doi: 10.1002/eji.1830240430. [DOI] [PubMed] [Google Scholar]
- Tsikas D., Böger R. H., Bode-Böger S. M., Gutzki F. M., Frölich J. C. Quantification of nitrite and nitrate in human urine and plasma as pentafluorobenzyl derivatives by gas chromatography-mass spectrometry using their 15N-labelled analogs. J Chromatogr B Biomed Appl. 1994 Nov 18;661(2):185–191. doi: 10.1016/0378-4347(94)00374-2. [DOI] [PubMed] [Google Scholar]
- Wei X. Q., Charles I. G., Smith A., Ure J., Feng G. J., Huang F. P., Xu D., Muller W., Moncada S., Liew F. Y. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995 Jun 1;375(6530):408–411. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- Yan L., Vandivier R. W., Suffredini A. F., Danner R. L. Human polymorphonuclear leukocytes lack detectable nitric oxide synthase activity. J Immunol. 1994 Aug 15;153(4):1825–1834. [PubMed] [Google Scholar]
- Zembala M., Siedlar M., Marcinkiewicz J., Pryjma J. Human monocytes are stimulated for nitric oxide release in vitro by some tumor cells but not by cytokines and lipopolysaccharide. Eur J Immunol. 1994 Feb;24(2):435–439. doi: 10.1002/eji.1830240225. [DOI] [PubMed] [Google Scholar]



