Abstract
Mcm10 is an essential protein that participates in both the initiation and the elongation of DNA replication. In this study we demonstrate a role for Mcm10 in the maintenance of heterochromatic silencing at telomeres and HM loci of budding yeast. Two mcm10 mutants drastically reduce silencing of both URA3 and ADE2 reporter genes integrated into these silent loci. When exposed to α-factor, mcm10 mutant cells display a “shmoo-cluster” phenotype associated with a defect in the maintenance of silencing. In addition, when combined with a defect in the establishment of silent chromatin, mcm10 mutants demonstrate a synergistic defect in HML silencing. Consistent with a direct silencing function, Mcm10p shows a two-hybrid interaction with Sir2p and Sir3p that is destroyed by the mcm10-1 mutation and dependent on the C-terminal 108 amino acids. Tethering GBD-MCM10 to a defective HMR-E silencer is not sufficient to restore silencing. Furthermore, mutations in MCM10 inhibit the ability of GBD-SIR3 to restore silencing when tethered to a defective HMR-E. Suppressor mutations in MCM2, which suppress the temperature sensitivity of mcm10-1, fail to overcome the mcm10-1 silencing defect, suggesting that MCM10's role in transcriptional silencing may be separate from its essential functions in DNA replication.
TRANSCRIPTIONAL silencing in the budding yeast Saccharomyces cerevisiae renders large regions of the genome inactive in a fashion similar to that in heterochromatic regions in higher eukaryotes (Grewal and Elgin 2002). Transcriptional silencing occurs at yeast telomeres and at silent mating-type (HM) loci, HML and HMR (reviewed in Rusche et al. 2003), which house silent endogenous copies of the mating-type genes (reviewed in Haber 1998). The HM loci are flanked by cis-acting regulatory elements called silencers (Feldman et al. 1984; Brand et al. 1985, 1987). These elements consist of A, E, and B sites that are bound by the origin recognition complex (ORC) (Bell et al. 1993; Micklem et al. 1993; Loo et al. 1995), Rap1 (Shore and Nasmyth 1987), and Abf1 (Diffley and Stillman 1988), respectively. Once bound, these factors recruit the silent information regulator (Sir) proteins, Sir1–4. Although Orc1 directly interacts with Sir1 and Rap1 with Sir4 to establish a silencing complex that includes Sir2 and Sir3, it is unclear if each of these proteins can be recruited to the silencer independently of each other. A stepwise spreading of the Sir2/3/4 complex from the silencer leads to the silencing of nearby genes (Hecht et al. 1996; Hoppe et al. 2002; Rusche et al. 2002). Telomeric silencing is similar to silencing at HM loci, with the exception that the Sir2/3/4 complex is recruited by Rap1 bound to telomeric DNA (Aparicio et al. 1991; Kyrion et al. 1993; Moretti et al. 1994; Rusche et al. 2003). Silencing is thought to consist of two phases, establishment and maintenance (Miller and Nasmyth 1984; Pillus and Rine 1989). During the establishment phase, silent chromatin is formed on nascent DNA and in the maintenance phase it has to be sustained until the next establishment event. Mutations in genes such as SIR1 and RAP1 can lead to defects in the establishment but not maintenance of silent chromatin (Pillus and Rine 1989; Mahoney et al. 1991; Sussel and Shore 1991), and mutations in genes such as CAC1 and SIR3 can cause maintenance but not establishment defects (Enomoto and Berman 1998; Enomoto et al. 2000).
A link between transcriptional silencing and DNA replication has been suggested by the observation that passage through S phase is required for the establishment of transcriptional silencing (Miller and Nasmyth 1984). Another connection between silencing and DNA replication is evidenced by the fact that silencers can be used to initiate replication of plasmids (Brand et al. 1987) and that some origins have silencer activity (Grunweller and Ehrenhofer-Murray 2002). However, other studies have demonstrated that the S-phase requirement does not involve DNA replication (Kirchmaier and Rine 2001; Li et al. 2001). In S. cerevisiae, DNA replication is a tightly regulated process initiated in early S phase at specific loci called origins of replication or autonomously replicating sequences (ARSs) (reviewed in Dutta and Bell 1997). Budding yeast have ∼400 such replication origins, but most have not been shown to play a role in silencing. Although the connection between DNA replication and transcriptional silencing is not completely understood, several DNA replication proteins have been implicated in silencing. These include S. cerevisiae ORC (Bell et al. 1993; Micklem et al. 1993), PCNA, RF-C, Cdc45 (Ehrenhofer-Murray et al. 1999; Zhang et al. 2000), and Mcm5 (Dziak et al. 2003). Similarly, DNA replication factors have also been implicated in the organization of chromatin structure in higher eukaryotes such as the fruit fly Drosophila melanogaster (Pflumm and Botchan 2001; Christensen and Tye 2003) and Schizosaccharomyces pombe (Bailis et al. 2003). One of these proteins, Mcm10, physically interacts with the heterochromatin factor HP1 (Christensen and Tye 2003; Kellum 2003) in D. melanogaster, indicating a possible direct role in chromatin structure.
MCM10 was originally identified in yeast screens that searched for mutants in DNA replication (Dumas et al. 1982; Solomon et al. 1992) and minichromosome maintenance (Maine et al. 1984). Mcm10 is an essential chromatin-associated protein (Homesley et al. 2000; Ricke and Bielinsky 2004; Yang et al. 2005). Temperature-sensitive mcm10 mutants show sluggish passage through S phase (Kawasaki et al. 2000), an inability to complete S phase (Merchant et al. 1997), and a replication fork pause phenotype at inactive origins of replication (Merchant et al. 1997). MCM10 has also been implicated in the initiation of DNA replication (Homesley et al. 2000; Wohlschlegel et al. 2002; Sawyer et al. 2004). While its exact roles in DNA replication are not yet completely understood, Mcm10 shows physical and genetic interactions with subunits of ORC (Merchant et al. 1997; Homesley et al. 2000; Kawasaki et al. 2000; Douglas 2003), which has been implicated in heterochromatin-induced silencing. Intriguingly, there are estimated to be ∼40,000 copies of Mcm10p per cell (Kawasaki et al. 2000), far exceeding in abundance the ∼400 DNA replication origins in budding yeast. Although the reason for such abundance is not clear, it seems likely that Mcm10 may have other, previously unidentified functions.
In this study we demonstrate that Mcm10 plays a role in heterochromatin silencing in S. cerevisiae. Two mutant alleles of MCM10 manifest significant defects in silencing at both the telomeric and HMR loci. Consistent with this observation, when mcm10 mutant cells are exposed to α-factor, they show a “shmoo-cluster” phenotype, signifying a defect in the maintenance of silent chromatin at the HML locus (Enomoto and Berman 1998). We also found two-hybrid interactions between Mcm10 and components of the silencing machinery and that these interactions are disrupted by the Mcm10-1 and Mcm10-43 mutant proteins. Interestingly, the temperature-sensitive phenotype of mcm10 mutant cells can be genetically separated from the silencing defect by second-site mutations that suppress the temperature sensitivity but not the silencing defect. Together our findings demonstrate a novel function for MCM10 in the maintenance of silent chromatin that may be separate from its essential role in DNA replication.
MATERIALS AND METHODS
Strains and plasmids:
Strains used in this study are listed in Table 1. All strains were isogenic derivatives of W303-1A, unless otherwise indicated. All procedures were performed according to standard yeast methodology (Sherman 1991). Strains carrying silencing reporters were made by crossing strain YB541 or YB697 to the appropriate mutant strain and selecting desired segregants by their conditional phenotypes and/or auxotrophy. Genotypes were confirmed by PCR or by plasmid complementation where applicable. Plasmids used in this study are listed in Table 2. Plasmids used for the expression of tethering fusions were constructed by the Gateway system (Invitrogen, San Diego). To create pGBKgw, the kanr gene of plasmid pGBKT7 (CLONTECH, Palo Alto, CA) was replaced with an ampr gene and a Gateway (Invitrogen) cassette was inserted into the multiple-cloning sequence. Gateway cassettes were ligated into plasmids pBTM116 and pGAD2F, creating pBTMgw and pGADgw, respectively. Gateway cassette B was ligated into the SmaI site of pGBT9.C (Bartel et al. 1996), creating plasmid pGBT9gw. pDONR201 entry clones containing MCM10 and SIR1 ready for N-terminal fusions were constructed according to Invitrogen instructions and sequenced. LR recombination reactions (Invitrogen) were set up between pGBT9gw, pGADgw, pBTMgw, or pGBKgw and each of the aforementioned entry clones. All yeast transformations were carried out using standard lithium acetate protocols.
TABLE 1.
Strains used in this study
| Strains | Genotype | Source |
|---|---|---|
| Isogenic to W303 | ||
| W303-1A | MATaade2-1 trp1-1 can1-100 leu2-3,112 his3-11,15 ura3-1 | |
| W303-1B | MATα ade2-1 trp1-1 can1-100 leu2-3,112 his3-11,15 ura3-1 | |
| BTY100 | W303 MATamcm10-1 | This lab |
| BTY101 | W303 MATα mcm10-1 | This lab |
| BTY103 | W303 MATamcm10-43 | This lab |
| BTY102 | W303 MATα mcm10-43 | This lab |
| YB541 | MATaadh4∷URA3 Tel (VII-L) | B. Stillman |
| YB697 | MATα hmr∷ADE2 | B. Stillman |
| ILY171 | W303 MATahmr∷ADE2 adh4∷URA3 Tel (VII-L) | This study |
| ILY180 | ILY171 mcm10-1 | This study |
| ILY270 | ILY171 mcm10-43 | This study |
| GCY23 | W303 MATaRDN1∷MET15 ΔAhmr∷TRP1 adh4∷URA3 Tel (VII-L) sir2Δ met15 ADE2 | D. Shore |
| ILY163 | W303 MATaadh4∷ADE2 Tel (VII-L) | This study |
| ILY165 | ILY163 mcm10-1 | This study |
| ILY167 | ILY163 mcm10-43 | This study |
| YJB1838 | W303 MATacac1∷LEU2 | J. Berman |
| YJB1940 | W303 MATasir1∷HIS3 | J. Berman |
| ILY153 | W303 MATα sir1∷HIS3 | This study |
| ILY154 | W303 MATα cac1∷LEU2 | This study |
| ILY155 | YJB1940 mcm10-1 | This study |
| ILY156 | ILY153 mcm10-1 | This study |
| ILY157 | YJB1940 mcm10-43 | This study |
| ILY158 | ILY153 mcm10-43 | This study |
| ILY159 | YJB1838 mcm10-1 | This study |
| ILY160 | ILY154 mcm10-1 | This study |
| ILY161 | YJB1838 mcm10-43 | This study |
| ILY162 | ILY154 mcm10-43 | This study |
| ILY155 | YJB1940 mcm10-1 | This study |
| ILY157 | YJB1940 mcm10-43 | This study |
| ILY159 | YJB1838 mcm10-1 | This study |
| ILY161 | YJB1838 mcm10-43 | This study |
| ILY212 | W303 MATasir1∷HIS3 cac1∷LEU2 | This study |
| ILY235 | YB541 sir1∷HIS3 | This study |
| ILY237 | YB541 cac1∷LEU2 | This study |
| ILY239 | ILY235 mcm10-1 | This study |
| ILY241 | ILY237 mcm10-1 | This study |
| ILY220 | ILY171 Mcm2-S619Y | This study |
| ILY221 | ILY171 Mcm2-S619F | This study |
| ILY222 | ILY171 Mcm2-S619Y mcm10-1 | This study |
| ILY223 | ILY171 Mcm2-S619F mcm10-1 | This study |
| YEA80 | MATα Aeb∷UASg hmr∷URA3 | R. Sternglanz |
| YEA82 | MATα aeB∷UASg hmr∷URA3 | R. Sternglanz |
| ILY137 | YEA80 MATamcm10-1 | This study |
| ILY139 | YEA80 MATamcm10-43 | This study |
| Other backgrounds | ||
| EGY40[pSH18-34] | MATaura3-52 trp1-1 leu2-3,112 [pSH18-34] | E. Golemis |
| ILY267 | EGY40[pSH18-34] sir4∷HIS3 | This study |
| ILY268 | EGY40[pSH18-34] sir3∷HIS3 | This study |
| ILY269 | EGY40[pSH18-34] sir2∷HIS3 MATaura3-52 his3-Δ200 leu2-3,112 trp1-901 gal4Δ gal80Δ | This study |
| PJ69-4a | LYS2∷GAL1-HIS3 GAL2-ADE2 met2∷GAL7-LacZ | P. James |
| 6697-1 | MATamet4 | This lab |
| 6697-3 | MATα met4 | This lab |
TABLE 2.
Plasmids used in this study
| Plasmid name | Description | Source |
|---|---|---|
| pRS315 | YCP LEU2 | New England Biolabs |
| pRS315MCM10 | YCP LEU2 MCM10 | This lab |
| pGAD2F | 2μ LEU2 GAD4-AD | S. Fields |
| pBTM116 | 2μ TRP1 LEXA-DBD | S. Fields |
| pSH18-34 | URA3 LacZ with LEXA binding sites | S. Fields |
| pGADgw | pGAD2F with Gateway cassette | This study |
| pBTMgw | pBTM116 with Gateway cassette | This study |
| pGBKgw | pGBKT7 with Gateway cassette ampr | This study |
| pBTMMCM10 | pBTMgw MCM10 | This study |
| pBTMmcm10-1 | pBTMgw mcm10-1 | This study |
| pBTMmcm10-43 | pBTMgw mcm10-43 | This study |
| pBTMMCM10-N128 | pBTMgw MCM10 (128-571) | This study |
| pBTMMCM10-RIGHT | pBTMgw MCM10 (292-571) | This study |
| pBTMMCM10-C108 | pBTMgw MCM10 (1-463) | This study |
| pBTMMCM10-LEFT | pBTMgw MCM10 (1-291) | This study |
| pBTMMCM10-MIN | pBTMgw MCM10 (128-463) | This study |
| pBTMSIR2 | pBTMgw SIR2 | This study |
| pBTMSIR3 | pBTMgw SIR3 | This study |
| pBTMSIR4 | pBTMgw SIR4 | This study |
| pGADSIR1 | pGADgw SIR1 | This study |
| pGADSIR2 | pGADgw SIR2 | This study |
| pGADSIR3 | pGADgw SIR3 | This study |
| pGADSIR4 | pGADgw SIR4 | This study |
| pGADRAP1 | pGADgw RAP1 | This study |
| pGADMCM10 | pGADgw MCM10 | This study |
| pGADmcm10-1 | pGADgw mcm10-1 | This study |
| pGBKMCM10 | pGBKgw MCM10 | This study |
| pGBKmcm10-1 | pGBKgw mcm10-1 | This study |
| pGBT9.C | YCP GBD cloning vector TRP1 | P. Bartel |
| pGBT9gw | pGBT9.C with Gateway cassette | This study |
| pGBT9MCM10 | pGBDgw MCM10 | This study |
| pGBT9SIR1 | pGBDgw SIR1 | This study |
| pM165 | GBD-Sir1p HIS3 2μm | D. Shore |
| pM2046 | GBD-Sir2p HIS3 RAP1prom | D. Shore |
| pM1588 | GBD-Sir4p HIS3 RAP1prom | D. Shore |
Silencing assays:
Strains bearing the URA3 reporter were grown overnight in appropriate dropout media. Tenfold serial dilutions were set up in sterile 96-well plates and ∼25 μl of each dilution was spotted onto the appropriate plate using a multipipettor. 5-Fluoroorotic acid (5-FOA) was used at a concentration of 1 mg/ml unless otherwise stated. In cases where the ADE2 reporter gene was used, cultures were diluted to ∼300 cells/dropout plate. Colonies were grown for 3 days at 30° and incubated for 3 days at 4° for color development before being counted.
α-Factor confrontation (shmooing) and mating assays:
For the shmooing assay, 50 μl of a saturated MATα culture was streaked along the middle of a YPD plate and incubated at 30° for 4–6 hr for the α-factor to diffuse into the media. A small sample of MATa culture being tested was then spotted near the edge of the plate and a dissecting microscope needle was used to line up individual unbudded MATa cells approximately one-half of an optical field (at ×160 magnification) away from the MATα streak. Cells were incubated at 30° for 16–18 hr and the resulting phenotypes of MATa cells were scored. For patch-mating assays, the strains being tested were streaked in a parallel pattern on YPD plates, grown overnight at 30°, and then replica plated onto tester strains 6697-1 or 6697-3, of opposite mating type on a minimal media plate. Plates were then incubated for 2 days at 30°.
Protein-protein interactions:
The pGADgw SIR and RAP1 two-hybrid plasmids were constructed using the Invitrogen Gateway cloning system. pGAD2F and pBTM116 plasmids (Fields and Song 1989) were converted into destination vectors by insertion of the Gateway recombination cassette into the BamHI site. The full-length pBTM.MCM10 was described in Merchant et al. (1997). pGAD2F and pBTM116 constructs were transformed into the two-hybrid strain EGY40 carrying the pSH18-34 reporter plasmid (Fields and Song 1989). Interactions were assessed by the appearance of blue colonies on plates containing X-gal (Sigma, St. Louis). Ten microliters of a saturated culture started from 10 to 20 colonies per transformation was spotted onto X-gal plates for photographs.
Microcolony growth rate assay:
Individual, unbudded YB541 cells from an overnight culture were lined up in a row parallel to a row of ILY100 cells, using a dissecting microscope. Cells were incubated on media plates as indicated and pictures were taken at ×160 magnification.
mcm10-1 suppressor screen:
One-hundred-microliter samples of overnight cultures of a mcm10-1 strain were plated on YPD plates and incubated for 2 days at 37°. Resulting colonies were screened for recessive cold sensitivity at 14°. Tetrad analysis was used to identify single-gene second-site suppressors. The mutations were cloned by transforming a YCp50 yeast genomic library (Rose et al. 1987) and screening for complementation of the cold-sensitive defect followed by sequencing.
RESULTS
mcm10 mutations affect silencing at telomeric and HMR loci:
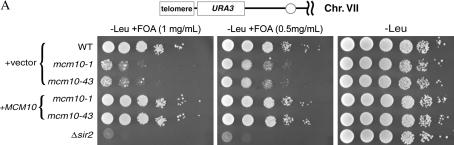
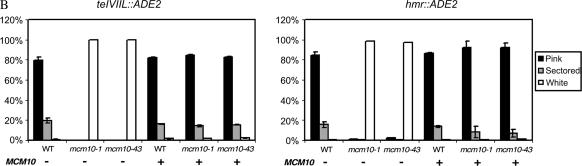
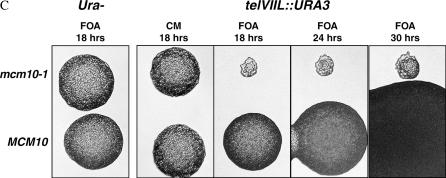
To investigate the effects of mcm10 mutants on silencing, we constructed a series of isogenic strains containing recessive, temperature-sensitive mcm10 mutations (mcm10-1 or mcm10-43) as well as reporter genes (URA3 and ADE2) integrated at the telomeric and HMR silent loci in the yeast genome. In the URA3 constructs, silencing is assayed by measuring the sensitivity of cells to 5-FOA, a toxic precursor of uracil that kills cells expressing the URA3 gene product. If URA3 is silenced, more cells will survive on 5-FOA and if silencing is defective, more cells become inviable. In strains containing a telVIIL∷URA3 reporter, mcm10 mutant cells showed a sensitivity to 5-FOA that is approximately 4 orders of magnitude greater than that observed in wild-type cells (Figure 1A). In addition, a larger proportion of mcm10 cells with the telomeric URA3 reporter were able to form colonies on media lacking uracil than that observed in MCM10 cells with the same reporter (data not shown). Derepression of the URA3 reporter in these experiments indicates that the mcm10 mutants are defective in telomeric silencing. To ensure that the silencing defect of the mcm10 mutants is not promoter specific, we also assayed telomeric silencing by utilizing a telVIIL∷ADE2 reporter (Figure 1B, left). If the ADE2 gene is silenced, the resulting yeast cells will form pink colonies, whereas loss of ADE2 silencing will give rise to white colonies. In wild-type cells, the vast majority of the colonies were either pink or white/pink sectored, but mcm10 colonies were exclusively white. The results from this experiment showed that mcm10 mutations engender a dramatic derepression of ADE2 at the telomere.
Figure 1.
MCM10 is required for proper telomeric and HMR silencing. (A) Telomeric silencing assays with mcm10 mutants. Strains ILY171, ILY180, ILY270, and GCY23, transformed with either pRS315 (top three rows and bottom row) or pRS315MCM10 (rows labeled “+MCM10”) are shown. Tenfold serial dilutions of overnight cultures were plated on CM-LEU media with or without the indicated concentration of 5-FOA. Plates were grown for 2 days at 30°. (B) Silencing assays of mcm10 mutants using the ADE2 reporter gene. Cultures were grown overnight in liquid selective media, diluted, and plated on CM-URA plates at ∼300 cells/plate. Plates were grown for 3 days at 30° and then incubated at 4° for 3 days before colonies were scored for color. Strains ILY171, ILY180, and ILY270 were used for the left and ILY163, ILY165, and ILY167 for the right. All strains were transformed with pRS315 (−) or pRS315MCM10 (+). All data points are the averages of at least two trials. Error bars that appear to be missing are too small to be displayed.
To investigate whether the silencing defect of the mcm10 mutants also applies to other silent loci, we integrated ADE2 into the HMR locus. Results from this experiment were similar to those observed at the telomeric locus; mcm10 strains with a hmr∷ADE2 reporter gave rise to almost exclusively white colonies, whereas wild-type strains with the same reporter were predominantly pink (Figure 1B, right). These results indicate that the silencing defect of mcm10 mutants affects both HMR and telomeric loci.
To investigate whether the silencing defects caused by these mcm10 mutations are dominant or recessive, single-copy ARS/CEN plasmids with or without a wild-type copy of MCM10 were transformed into each of the reporter strains. The presence of a MCM10-expressing plasmid restored silencing in all mcm10 mutant strains to wild-type levels whereas empty vectors alone did not (Figure 1, A and B). Together these findings show that the silencing defects caused by both mcm10-1 and mcm10-43 mutations are recessive and independent of reporter genes used and that they have a global effect on telomeric and HMR loci alike.
Mcm10p plays a role in the maintenance but not the establishment of silent chromatin at HML:
To address the role of MCM10 in HML silencing, we asked whether MCM10 is required for proper silencing of the endogenous MATα1 and MATα2 genes present at the HML locus. A wild-type MATa cell responds to a pheromone (α-factor) produced by MATα cells by arresting and forming a projection called a “shmoo.” A MATa cell where the HMLα is derepressed expresses information of both mating types and is no longer responsive to α-factor (Pillus and Rine 1989). Therefore, it is possible to measure the silencing of HMLα in individual MATa cells by assaying their response to α-factor. Furthermore, by comparing how mcm10 mutant cells respond to α-factor to known silencing mutants it is possible to determine whether mcm10 mutants are defective in the establishment or in the maintenance of HML silencing.
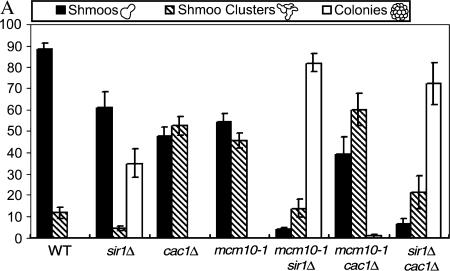
A sir1 mutant, which has a defect in establishment, but not in maintenance of the silent state (Pillus and Rine 1989; Rusche et al. 2002), formed shmoos and colonies representing the silenced and derepressed populations of cells, respectively (Figure 2A). In our study, ∼30% of the sir1Δ cells formed colonies, consistent with previous results (Pillus and Rine 1989). Cac1 is the largest subunit of the chromatin assembly factor 1 (CAF-1) complex that deposits histones H3/H4 onto newly replicated DNA (Verreault et al. 1996). A cac1Δ strain, which is defective in the maintenance of the silent state, but not in its establishment (Enomoto et al. 1997; Enomoto and Berman 1998) did not form any colonies, but formed an increased number of shmoo clusters (Figure 2A). This phenotype is indicative of cells that are able to respond to α-factor, but are not able to maintain the arrested phenotype for an extended period of time and divide before arresting and becoming shmoos again. Both shmoos and shmoo clusters represent cells that can respond to α-factor, indicating a stable establishment of silencing. The difference is that the cells that form shmoos are able to stably maintain their silent state for a prolonged period of time, whereas cells that form shmoo clusters are not. Both mcm10 mutants (only mcm10-1 data shown) displayed a phenotype similar to that of the cac1Δ strain but not to that of the sir1Δ strain (Figure 2A). Since mcm10 cells did not form colonies, it is likely that the establishment pathway in these mutants is still intact, since all cells are able to respond to α-factor. These results suggest that Mcm10p plays a role in maintaining the HML silent chromatin, rather than establishing it.
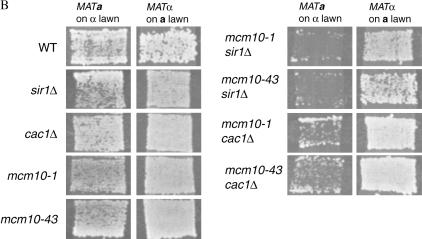
Figure 2.
Mcm10 is involved in the maintenance of HML silencing. (A) Shmoo phenotype analysis of mcm10-1. Individual, unbudded cells from strains W303-1A, YJB1940, YJB1838, BTY100, ILY155, ILY159, and ILY212 were exposed to α-factor overnight on YPD plates before their shmooing phenotypes were scored. Approximately 150 cells of each strain were analyzed in three independent trials. (B) Patch-mating assays of various mcm10 and silencing mutants. Cells of the indicated relevant genotype were mated to tester strains. MATα strains were mated to tester strain 6697-1 (a-lawn) and MATa strains were mated to 6697-3 (α-lawn). Mated cells were allowed to grow on SD plates at 30° for 2 days. (C) mcm10 mutants are defective in the maintenance/inheritance of telomeric silencing. The rates of colony size increase of mutant (top) and wild-type (bottom) strains were compared by lining up individual unbudded cells on synthetic complete media with or without 5-FOA. BTY100 (top, left), W303-1A (bottom, left), ILY180 (top, right four panels), and YB541 (bottom, right four panels) are shown. The three right panels show pictures of the same two colonies at different time intervals. At least 30 such cells were analyzed. Plates were incubated at 30°.
To confirm our previous results, we tested the response of double mutants to α-factor. sir1Δ mcm10 cells showed a striking ablation of silencing, just like a sir1Δ cac1Δ strain (Figure 2A). In these strains, cells were almost completely unresponsive to α-factor, with ∼85% of cells becoming colonies, whereas only ∼5% of cells were able to form shmoos and ∼10% formed shmoo clusters. This observation indicates a much greater derepression of the HMLα locus in the double mutants than in either of the single mutants. In strains bearing both cac1Δ and mcm10 mutations there was a 10–20% increase in the number of shmoo clusters formed (Figure 2A), signifying a slightly greater defect in the maintenance of the silent state than in either of the two mutants alone. The absence of colonies formed by the mcm10 cac1Δ double mutant supports the notion that both proteins play a role in maintenance, but not establishment of silencing at HML.
Another way to measure HML silencing is to measure the ability of MATa cells bearing appropriate mutations to mate with MATα cells in patch-mating assays (Figure 2B). If the HMLα region is derepressed in MATa cells, the cells will not mate efficiently. This experiment can also be done in reverse to measure silencing of the HMRa locus by assaying the ability of MATα cells to mate. In our studies none of the single mutants of MCM10, SIR1, or CAC1 showed mating defects for either mating type, whereas the double mutants did. sir1Δ mcm10 double mutants showed significant MATa-specific mating defects (Figure 2B), consistent with an abolishment of HMLα silencing observed in Figure 2A. cac1Δ mcm10 double mutants showed a less dramatic MATa-specific mating defect. Similar effects have been observed before with other maintenance mutants (Enomoto et al. 2000). Together these findings are consistent with a role for Mcm10 in the maintenance of silencing.
mcm10 mutants have a defect in the maintenance and/or inheritance of telomeric silencing:
Pillus and Rine (1989) showed that HM chromatin in sir1 mutant cells can stably exist in one of two epigenetic states—silent and active. These cells are able to propagate this state to their progeny for many generations. To stably propagate silencing to the next generation, a mother cell must also maintain the silent chromatin until it is inherited by the daughter cell. Inheritance of the silent state can be assayed by measuring how often a silenced mother cell gives rise to a derepressed daughter cell (Monson et al. 1997). In the case where a telomeric URA3 silencing reporter is used, when a cell divides, if the silencing is not properly inherited by the daughter cell, then it will be Ura+ and will die in the presence of 5-FOA. Thus, a telVIIL∷URA3 cell with a defect in the inheritance or maintenance of silencing will lead to a slower growing colony, since a proportion of its progeny will be dying on 5-FOA. By observing the rate of colony growth on 5-FOA it is possible to assay maintenance/inheritance of telomeric silencing in cells bearing a URA3 reporter. However, this assay is not able to adequately distinguish between defects in the inheritance and the maintenance of silent chromatin, since the silencing may be lost due to a maintenance defect before the inheritance step takes place.
To investigate whether mcm10 mutant cells properly propagate the telomeric silent state, we placed individual wild-type and mutant unbudded cells containing the telVIIL∷URA3 reporter on media containing 5-FOA and compared growth rates by colony size (Figure 2C). MCM10 ura3 cells bearing the telomeric URA3 reporter proliferated at a rate comparable to that of isogenic cells without the reporter whether on 5-FOA or on regular complete media. Similarly, mcm10-1 ura3 cells grew into colonies as fast as MCM10 ura3 cells on 5-FOA. In contrast, although mcm10-1 cells bearing the URA3 reporter proliferated at a rate comparable to that of their wild-type counterparts on media without 5-FOA, 5-FOA significantly retarded their proliferation. Similar results were observed for mcm10-43 cells (not shown). These results suggest that mcm10 mutants are defective in the maintenance and/or inheritance of silent telomeric chromatin.
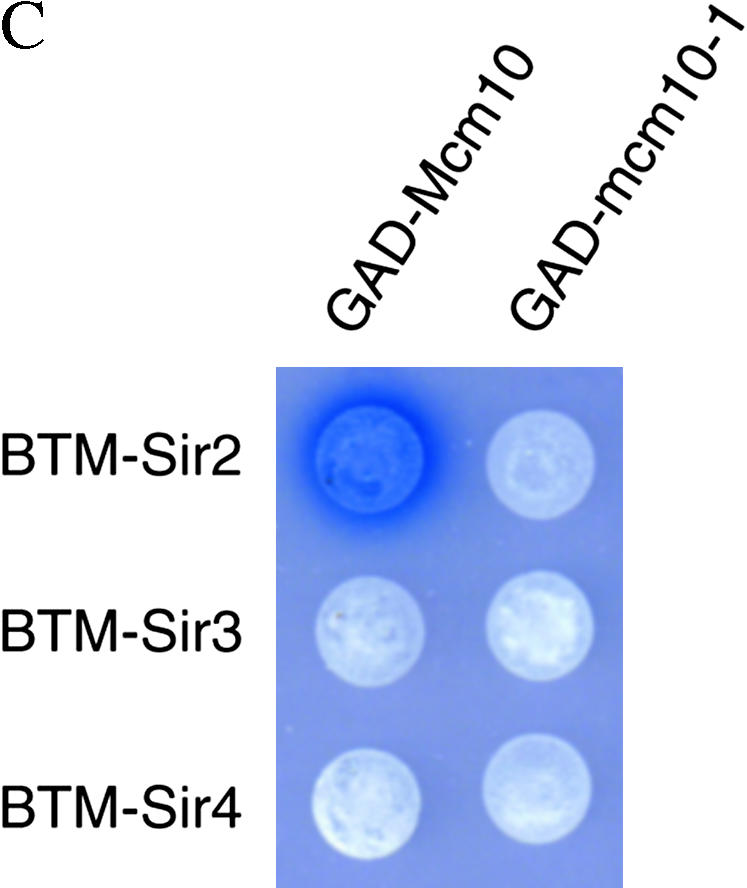
Mcm10 interacts with Sir2 and Sir3:
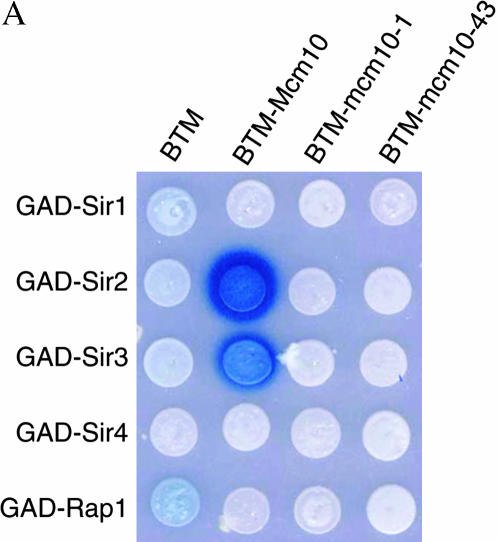
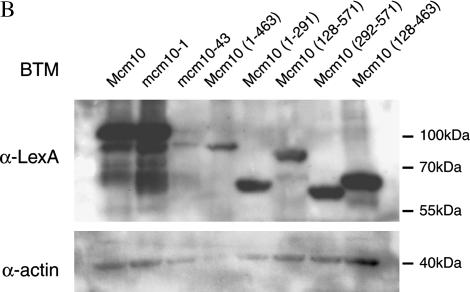
If Mcm10 plays a direct role in silencing, it should interact with components of the silencing machinery. To test this possibility, we used yeast two-hybrid assays to investigate whether Mcm10 interacts with Sir1, Sir2, Sir3, Sir4, or Rap1. The combinations of BTM-MCM10 and GAD-SIR2 or GAD-SIR3 showed activation of the LacZ reporter indicated by the blue color of the colonies formed on media containing X-gal (Figure 3A), signifying Mcm10 interactions with Sir2 and Sir3. These interactions are disrupted by two single-amino-acid substitution mutations, mcm10-1 (P269L) and mcm10-43 (C320Y). Western blot analysis of BTM-mcm10-1 and BTM-mcm10-43 indicated that these fusion proteins are stably expressed (Figure 3B). These results were largely confirmed by switching the bait/prey orientation in the two-hybrid experiment (Figure 3C). The lack of interaction between BTM-SIR3 and GAD-MCM10 is probably due to interference of the BTM tag with Sir3 function as previously observed (A. Lustig, personal communication). None of the other plasmid combinations showed an increase in LacZ activity (Figure 3A).
Figure 3.
The C terminus of Mcm10 interacts with silencing proteins. Yeast two-hybrid bait/prey constructs were transformed into reporter strains EGY40[pSH18-34] (A, B, D, and E), or PJ69-4a (C). (A, B, D, and E) Cultures were spotted onto selective X-gal plates. An interaction is indicated by blue color resulting from activation of the LacZ reporter. (B) Cultures of EGY40[pSH18-34] expressing BTM-Mcm10 fusions were grown overnight in CM-TRP media. Cells from 1-ml aliquots of these cultures were boiled and run on Western blots according to standard protocols. The blot was probed with rabbitt anti-LexA antibody (a gift from Jeff Roberts), stripped, and reprobed with rabbit anti-actin antibody (a gift from Tim Huffaker). (C) Saturated cultures were serially diluted and spotted on selective plates. An interaction is indicated by growth on CM-HIS media caused by activation of the HIS3 reporter. (E) Two-hybrid experiments were performed in Δsir2, Δsir3, or Δsir4 deletion strains ILY267, ILY268, and ILY269.
We also set up two-hybrid experiments in another system, using GBK-tagged proteins as bait and a HIS3 gene as reporter (Figure 3D). In this system, GBK-MCM10 showed interactions with GAD-SIR2 and GAD-SIR3 while GBK-mcm10-1 disrupted these interactions, further confirming results observed in Figure 3A.
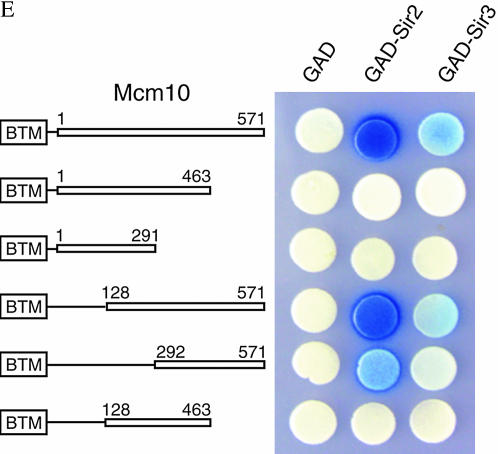
To identify the Sir2- and Sir3-interacting domain of Mcm10, we carried out two-hybrid analysis with truncations of Mcm10 (Figure 3E). As shown above, full-length Mcm10 showed strong interaction with Sir2 and significant interaction with Sir3. Interactions with Sir2 and Sir3 were uncompromised by the N-terminal deletion of 128 amino acids from Mcm10 (128-571) and somewhat compromised by deletion from Mcm10 (292-571). In contrast, C-terminal deletion of 108 amino acids from Mcm10 (1-463) completely destroyed interactions with both Sir2 and Sir3. Western blot analysis indicated that all of these truncation fusions are stably expressed (Figure 3B). These results indicate that the interacting domain of Mcm10 with Sir2 and Sir3 is located at the C-terminal region. We speculate that disruption of this interaction by mcm10-1(P269L) may be the result of an overall structural change in the Mcm10 protein that compromises the C-terminal interacting domain.
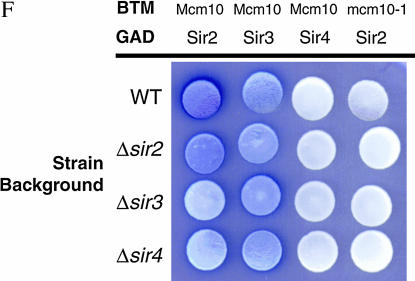
The finding that both Sir2 and Sir3 interact with Mcm10 through the C-terminal domain of Mcm10 could be the result of indirect interaction of one Sir protein mediated by another. To investigate if Sir2 is mediated by Sir3 or vice versa in their interactions with Mcm10, we carried out the two-hybrid analysis in strains deleted in SIR2, SIR3, or SIR4 (Figure 3F). We found that Sir2 and Sir3 interacted with Mcm10 independent of any of the Sir proteins.
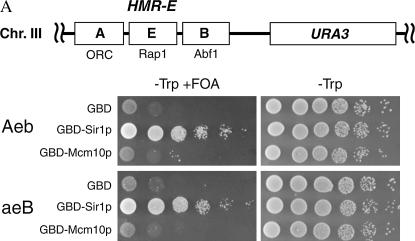
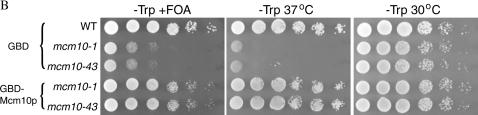
Tethering Mcm10p to a defective HMR-E silencer is not sufficient to restore silencing. Directly recruiting one of the Sir proteins or Rap1 to a defective silencer can restore silencing (Chien et al. 1993; Buck and Shore 1995; Lustig et al. 1996; Marcand et al. 1996). To investigate whether tethering Mcm10 is sufficient to restore silencing, we used strains in which the silencer was disrupted and replaced by Gal4 binding sites (Andrulis et al. 2002). The mating-type gene cassette at the HMR was replaced with URA3, providing a way to measure silencing at this locus. We made a plasmid construct expressing a GBD-MCM10 fusion. This hybrid gene is unable to restore silencing to an HMR-E, where either the Rap1 and Abf1 binding sites (Aeb, Figure 4A, top) or the ORC and Rap1 binding sites (aeB, Figure 4A, bottom) are deleted, while expression of GBD-SIR1 was able to restore silencing in both cases (Figure 4A). Since it is possible that the fusion protein is unstable or otherwise defective, we checked its ability to complement mcm10 mutations. GBD-MCM10 was able to complement both the temperature sensitivity and the silencing defect of mcm10-1 and mcm10-43 (Figure 4B), suggesting that the hybrid protein retains its silencing function. Taken together, these data show that recruiting Mcm10 is not sufficient to restore silencing to a defective silencer.
Figure 4.
Tethering Mcm10 to a defective silencer is not sufficient to restore silencing. (A) GBD-Mcm10 is not able to restore silencing at HMR-E (depicted in schematic). Strains YEA80 (top) or YEA82 (bottom) were transformed with pGBT9, pGBT9SIR1, or pGBT9MCM10. Tenfold serial dilutions were plated on the indicated media and grown at 30° for 2 days. (B) GBD-MCM10 construct is able to restore both the temperature sensitivity and the silencing defect of mcm10 strains. Strains YB541, ILY180, and ILY102 were transformed with either pGBT9 or pGBT9MCM10 (two bottom rows). Tenfold serial dilutions were plated on selective media with or without 5-FOA at 30° (permissive temperature) or on complete media at 37° (restrictive temperature).
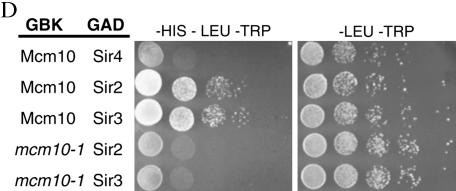
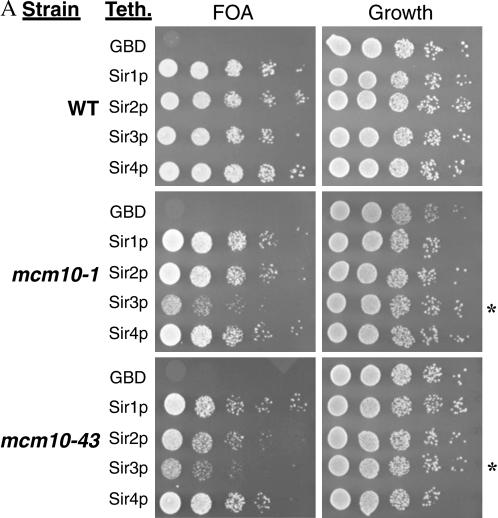
Mutations in MCM10 inhibit the ability of GBD-SIR3 to restore silencing:
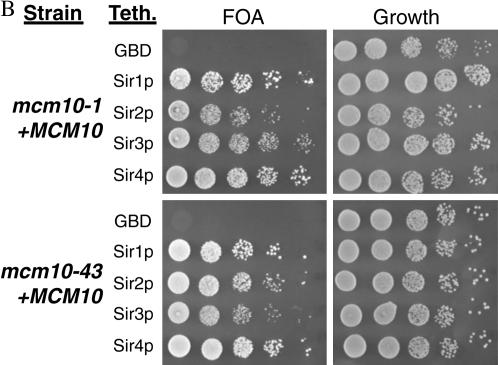
Normally, tethering any of the Sirs to a defective silencer is sufficient to restore silencing (Chien et al. 1993; Lustig et al. 1996; Marcand et al. 1996). If Mcm10 functions downstream of the Sirs, then a mcm10 mutation may inhibit the ability of GBD-SIR constructs to restore silencing to a defective HMR-E. We constructed tethering yeast strains containing either mcm10-1 or mcm10-43 mutations as well as the defective silencer with GBD binding sites and HMR marked with URA3. As expected, tethering any of the Sirs in a MCM10 strain restored silencing to approximately wild-type levels (Figure 5A, top). On the other hand, targeted silencing by GBD-SIR3 was defective in both mcm10 mutant strains (Figure 5A, bottom). We were able to complement this defect by adding a plasmid containing a single copy of MCM10 (Figure 5B). Interestingly, mutations in MCM10 did not have an effect on targeted silencing by GBD-SIR1, GBD-SIR2, or GBD-SIR4, all of which would require the participation of Sir3 for silencing. Together these findings suggest that the silencing function of MCM10 may lie downstream of the Sir proteins, with Mcm10 regulating the silencing activity of Sir3.
Figure 5.
Mutations in MCM10 inhibit the ability of GBD-Sir3 to restore silencing when tethered to a defective silencer. (A) Tethering assays in the presence of mcm10 mutations. Strains YEA80 (WT), ILY137 (mcm10-1), and ILY139 (mcm10-43) were transformed with pM165, pM2046, pM1587, and pM1588, respectively. Tenfold dilutions of overnight cultures were spotted on appropriate dropout media with or without 5-FOA. The strain background (Strain) and tethering plasmid used (Teth.) are indicated. All strains were also transformed with empty vector pRS315 (GBD) as controls. The strains that show a silencing defect are indicated by an asterisk. (B) MCM10 restores silencing to strains from A by transforming these strains with pRS315MCM10. Silencing was assayed as in A.
The temperature sensitivity and silencing defects of mcm10-1 can be genetically separated:
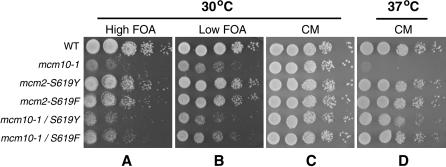
Since Mcm10 plays a role in DNA replication, it is possible that the silencing defect caused by mcm10 mutations is a by-product of a replication defect or of an overall defective S phase. To address this point we asked if the temperature sensitivity (which is attributed to a defect in replication) and silencing defects of mcm10-1 could be separated. In our studies of Mcm10 function we conducted a screen for second-site suppressors of the temperature-sensitive phenotype of mcm10-1. We screened for spontaneous reversion of the mcm10-1 temperature sensitivity that also conferred a second phenotype (cold sensitivity) so that the mutation could be easily identified. We isolated two mutants, MCM2-S619Y and MCM2-S619F. These suppressors are capable of overcoming the temperature sensitivity of mcm10-1 (Figure 6D) in a dominant fashion (data not shown). We constructed strains that contain the HMR and telomeric silencing reporters, mcm10-1, and/or the suppressor mutations to assay whether these mutations can also suppress the telomeric silencing defect conferred by mcm10-1. The mutations Mcm2-S619Y or Mcm2-S619F did not confer a silencing defect on their own (Figure 6, A and B). While these mcm2 alleles were able to suppress the temperature-sensitive phenotype caused by the mcm10-1 mutation, they failed to restore proper telomeric (Figure 6, A and B) and HMR (data not shown) silencing to a mcm10-1 strain. These results suggest that the temperature-sensitive phenotype of mcm10-1 is not linked to the silencing defect.
Figure 6.
The temperature sensitivity of mcm10-1 can be separated from its silencing defect. MCM2-S619Y and MCM2-S619F suppress the temperature sensitivity of mcm10-1, but not the silencing defect. Tenfold serial dilutions of overnight cultures of strains ILY171, ILY180, ILY220, ILY221, ILY222, and ILY223 were plated on complete media plates at 37° (D) or 30° (A–C). A and B show growth on synthetic complete media containing 0.5 mg/ml (low FOA) or 1 mg/ml (high FOA). Plates were grown for ∼2 days.
DISCUSSION
Involvement of numerous DNA replication proteins such as the ORC, PCNA, RF-C, Cdc45, and Mcm5 in transcriptional silencing has been implicated in S. cerevisiae. Recently, involvement of silencing proteins such as Sir2 and Sir3 in the regulation of replication initiation has also been reported (Pappas et al. 2004). The relationship between these two multistep processes and their participating components remains an enigma. Mcm10 is a DNA replication factor involved in both the initiation and elongation steps of DNA replication (Homesley et al. 2000; Wohlschlegel et al. 2002; Lee et al. 2003; Fien et al. 2004; Sawyer et al. 2004). Although Mcm10 has previously been shown to interact with DNA replication proteins implicated in silencing (Homesley et al. 2000; Kawasaki et al. 2000), a direct silencing function has never been described. The results presented in this study suggest that Mcm10 interacts with Sir2 and Sir3 in the maintenance of transcriptional silencing and that this function appears to be separable from its functions essential for DNA replication.
We have demonstrated that two recessive alleles of MCM10 were able to derepress URA3 and ADE2 reporter genes at telomeric and HMR loci (Figure 1). In addition, these mutations were able to derepress endogenous genes at HML (Figure 2, A and B). Thus, Mcm10 has a rather ubiquitous role in silencing, in contrast to ORC, which plays a role only in HM silencing. Although silencing in S. cerevisiae also occurs in ribosomal DNA loci, the effects of Mcm10 on rDNA silencing were not examined in this study. Using reporter genes at HMR, mcm10 mutants clearly showed derepression of HMR (Figure 1B). However, mating assays in the mcm10 sir1Δ double mutants showed a MATa-specific mating defect supporting the notion that these mutants derepress HML more efficiently than HMR (Figure 2B). This phenomenon has been observed before with other maintenance mutants (Enomoto et al. 2000) and can most likely be attributed to the fact that silencing is stronger at the HMR than at the HML.
The possibility that Mcm10 may have a separate silencing function is supported by the observation that dominant extragenic suppressors (MCM2-S619Y and MCM2-S619F) of the temperature sensitivity of mcm10-1 are not able to suppress the silencing defect (Figure 6). In fact, it is possible that Mcm10 may execute its silencing function at the replication fork and yet its silencing function could be uncoupled from it replication activity. However, we cannot rule out the unlikely possibility that these two apparently independent phenotypes are a manifestation of the same defect at different levels of severity. Future experiments with the aid of separation-of-function mutations in MCM10 should clarify this point.
Tethering silencing proteins to a defective silencer can restore silencing. However, tethering GBD-Mcm10 to a defective silencer could not restore silencing (Figure 5). This finding suggests that Mcm10 functions downstream of the establishment of silent chromatin in the propagation or maintenance of silent chromatin. This hypothesis is supported by epistasis analysis with sir1 and cac1 in the shmoo assay and the reporter silencing assays (Figure 2, A and B). MCM10 is in the same epistasis group as CAC1, which encodes a subunit of the histone chaperone CAF-1 known to be involved in the maintenance of heterochromatin. It is in a different epistasis group from SIR1, whose role in silencing is restricted to establishment. Previous tethering experiments with Sir proteins showed that recruiting any of the four Sirs to a defective silencer can restore silencing to wild-type levels (Chien et al. 1993; Marcand et al. 1996; Triolo and Sternglanz 1996). When we conducted similar experiments in mcm10 strains, we noted that GBD-Sir3 constructs lose their ability to properly restore silencing (Figure 5). This result suggests that Mcm10 may modulate Sir3 activity and is consistent with the two-hybrid interaction between Mcm10 and Sir3 (Figure 3). However, although Mcm10 also interacts with Sir2, silencing by GBD-Sir2 is not inhibited. One explanation is that the GBD fusion may be altering the structure of Sir3 in such a way to make it hypersensitive to mutations in MCM10. Another explanation is that Sir2 and Mcm10 interact indirectly, through Sir3 or another yet unidentified factor. However, deletion of SIR3 had no effect on the two-hybrid interaction of Sir2 and Mcm10, suggesting that Sir3 cannot be the mediator. Taken together, these data indicate that the nature of the Mcm10-Sir2 interaction may be different from that of the Mcm10-Sir3 interaction. Sir3 has been shown to have homology to Orc1, a member of the origin recognition complex (Zhang et al. 2002). Mcm10 also interacts with members of the ORC including Orc1 (Kawasaki et al. 2000), Orc2 (Kawasaki et al. 2000; Christensen and Tye 2003), Orc3, Orc5, and Orc6 (Douglas 2003). It is possible that functional and/or structural similarities between silencing and replication proteins may mediate the Mcm10-Sir3 interaction.
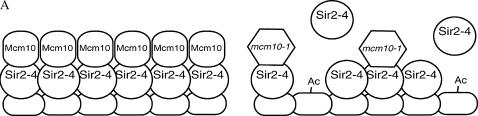
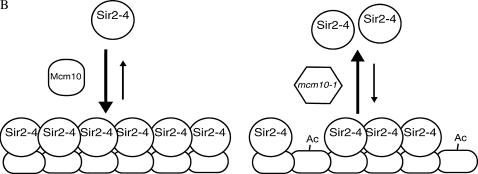
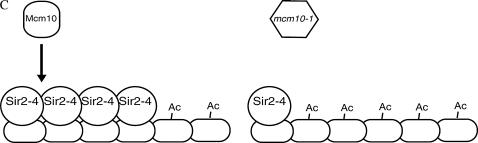
The fact that Mcm10's function lies in the maintenance of silent chromatin and yet tethering Mcm10 does not restore silencing suggests that Mcm10 may act to stabilize silent chromatin structures, such as Sir2-4 complexes, after they have been recruited. Stabilization may be accomplished by Mcm10 binding to assembled Sir2-4 complexes on chromatin (Figure 7A) or escorting soluble Sir2-4 to chromatin (Figure 7B), consistent with the interaction of Mcm10 with both Sir2 and Sir3. Deposition of Mcm10 on propagating heterochromatin may be mediated by the elongation machinery (Figure 7A) or drawn directly from soluble Mcm10 (Figure 7, A and B). However, if the function of Mcm10 is to simply stabilize established silent chromatin, it should not matter which GBD-SIR fusion is used to initiate silencing in our tethering experiments. Our data suggest that in the absence of wild-type Mcm10, GBD-Sir3 has difficulty nucleating the spread of silent chromatin. The fact that only GBD-Sir3-mediated silencing is affected in mcm10 strains may indicate that this defect occurs during the steps when GBD-Sir3 first binds DNA and initiates silencing before the other Sirs are recruited. Perhaps mcm10 mutations are inhibiting the first step in this artificial establishment of silencing such that GBD-Sir3 has trouble recruiting the other Sirs to begin the assembly and spreading of Sir complexes from the silencer (Figure 7C). In other words, GBD-Sir3 alone may not completely fulfill its proper function in mcm10 strains and that transition of establishment to maintenance requires the participation of the other Sirs if Mcm10 is compromised. Furthering this idea (Figure 7C), we believe that Mcm10 cooperates with Sir3 during the normal transition of nucleation to spreading of heterochromatin at the silent mating-type and telomeric loci (Figures 1 and 2) independent of GBD-Sir3. This explanation is consistent with the established model of a cooperative mechanism of silencing between the Sir proteins. This explanation is also consistent with the hypothesized role of Mcm10 at replication origins as a stabilizing scaffold in the transition of the pre-replication complex to the pre-initiation complex (IC) and subsequently from the pre-IC to the elongation complex (Homesley et al. 2000; Wohlschlegel et al. 2002; Lee et al. 2003; Fien et al. 2004; Sawyer et al. 2004). By studying the roles of Mcm10 in DNA replication and in gene silencing we may begin to elucidate the connection between DNA replication and heterochromatin assembly.
Figure 7.
Hypotheses for Mcm10 function in maintenance of silencing. (A) Mcm10 associates with the chromatin-bound Sir2-4 complex to stabilize silent chromatin. Mutant Mcm10 proteins are not able to maintain interaction with the Sirs. Dissociation of Sirs from chromatin results in derepression of silent loci. (B) Mcm10p acts to recruit Sir proteins to silent chromatin where needed. In the case of mcm10 mutants, inability of Mcm10-1p to facilitate the binding of a sufficient number of Sir complexes to the silent region leads to the degradation of silent chromatin over time. (C) Mcm10 facilitates the transition from the nucleation of Sir2-4 complexes at the silencer to the spreading of Sir2-4 complexes along the silenced region. A defective Mcm10 does not allow the proper spreading of silent chromatin.
Acknowledgments
We thank Nancy Douglas and Erik Andrulis for many of the strains, plasmids, and reagents used in this study and for their discussions and critical reading of this manuscript. We also thank Bruce Stillman, Rolf Sternglanz, Erica Golemis, Judith Berman, David Shore, Ravi Ram, Jeff Roberts, and Tim Huffaker for providing strains, plasmids, and antibodies. This work is supported by the National Institutes of Health [(NIH)GM34190]. I.L. is supported by a Graduate Assistance in Area of National Need fellowship (P200A030105 and NIHGM7617).
References
- Andrulis, E. D., D. C. Zappulla, A. Ansari, S. Perrod, C. V. Laiosa et al., 2002. Esc1, a nuclear periphery protein required for Sir4-based plasmid anchoring and partitioning. Mol. Cell. Biol. 22: 8292–8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aparicio, O., B. Billington and D. Gottschling, 1991. Modifiers of position effect are shared between telomeric and silent mating-type loci in S. cerevisiae. Cell 66: 1279–1287. [DOI] [PubMed] [Google Scholar]
- Bailis, J., P. Bernard, R. Antonelli, R. Allshire and S. Forsburg, 2003. Hsk1-Dfp1 is required for heterochromatin-mediated cohesion at centromeres. Nat. Cell Biol. 5: 1111–1116. [DOI] [PubMed] [Google Scholar]
- Bartel, P., J. Roecklein, D. SenGupta and S. Fields, 1996. A protein linkage map of Escherichia coli bacteriophage T7. Nat. Genet. 12: 72–77. [DOI] [PubMed] [Google Scholar]
- Bell, S., R. Kobayashi and B. Stillman, 1993. Yeast origin recognition complex functions in transcription silencing and DNA replication. Science 262: 1844–1849. [DOI] [PubMed] [Google Scholar]
- Brand, A., L. Breeden, J. Abraham, R. Sternglanz and K. Nasmyth, 1985. Characterization of a “silencer” in yeast: a DNA sequence with properties opposite to those of a transcriptional enhancer. Cell 41: 41–48. [DOI] [PubMed] [Google Scholar]
- Brand, A., G. Micklem and K. Nasmyth, 1987. A yeast silencer contains sequences that can promote autonomous plasmid replication and transcriptional activation. Cell 51: 709–719. [DOI] [PubMed] [Google Scholar]
- Buck, S., and D. Shore, 1995. Action of a RAP1 carboxy-terminal silencing domain reveals an underlying competition between HMR and telomeres in yeast. Genes Dev. 9: 370–384. [DOI] [PubMed] [Google Scholar]
- Chien, C., S. Buck, R. Sternglanz and D. Shore, 1993. Targeting of SIR1 protein establishes transcriptional silencing at HM loci and telomeres in yeast. Cell 75: 531–541. [DOI] [PubMed] [Google Scholar]
- Christensen, T. W., and B. K. Tye, 2003. Drosophila Mcm10 interacts with members of the prereplication complex and is required for proper chromosome condensation. Mol. Biol. Cell 14: 2206–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diffley, J., and B. Stillman, 1988. Purification of a yeast protein that binds to origins of DNA replication and a transcriptional silencer. Proc. Natl. Acad. Sci. USA 85: 2120–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas, N., 2003. Functional dissection of Saccharomyces cerevisiae MCM10 Ph.D. Thesis, Cornell University, Ithaca, NY.
- Dumas, L., J. Lussky, E. McFarland and J. Shampay, 1982. New temperature-sensitive mutants of Saccharomyces cerevisiae affecting DNA replication. Mol. Gen. Genet. 187: 42–46. [DOI] [PubMed] [Google Scholar]
- Dutta, A., and S. P. Bell, 1997. Initiation of DNA replication in eukaryotic cells. Annu. Rev. Cell Dev. Biol. 13: 293–332. [DOI] [PubMed] [Google Scholar]
- Dziak, R., D. Leishman, M. Radovic, B. K. Tye and K. Yankulov, 2003. Evidence for a role of MCM (mini-chromosome maintenance)5 in transcriptional repression of sub-telomeric and Ty-proximal genes in Saccharomyces cerevisiae. J. Biol. Chem. 278: 27372–27381. [DOI] [PubMed] [Google Scholar]
- Ehrenhofer-Murray, A. E., R. T. Kamakaka and J. Rine, 1999. A role for the replication proteins PCNA, RF-C, polymerase ɛ and Cdc45 in transcriptional silencing in Saccharomyces cerevisiae. Genetics 153: 1171–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto, S., and J. Berman, 1998. Chromatin assembly factor I contributes to the maintenance, but not the re-establishment, of silencing at the yeast silent mating loci. Genes Dev. 12: 219–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto, S., P. McCune-Zierath, M. Gerami-Nejad, M. Sanders and J. Berman, 1997. RLF2, a subunit of yeast chromatin assembly factor-I, is required for telomeric chromatin function in vivo. Genes Dev. 11: 358–370. [DOI] [PubMed] [Google Scholar]
- Enomoto, S., S. D. Johnston and J. Berman, 2000. Identification of a novel allele of SIR3 defective in the maintenance, but not the establishment, of silencing in Saccharomyces cerevisiae. Genetics 155: 523–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman, J., J. Hicks and J. Broach, 1984. Identification of sites required for repression of a silent mating type locus in yeast. J. Mol. Biol. 178: 815–834. [DOI] [PubMed] [Google Scholar]
- Fields, S., and O. Song, 1989. A novel genetic system to detect protein-protein interactions. Nature 340: 245–246. [DOI] [PubMed] [Google Scholar]
- Fien, K., Y.-S. Cho, J.-K. Lee, S. Raychaudhuri, I. Tappin et al., 2004. Primer utilization by DNA polymerase alpha-primase is influenced by its interaction with Mcm10p. J. Biol. Chem. 279: 16144–16153. [DOI] [PubMed] [Google Scholar]
- Grewal, S. I. S., and S. C. R. Elgin, 2002. Heterochromatin: new possibilities for the inheritance of structure. Curr. Opin. Genet. Dev. 12: 178–187. [DOI] [PubMed] [Google Scholar]
- Grunweller, A., and A. E. Ehrenhofer-Murray, 2002. A novel yeast silencer: the 2μ origin of Saccharomyces cerevisiae has HST3-, MIG1- and SIR-dependent silencing activity. Genetics 162: 59–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber, J. E., 1998. Mating-type gene switching in Saccharomyces cerevisiae. Annu. Rev. Genet. 32: 561–599. [DOI] [PubMed] [Google Scholar]
- Hecht, A., S. Strahl-Bolsinger and M. Grunstein, 1996. Spreading of transcriptional repressor SIR3 from telomeric heterochromatin. Nature 383: 92–96. [DOI] [PubMed] [Google Scholar]
- Homesley, L., M. Lei, Y. Kawasaki, S. Sawyer, T. Christensen et al., 2000. Mcm10 and the MCM2–7 complex interact to initiate DNA synthesis and to release replication factors from origins. Genes Dev. 14: 913–926. [PMC free article] [PubMed] [Google Scholar]
- Hoppe, G. J., J. C. Tanny, A. D. Rudner, S. A. Gerber, S. Danaie et al., 2002. Steps in assembly of silent chromatin in yeast: Sir3-independent binding of a Sir2/Sir4 complex to silencers and role for Sir2-dependent deacetylation. Mol. Cell. Biol. 22: 4167–4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki, Y., S.-i. Hiraga and A. Sugino, 2000. Interactions between Mcm10p and other replication factors are required for proper initiation and elongation of chromosomal DNA replication in Saccharomyces cerevisiae. Genes Cells 5: 975–989. [DOI] [PubMed] [Google Scholar]
- Kellum, R., 2003. HP1 complexes and heterochromatin assembly. Curr. Top. Microbiol. Immunol. 274: 53–77. [DOI] [PubMed] [Google Scholar]
- Kirchmaier, A. L., and J. Rine, 2001. DNA replication-independent silencing in S. cerevisiae. Science 291: 646–650. [DOI] [PubMed] [Google Scholar]
- Kyrion, G., K. Liu, C. Liu and A. Lustig, 1993. RAP1 and telomere structure regulate telomere position effects in Saccharomyces cerevisiae. Genes Dev. 7: 1146–1159. [DOI] [PubMed] [Google Scholar]
- Lee, J.-K., Y.-S. Seo and J. Hurwitz, 2003. The Cdc23 (Mcm10) protein is required for the phosphorylation of minichromosome maintenance complex by the Dfp1-Hsk1 kinase. Proc. Natl. Acad. Sci. USA 100: 2334–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y.-C., T.-H. Cheng and M. R. Gartenberg, 2001. Establishment of transcriptional silencing in the absence of DNA replication. Science 291: 650–653. [DOI] [PubMed] [Google Scholar]
- Loo, S., C. Fox, J. Rine, R. Kobayashi, B. Stillman et al., 1995. The origin recognition complex in silencing, cell cycle progression, and DNA replication. Mol. Biol. Cell 6: 741–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig, A., C. Liu, C. Zhang and J. Hanish, 1996. Tethered Sir3p nucleates silencing at telomeres and internal loci in Saccharomyces cerevisiae. Mol. Cell. Biol. 16: 2483–2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney, D., R. Marquardt, G. Shei, A. Rose and J. Broach, 1991. Mutations in the HML E silencer of Saccharomyces cerevisiae yield metastable inheritance of transcriptional repression. Genes Dev. 5: 605–615. [DOI] [PubMed] [Google Scholar]
- Maine, G. T., P. Sinha and B.-K. Tye, 1984. Mutants of S. cerevisiae defective in the maintenance of minichromosomes. Genetics 106: 365–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcand, S., S. Buck, P. Moretti, E. Gilson and D. Shore, 1996. Silencing of genes at nontelomeric sites in yeast is controlled by sequestration of silencing factors at telomeres by Rap 1 protein. Genes Dev. 10: 1297–1309. [DOI] [PubMed] [Google Scholar]
- Merchant, A., Y. Kawasaki, Y. Chen, M. Lei and B. Tye, 1997. A lesion in the DNA replication initiation factor Mcm10 induces pausing of elongation forks through chromosomal replication origins in Saccharomyces cerevisiae. Mol. Cell. Biol. 17: 3261–3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micklem, G., A. Rowley, J. Harwood, K. Nasmyth and J. Diffley, 1993. Yeast origin recognition complex is involved in DNA replication and transcriptional silencing. Nature 366: 87–89. [DOI] [PubMed] [Google Scholar]
- Miller, A., and K. Nasmyth, 1984. Role of DNA replication in the repression of silent mating type loci in yeast. Nature 312: 247–251. [DOI] [PubMed] [Google Scholar]
- Monson, E. K., D. de Bruin and V. A. Zakian, 1997. The yeast Cac1 protein is required for the stable inheritance of transcriptionally repressed chromatin at telomeres. Proc. Natl. Acad. Sci. USA 94: 13081–13086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti, P., K. Freeman, L. Coodly and D. Shore, 1994. Evidence that a complex of SIR proteins interacts with the silencer and telomere-binding protein RAP1. Genes Dev. 8: 2257–2269. [DOI] [PubMed] [Google Scholar]
- Pappas, Jr., D. L., R. Frisch and M. Weinreich, 2004. The NAD+-dependent Sir2p histone deacetylase is a negative regulator of chromosomal DNA replication. Genes Dev. 18: 769–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflumm, M., and M. Botchan, 2001. Orc mutants arrest in metaphase with abnormally condensed chromosomes. Development 128: 1697–1707. [DOI] [PubMed] [Google Scholar]
- Pillus, L., and J. Rine, 1989. Epigenetic inheritance of transcriptional states in S. cerevisiae. Cell 59: 637–647. [DOI] [PubMed] [Google Scholar]
- Ricke, R. M., and A.-K. Bielinsky, 2004. Mcm10 regulates the stability and chromatin association of DNA polymerase-alpha. Mol. Cell 16: 173–185. [DOI] [PubMed] [Google Scholar]
- Rose, M. D., P. Novick, J. H. Thomas, D. Botstein and G. R. Fink, 1987. A Saccharomyces cerevisiae genomic plasmid bank based on a centromere-containing shuttle vector. Gene 60: 237–243. [DOI] [PubMed] [Google Scholar]
- Rusche, L. N., A. L. Kirchmaier and J. Rine, 2002. Ordered nucleation and spreading of silenced chromatin in Saccharomyces cerevisiae. Mol. Biol. Cell 13: 2207–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusche, L. N., A. L. Kirchmaier and J. Rine, 2003. The establishment, inheritance, and function of silenced chromatin in Saccharomyces cerevisiae. Annu. Rev. Biochem. 72: 481–516. [DOI] [PubMed] [Google Scholar]
- Sawyer, S. L., I. H. Cheng, W. Chai and B. K. Tye, 2004. Mcm10 and Cdc45 cooperate in origin activation in Saccharomyces cerevisiae. J. Mol. Biol. 340: 195–202. [DOI] [PubMed] [Google Scholar]
- Sherman, F., 1991. Getting started with yeast. Methods Enzymol. 194: 3–21. [DOI] [PubMed] [Google Scholar]
- Shore, D., and K. Nasmyth, 1987. Purification and cloning of a DNA binding protein from yeast that binds to both silencer and activator elements. Cell 51: 721–732. [DOI] [PubMed] [Google Scholar]
- Solomon, N., M. Wright, S. Chang, A. Buckley, L. Dumas et al., 1992. Genetic and molecular analysis of DNA43 and DNA52: two new cell-cycle genes in Saccharomyces cerevisiae. Yeast 8: 273–289. [DOI] [PubMed] [Google Scholar]
- Sussel, L., and D. Shore, 1991. Separation of transcriptional activation and silencing functions of the RAP1-encoded repressor/activator protein 1. Isolation of viable mutants affecting both silencing and telomere length. Proc. Natl. Acad. Sci. USA 88: 7749–7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triolo, T., and R. Sternglanz, 1996. Role of interactions between the origin recognition complex and SIR1 in transcriptional silencing. Nature 381: 251–253. [DOI] [PubMed] [Google Scholar]
- Verreault, A., P. D. Kaufman, R. Kobayashi and B. Stillman, 1996. Nucleosome assembly by a complex of CAF-1 and acetylated histones H3/H4. Cell 87: 95–104. [DOI] [PubMed] [Google Scholar]
- Wohlschlegel, J., S. Dhar, T. Prokhorova, A. Dutta and J. Walter, 2002. Xenopus Mcm10 binds to origins of DNA replication after Mcm2–7 and stimulates origin binding of Cdc45. Mol. Cell 9: 233–240. [DOI] [PubMed] [Google Scholar]
- Yang, X., J. Gregan, K. Lindner, H. Young and S. E. Kearsey, 2005. Nuclear distribution and chromatin association of DNA polymerase alpha-primase is affected by TEV protease cleavage of Cdc23 (Mcm10) in fission yeast. BMC Mol. Biol. 6: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Z., K. Shibahara and B. Stillman, 2000. PCNA connects DNA replication to epigenetic inheritance in yeast. Nature 408: 221–225. [DOI] [PubMed] [Google Scholar]
- Zhang, Z., M. K. Hayashi, O. Merkel, B. Stillman and R.-M. Xu, 2002. Structure and function of the BAH-containing domain of Orc1p in epigenetic silencing. EMBO J. 21: 4600–4611. [DOI] [PMC free article] [PubMed] [Google Scholar]