Abstract
A rapid route to gene molecular identification involves using recombination frequencies in locating mutational sequence changes. We describe a case where the recombination frequency is deceptively low, probably reflecting centromere proximity. Recombination frequencies are greatly reduced near the centromeres on the right arms of chromosomes III and IV of Aspergillus nidulans.
WITH the advent of whole-genome sequencing, an attractive route for identifying the sequence of a gene for which a mutant allele exists involves mapping the mutation and sequencing plausible candidate open reading frames (ORFs) to locate the mutational sequence change. Sometimes, however, recombination frequencies can be deceptive, as in the case described here.
As part of a project to investigate nuclear localization of the PacC transcription factor of the ascomycete Aspergillus nidulans, we sought spontaneous mutations that suppress the molybdate hypersensitivity phenotype of a pacC allele whose translation product has a K159M mutational change inactivating the PacC nuclear localization signal (described by Fernández-Martinez et al. 2003). We selected for tolerance of 20 mm Na2MoO4 on supplemented minimal medium (Cove 1966). Among the mutations obtained, one, designated halA24, had no effect on PacC nuclear localization or on other aspects of the highly pleiotropic (see reviews by Peñalva and Arst 2002, 2004) mutant pacC allele phenotype apart from molybdate hypersensitivity. However, it has an otherwise interesting phenotype (to be described elsewhere). Using conventional parasexual and sexual mapping techniques (Clutterbuck 1974) facilitated by the molybdate resistance of halA24 pacC+ strains, halA24 was located between phenA and cbxA/carC on the right arm of chromosome III. Two preliminary crosses, with 99 and 100 progeny analyzed, respectively, gave recombination frequencies of 1 cM to phenA2 and 13 and 17 cM, respectively, to cbxA17. Using the A. nidulans genome sequence (http://www.broad.mit.edu/annotation/fungi/aspergillus) to identify likely ORFs, mutational sequence changes were determined for identifying definitively the phenA and cbxA/carC genes (Table 1). Following failure to find the halA24 sequence change in plausible candidate ORFs near phenA on the cbxA side, it was eventually located at >35% of the physical distance between phenA and cbxA (Figure 1). The gene identity has been firmly established and its characterization will be described in detail elsewhere. Its predicted product is very likely to be a Ser/Thr protein kinase (http://www.sanger.ac.uk/Software/Pfam; E-value 5.7e-78) and halA24 tandemly duplicates four residues within the predicted kinase domain (Table 1).
TABLE 1.
Mutant sequence changes determined in this work
| Gene | Database notation | Allele | Nucleotide change | Amino acid change | Mutant protein |
|---|---|---|---|---|---|
| phenA | NAa | phenA2 | A100Gd | IVS1g | 1–18 + 15 |
| phenA4 | A100G | IVS1g | 1–18 + 15 | ||
| phenA5 | 859insTT | F268fsh | 1–267 + 19 | ||
| phenA7 | G29A | G10D | G10D | ||
| phenA8 | G421A | W121i | 1–120 | ||
| halA | AN8830.2 | halA24 | 1566insATCAACTCGTCC | insINSS | 1–503 + INSS + 504–524 |
| cbxA/carC | AN8793.2b | cbxA17 | A684G | H156R | H156R |
| carC9 | T483C | L89P | L89P | ||
| suaB | Gln-tRNAc | suaB111 | G145,904Ae | — | — |
| hisA | AF159463 | hisA10 | T973C | L296S | L296S |
| AN7430.2 | |||||
| gdhB | AY223544 | gdhB1 | ΔT1014-T1023 | M318fs | 1–317 + 12 |
| AN7451.2 | |||||
| meaA | AY049706 | meaA8 | C855Tf | Q231i | 1–230 |
| AN7463.2 |
Gene not annotated by the database. Predicted product is prephenate dehydratase; cDNA was sequenced.
Predicted product is cytochrome B-560 subunit of succinate dehydrogenase; cDNA was sequenced.
Predicted glutamine tRNA, anticodon CUG. Start is at 145,869, end at 145,954. Contig 1.161. Amber suppressor. G-to-A change in the anticodon sequence in suaB111.
Nucleotide coordinates are based on the coding region in genomic DNA.
Contig 1.161 coordinate; change in Figure 1 coordinate is G283,287A.
Our nucleotide sequence change corrects that given by Monahan et al. (2002).
Intron lariat sequence was altered; splicing defect is likely. The predicted protein would terminate after translation of the (mutant) intron and the first eight nucleotides of exon 2.
fs, frameshift.
Stop codon.
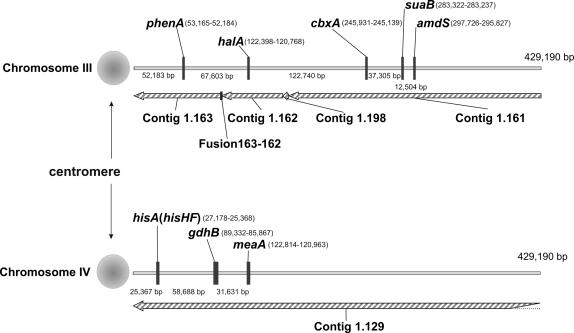
Figure 1.
Physical maps of the centromere-proximal regions of the right arms of chromosomes III and IV. Contig numbering follows that of the Broad Institute (http://www.broad.mit.edu/annotation/fungi/aspergillus/). Distances between genes or to the end of the available genomic sequence are given. Coordinates following the gene name indicate the coding region with that of the first base of the initiation codon appearing first. Contigs 1.161 and 1.162 were joined by alignment with contig 1.198. To join contigs 1.162 and 1.163, genomic DNA was PCR amplified using oligonucleotides annealing at a distance of 170 nt from the end of 1.162 and the beginning of 1.163. Sequencing of the PCR fragment revealed database errors at the ends of both contigs (a consequence of shotgun cloning) and, after deletion of a total of 36 incorrect nt, a 34-nt addition was necessary to join the two contigs. The chromosome III contigs thus joined in total 429,190 bp, but only 429,190 bp of the 627,835 bp of contig 1.129 from chromosome IV are indicated.
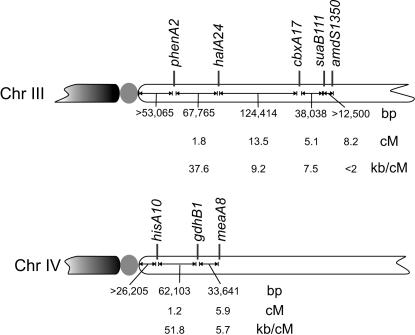
To better document the highly skewed relationship between recombination frequency and physical distance in the phenA-cbxA interval, 392 random progeny were analyzed from the cross biA1; phenA2 cbxA17 × halA24; inoB2; fwA1 (see Clutterbuck 1993 for gene symbols), giving 1.8 ± 0.7 cM between phenA2 and halA24 and 13.5 ± 1.7 cM between halA24 and cbxA17, a 7.5-fold difference. The physical distances are 67,765 and 124,414 bp, respectively (Table 1 and Figure 1), a 1.84-fold difference. Between phenA2 and halA24 1 cM corresponds to 37.6 kb and between halA24 and cbxA17 1 cM corresponds to 9.2 kb. To ensure that this difference is a genuine feature of relative recombination frequencies in these two intervals and not simply the consequence of a previously unrecognized chromosomal rearrangement(s), the two parental strains from this cross were first shown to be translocation-free using the parasexual cycle. Second, a combination of Southern blotting and PCR fragment size determination demonstrated the absence of any significant rearrangement in either parental strain in a region of 96,747 bp extending from 17,847 bp centromere-proximal to phenA2 to 11,134 bp centromere-distal to halA24 (data not shown). It is possible that the recombination frequency continues to rise beyond cbxA. Sealy-Lewis (1987) determined a map distance of 5.1 ± 2.2 cM between cbxA17 and suaB111. Using the physical data in Figure 1 and Table 1 to calculate 38,038 bp between these mutations, this would equate to 7.5 kb/cM. Beyond suaB, the recombination frequency might be even higher in view of the 12.5-kb distance between suaB and amdS coding regions (Figure 1) and the suaB111–amdS1350 8.2 cM map distance (Sealy-Lewis 1987), which would result in a recombination frequency <2 kb/cM.
The most obvious explanation for this markedly nonlinear relationship between recombination frequency and physical distance is the proximity of the centromere. Reduced recombination in the vicinity of the centromere was reported first in Drosophila melanogaster (Dobzhansky 1930) and subsequently in other organisms, a particularly elegant demonstration being that of Lambie and Roeder (1986) in Saccharomyces cerevisiae. In A. nidulans a translocation shifting a region near the chromosome II centromere to a much more centromere-distal position on chromosome VIII increases the recombination frequency between two of the translocation-shifted genes by an order of magnitude (Arst et al. 1979). Arguably the most dramatic effect involves the silent mating-type loci of Schizosaccharomyces pombe where no recombination has been detected at a resolution of 0.001 cM in a 15-kb interval sharing homology with centromeric repeats (Egel et al. 1989; Grewal and Klar 1997 and references therein). All eight A. nidulans centromeres lie within gaps in the genomic sequence, but from the contiguous right arm sequence of chromosome III the minimum distance between phenA2 and the centromere is 53,065 bp.
A striking centromere effect on recombination frequency can also be seen on the right arm of chromosome IV, as previously noted by Aleksenko et al. (2001). With a gene order of centromere, hisA/hisHF, gdhB, and meaA and using mapping data from Arst et al. (1989) in conjunction with the genome sequence (see Figure 1) and mutational sequence changes (Table 1), the distances between hisA10 and gdhB1 are 1.2 ± 0.3 cM and 62,103 bp and those between gdhB1 and meaA8 are 5.9 ± 1.3 cM and 33,641 bp. Between hisA10 and gdhB1 there are 51.8 kb/cM, in good agreement with 49.3 kb/cM between hisA134 and gdhB1 reported by Aleksenko et al. (2001), and between gdhB1 and meaA8 1 cM corresponds to 5.7 kb. The higher rate of recombination between phenA2 and halA24 as compared to that between hisA10 and gdhB1 might be explained by the minimum distance of phenA2 to the centromere being approximately twice that of hisA10 to the centromere (53,065 bp vs. 26,205 bp). Caution is needed, however, because the actual distances to the centromere have not been determined. Figure 2 is a summary showing relationships between recombination frequencies and physical distances on the right arms of chromosomes III and IV.
Figure 2.
Summary indicating meiotic map distances and relationships between recombination frequencies and physical distances.
In the above cases it is very likely that centromere proximity is responsible for greatly reduced recombination frequencies. However, when recombination frequencies are used in conjunction with genome sequences for gene identification, it should be remembered that other factors can also affect the relationship between recombination frequency and physical distance. For brevity we give a few examples from fungi but the factors involved affect recombination frequencies across a wide range of organisms. Such factors include environmental influences such as nutritional state (e.g., Abdullah and Borts 2001), genetic factors including both trans-acting genes (e.g., Catcheside 1981; Yeadon et al. 2004) and cis-acting recombination hotspots (e.g., Pryce et al. 2005; Steiner and Smith 2005), and the long-known effects of chromosomal rearrangements (reviewed by Käfer 1977 and Perkins 1997). These effects can be considerable. For example, effects of more than an order of magnitude have been observed for both trans- and cis-acting genetic factors (Catcheside 1981; Steiner and Smith 2005), and there is evidence that, in the wild, selection operates on recombination frequencies (e.g., Saleem et al. 2001). Judicious choice of strains and crossing conditions and, in extremis, molecular screening for chromosomal rearrangements can facilitate the use of recombination data for gene identification.
Acknowledgments
We are grateful to Heather Sealy-Lewis for suaB111 and to John Clutterbuck for a number of other markers; to Miguel Peñalva, Claudio Scazzocchio, Joan Tilburn, and Ken Haynes for much helpful advice; and to Elena Reoyo and Lily Stanton for technical assistance. We thank the Ministerio de Ciencia y Tecnologia, the Fondo de Investigaciones Sanitarias, and the Wellcome Trust for support through grants BMC2003-00874 to E.A.E., Redemeth G054 to Miguel Peñalva, and 067878 to H.N.A.
References
- Abdullah, M. F. F., and R. H. Borts, 2001. Meiotic recombination frequencies are affected by nutritional states in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 98: 14524–14529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleksenko, A., M. L. Nielsen and A. J. Clutterbuck, 2001. Genetic and physical mapping of two centromere-proximal regions of chromosome IV in Aspergillus nidulans. Fungal Genet. Biol. 32: 45–54. [DOI] [PubMed] [Google Scholar]
- Arst, H. N., Jr., K. N. Rand and C. R. Bailey, 1979. Do the tightly linked structural genes for nitrate and nitrite reductases in Aspergillus nidulans form an operon? Evidence from an insertional translocation which separates them. Mol. Gen. Genet. 174: 89–100. [DOI] [PubMed] [Google Scholar]
- Arst, H. N., Jr., D. Tollervey and M. X. Caddick, 1989. A translocation associated loss-of-function mutation in the nitrogen metabolite repression regulatory gene of Aspergillus nidulans can revert intracistronically. Mol. Gen. Genet. 215: 364–367. [DOI] [PubMed] [Google Scholar]
- Catcheside, D. E. A., 1981. Genes in Neurospora that suppress recombination when they are heterozygous. Genetics 98: 55–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutterbuck, A. J., 1974. Aspergillus nidulans, pp. 447–510 in Handbook of Genetics, Vol. 1: Bacteria, Bacteriophage and Fungi, edited by R. C. King. Plenum Press, New York.
- Clutterbuck, A. J., 1993. Aspergillus nidulans, pp. 3.71–3.84 in Genetic Maps, Vol. 3: Locus Maps of Complex Genomes, Ed. 6, edited by S. J. O'Brien. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Cove, D. J., 1966. The induction and repression of nitrate reductase in the fungus Aspergillus nidulans. Biochim. Biophys. Acta 113: 51–56. [DOI] [PubMed] [Google Scholar]
- Dobzhansky, T., 1930. Translocations involving the third and fourth chromosomes of Drosophila melanogaster. Genetics 15: 347–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egel, R., M. Willer and O. Neilsen, 1989. Unblocking of meiotic crossing-over between the silent mating-type cassettes of fission yeast, conditioned by the recessive, pleiotropic mutant rik1. Curr. Genet. 15: 407–410. [Google Scholar]
- Fernández-Martinez, J., C. V. Brown, E. Díez, J. Tilburn, H. N. Arst, Jr. et al., 2003. Overlap of nuclear localisation signal and specific DNA-binding residues within the zinc-finger domain of PacC. J. Mol. Biol. 334: 667–684. [DOI] [PubMed] [Google Scholar]
- Grewal, S. I. S., and A. J. S. Klar, 1997. A recombinationally repressed region between mat2 and mat3 loci shares homology to centromeric repeats and regulates directionality of mating-type switching in fission yeast. Genetics 146: 1221–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käfer, E., 1977. Meiotic and mitotic recombination in Aspergillus and its chromosomal aberrations. Adv. Genet. 19: 33–131. [DOI] [PubMed] [Google Scholar]
- Lambie, E. J., and G. S. Roeder, 1986. Repression of meiotic crossing over by a centromere (CEN3) in Saccharomyces cerevisiae. Genetics 114: 769–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan, B. J., S. E. Unkles, I. T. Tsing, J. R. Kinghorn, M. J. Hynes et al., 2002. Mutation and functional analysis of the Aspergillus nidulans ammonium permease MeaA and evidence for interaction with itself and MepA. Fungal Genet. Biol. 36: 35–46. [DOI] [PubMed] [Google Scholar]
- Peñalva, M. A., and H. N. Arst, Jr., 2002. Regulation of gene expression by ambient pH in filamentous fungi and yeasts. Microbiol. Mol. Biol. Rev. 66: 426–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peñalva, M. A., and H. N. Arst, Jr., 2004. Recent advances in the characterization of ambient pH regulation of gene expression in filamentous fungi and yeasts. Annu. Rev. Microbiol. 58: 425–451. [DOI] [PubMed] [Google Scholar]
- Perkins, D. D., 1997. Chromosome rearrangements in Neurospora and other filamentous fungi. Adv. Genet. 36: 239–398. [DOI] [PubMed] [Google Scholar]
- Pryce, D. W., A. Lorenz, J. B. Smirnova, J. Loidl and R. J. McFarlane, 2005. Differential activation of M26-containing meiotic recombination hot spots in Schizosaccharomyces pombe. Genetics 170: 95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem, M., B. C. Lamb and E. Nevo, 2001. Inherited differences in crossing over and gene conversion frequencies between wild strains of Sordaria fimicola from “Evolution Canyon.” Genetics 159: 1573–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sealy-Lewis, H. M., 1987. Suppressor specificity in Aspergillus nidulans. Curr. Genet. 12: 141–148. [Google Scholar]
- Steiner, W. W., and G. R. Smith, 2005. Optimizing the nucleotide sequence of a meiotic recombination hotspot in Schizosaccharomyces pombe. Genetics 169: 1973–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeadon, P. J., F. J. Bowring and D. E. A. Catcheside, 2004. Alleles of the hotspot cog are codominant in effect on recombination in the his-3 region of Neurospora. Genetics 167: 1143–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]